Abstract
One of the leading causes of death among patients with malignancies is represented by bone cancer. According to current studies, the leading cause of death among these patients is represented by late diagnosis, poor response to therapy, and the lack of accuracy in terms of clinical evaluation. In this regard, there have been developed a series of methods of diagnosis and evaluation, the most investigated being represented by miRNA expression. In this updated work, we want to present a series of changes in the expression of miRNAs in bone cancer. Moreover, we want to present the implications of miRNAs in targeted therapy in such patients. Studies available in scientific databases such as PubMed and Scopus were examined. The studies were searched using the keywords “miRNAs expression”, “bone cancer”, “genetic therapy” and “genetic biomarkers.” For the evaluation and monitoring of bone cancer, the expression of miRNAs can be successfully used due to increased specificity. Using miRNAs as gene therapy can be also considered a therapeutic method of the future, mainly due to selective and targeted response of the body.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, worldwide, a high percentage of patients die of cancer disease. One of the cancers responsible for an increased rate of mortality is represented by bone cancer. The most common forms of primary bone cancer are the osteosarcoma, chondrosarcoma, and Ewing’s sarcoma (Driel and Leeuwen 2014). Among patients with bone cancer, the most representative causes of death are late detection, limited methods of investigation and monitoring, and a limited assessment of treatment response. In this regard, we have researched a few genetic biomarkers. Those who presented the most favorable characteristics in this regard are miRNAs (Bratu et al. 2016; Rogobete et al. 2016; Bedreag et al. 2016b). They become ideal candidates regarding the evaluation of bone cancer disease due to specificity, selectivity, and the increased accuracy they present. In this updated paper, we wish to present a series of changes in the expression of miRNAs in bone cancer, and a series of links between miRNA expressions and molecular mechanisms involved in the development and invasion of bone cancer.
Pathophysiological and Pathological Aspects of Bone Cancer
One of the most common forms of bone cancer is the osteosarcoma. Regarding the origin of cancer cells in the case of osteosarcoma, there are insufficient data presented in the literature. However, recent studies have shown a preponderant development of cancer cells in mesenchymal stem cells that contain mutations in the p53. Regarding the degree of invasiveness, osteosarcomas have an increased incidence in the pulmonary tissue. The most common form of cancer in the bones located in the pelvis or chest is represented by Ewing’s sarcoma (Dylla et al. 2013; Kinase et al. 2014; Sun et al. 2016). This type of cancer develops mainly in children and young people and has a prognosis for metastasis, featuring a lower survival rate. From the point of view of genetic changes, Ewing’s sarcoma is characterized by the t(11; 22)(q24; q12) translocation. Genetic translocations involved in developing this type of sarcoma are the result of fusing 5′ portion of Ewing’s gene with 3′ portion of one of the genes responsible for the transcription factors, erythroblast transformation-specific family. In this family, there are erythroblast transformation-specific translocation variant-1 (ETS-ETV-1), erythroblast transformation-specific translocation variant-4 (ETS-ETV-4), Erythroblast transformation-specific translocation fifth Ewing variant (ETS-FEV), friend leukemia integration-1 (FLI-1), and ETS-related gene (ERG) (Cho et al. 2008; Fey et al. 2012; Vimalraj et al. 2013; Mohan et al. 2014; Sun et al. 2016). Another type of bone cancer is the chondrosarcoma. From a morphological point of view, this type of cancer is represented by the production of a chondroid matrix. It develops mainly in the 30–60 age range and shows a low growth speed. Recent studies have identified several biochemical and genetic disorders in terms of developing chondrosarcomas, such as an increase in the expression of hypoxia pathway 1-alpha (HIF1-α) or the upregulation of Bcl-2 and Bcl-xl (Wang et al. 2013; Poulsen et al. 2014; Rippo et al. 2014). Regarding the location, neoplastic lesions are found in a high percentage intramedullary, and lower rates on the outer surface of the cortex. The latter ones are called surface osteosarcoma. Regarding classification, we can discuss about parosteal osteosarcoma, periosteal osteosarcoma, and high-grade surface osteosarcoma. Osteosarcoma is one of the most common malignant bone tumors, representing according to statistics approximately 17% of this type of tumors. Unfortunately, it shows a high incidence in adolescents and has poor prognosis. Regarding treatment regimens in the present, surgical methods and chemotherapy are used, but the response is weak, most likely due to the complex molecular mechanisms at that level. Regarding invasion and malignancy, the main sites are the bone marrow with production of leukemia, multiple myeloma, and lymphoma (Calin et al. 2008; Schnetzke et al. 2015).
Due to the characteristics, specific to the bone, it becomes an easy site to be invaded by cancer cells and therefore developing metastasis. These features include rich vasculature with numerous arteries, arterioles, and capillaries as well as complex venous plexus. The mechanism by which cancer cells get out of the vessels in the bone tissue is called mesenchymal to epithelial transition.
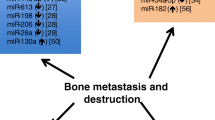
Several studies have revealed an important role regarding miRNAs in the development of metastases in patients with bone cancer (Zoni and Pluijm 2016). In this regard, the most important species of miRNAs involved in the development and invasion of neoplasm formations are miRNA-183, miRNA-21, miRNA-30, and miRNA-218 responsible for increasing osteoclastogenesis through the heme oxygenase-1 (HO-1), increased expression of matrix metallopeptidase 2 (MMP2), matrix metallopeptidase 9 (MMP9), matrix metallopeptidase 13 (MMP13), or the activation of vascular-endothelial molecule-1 (VEM) (Fig. 1) (Zoni and Pluijm 2016).
Biochemical Aspects of miRNAs
From the point of view of biosynthesis mechanisms of miRNAs, they begin in the cell nucleus (Bedreag et al. 2016a). Biosynthesis processes begin with the activation of RNA polymerase II and the action on specific genes. In this way, there are a series of biochemical reactions of transcription and pri-microRNAs are formed. Following these reactions, Rnase III endonuclease, also called Drosha with DiGeorge Syndrome Critical Region 8 (DGCR8), turns pri-miRNAs in pre-miRNAs (Ticlea et al. 2016). Subsequently, pre-miRNAs are transported into the cytoplasm through Exportin 5 protein. Once in the cytoplasm, pre-miRNAs are attacked by RNase III endonuclease, also called Dicer with RNA-binding protein (TRBP) leading to the formation of double-stranded miRNAs (Bedreag et al. 2015; Bratu et al. 2016; Papurica et al. 2016). Mature miRNA species generated are incorporated into the RNA-induced silencing complex (RISC). The last step that concerns miRNA biogenesis is extracellular transport under various epigenetic forms (Fig. 1) (Papurica et al. 2015).
miRNA Expression in Cancer Bone Disease
Another intensely studied species in terms of epigenetic expression in bone cancer is represented by miRNA-19a. Also, A statistically significant change in the expression of this species in several types of cancer, such as lung cancer, colorectal cancer, or esophageal squamous cell carcinoma was observed. From a functional perspective, miRNA-19a intervenes in the modulation of TIMP-2 expression, thus suppressing laryngeal squamous cell carcinoma apoptosis. STAT3 mechanisms are found in pulmonary cancer, intervening from a pathological point of view in the activation of non-small cell lung cancer. Zou et al. studied the activity of miRNA-19a in human osteosarcomas. In the study, they showed a statistically significant correlation between increasing the expression of miRNA-19a and decreasing mortality in patients with osteosarcoma (p < 0.05) (Table 1). They also reported significant correlations between increasing miRNA-19 and poor response to chemotherapy and degree of metastatic invasion (Zou et al. 2016).
Another intensively investigated genetic species in this area is miRNA-92a. Jiang et al. have reported an increase in miRNA-92a expression in osteosarcoma correlated with increasing mortality. Moreover, they have shown that miRNA-92a comes into the mechanism F-box and WD repeat-containing protein 7 (FBXW7) (Jiang et al. 2017).
Namløs et al. conducted a study on the expression of miRNAs in osteosarcoma cell lines. The study showed a decrease in the expression of miRNA-1, miRNA-133a, miRNA-144, miRNA-451, miRNA-195 and miRNA-497, miRNA-126, miRNA-126*, miRNA-142-3p, miRNA-150, miRNA-223, miRNA-486-5p, miRNA-133b, and miRNA-206 as opposed to healthy bone tissue. Also, they have identified increased expression for miRNA-17-92, miRNA-106b-25, miRNA-106a-92, miRNA-9, miRNA-9*, miRNA-21*, miRNA-31, miRNA-31 * miRNA-196a, miRNA-196b, and miRNA-374a in the osteosarcoma cell line (Namløs et al. 2012). Tang et al. conducted a similar study, reporting a change in the expression of miRNA-27a in patients with osteosarcoma (p < 0.001). Moreover, they show that modifying the expression of miRNA-27a can be correlated with positive distant metastasis (p = 0.01), respectively, with poor response to specific therapies (p = 0.008) (Tang et al. 2015).
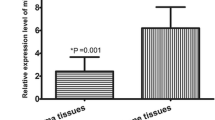
Sun et al. have identified a decrease in miRNA-217 expression in osteosarcoma cell lines and clinical specimens. They also reported significant correlations in terms of downregulation of miRNA-27, tumor size, and positive distance metastasis. An extraordinary thing they have highlighted in this study is the fact that miRNAs can serve as therapeutic agent. They showed in vitro that increasing miRNA-127 expression will inhibit proliferation, invasion, and tumor cell migration (Sun et al. 2015a). A similar study was conducted by Yang et al. (2015) who reported an aberrant increase in the expression of miRNA-221 in osteosarcoma tissues (p = 0.001). Regarding the implications of miRNAs in the principles of therapy based on miRNAs, Li et al. (2016) showed that miRNA-143 can inhibit the activity of Bcl-2 by activating Caspase-3, thus inducing apoptosis in osteosarcoma cells. Wang et al. reported in a similar study an increase in miRNA-214 expression in osteosarcoma tissue (p < 0.001). Moreover, they showed a statistically significant correlation between the upregulation of miRNA-214 and large tumor size (p = 0.01) or poor response to chemotherapy (p = 0.006) (Wang et al. 2014).
Regarding resistance to chemotherapy, Cai et al. (2013) have reported an increase in the expression of miRNA-210 and miRNA-221. A similar study was conducted by Song et al. (2009) which showed a statistically significant correlation regarding the poor response to chemotherapy and increased expression of miRNA-140. According to reports, the leading cause of death among bone cancer patients is represented by lung metastases (Sun et al. 2015b). Regarding the expression of miRNAs in Ewing’s sarcoma, Kawano et al. (2015) showed a decrease in the expression for miRNA-16, miRNA-29b, and let-7a.
Conclusions
Changes in the expressions of miRNAs may serve as a successfully epigenetic biomarker for evaluating and monitoring patients with bone cancer disease. Moreover, administration of specific miRNAs can alter the genetic and pathophysiologic response, and can be considered as therapeutic methods of the future. With all these, further studies are needed both in terms of getting a diagnostic panel, and in order to develop specific genetic therapies.
References
Bao Y, Yi Y, Peng L et al (2013) Roles of microRNA-206 in osteosarcoma pathogenesis and progression. Asian Pac J Cancer Prev 14(6):3751–3755
Bedreag OH, Rogobete AF, Dumache R et al (2015) The use of circulating microRNAs as biomarkers in critically ill polytrauma patient. A review. Biomark Genom Med 7:131–138
Bedreag OH, Rogobete AF, Cradigati CA et al (2016a) A novel evaluation of microvascular damage in critically ill polytrauma patients by using circulating microRNAs. Rev Rom Med Lab 24(1):21–30
Bedreag OH, Sandesc D, Chiriac SD et al (2016b) The use of circulating miRNAs as biomarkers for oxidative stress in critically ill polytrauma patients. Clin Lab 62(3):263–274
Bratu LM, Rogobete AF, Papurica M et al (2016) Literature research regarding miRNAs’ expression in the assessment and evaluation of the critically ill polytrauma patient with traumatic brain and spinal cord injury. Clin Lab 62(10):2019–2024
Cai H, Lin L, Cai H et al (2013) Prognostic evaluation of microRNA-210 expression in pediatric osteosarcoma. Med Oncol 30:499
Calin GA, Cimmino A, Fabbri M et al (2008) miR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci 105:5166–5171
Cho K, Wang X, Nie S et al (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–1316
Dong J, Liu Y, Liao W et al (2016) miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J Bone Oncol 5:74–79
Duan Z, Choy E, Harmon D et al (2011) MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther 10(8):1337–1345
Dylla L, Moore C, Jedlicka P (2013) MicroRNAs in Ewing sarcoma. Front Oncol 28(3):65
Fey V, Ka S, Pollari S et al (2012) Identification of MicroRNAs inhibiting TGF- b-induced IL-11 production in bone metastatic breast cancer cells. PLoS ONE 7(5):e37361
Ji F, Zhang H, Wang Y et al (2013) MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone 56:220–226
Jiang X, Li X, Wu F et al (2017) Overexpression of miR-92a promotes the tumor growth of osteosarcoma by suppressing F-box and WD repeat-containing protein 7. Gene 606:10–16. doi:10.1016/j.gene.2017.01.002
Kawano M, Tanaka K, Itonaga I, Iwasaki T, Tsumura H (2015) c-Myc represses tumor-suppressive microRNAs, let-7a, miR-16 and miR-29b, and induces cyclin D2-Mediated cell proliferation in Ewing’s sarcoma cell line. PLoS ONE 10(9):e0138560
Kinase C, Huang S, Han Z, Cao K (2014) Let-7a functions as a tumor suppressor in Ewing’ s sarcoma cell lines partly by targeting. DNA Cell Biol 33(3):136–147
Li R, Wang L (2016) Decreased microRNA-452 expression and its prognostic significance in human osteosarcoma. World J Surg Oncol 18(14):150
Li W, Wu H, Li Y et al (2016) Science direct MicroRNA-143 promotes apoptosis of osteosarcoma cells by caspase-3 activation via targeting Bcl-2. Biomed Pharmacother 80:8–15
Lian F, Cui Y, Zhou C et al (2015) Identification of a plasma four-microRNA panel as potential noninvasive biomarker for osteosarcoma. PLoS ONE 10(3):e0121499
Lian D, Wang Z, Liu N (2016) MicroRNA-1908 is a biomarker for poor prognosis in human osteosarcoma. Eur Rev Med Pharmacol Sci 20(7):1258–1262
Liu W, Zhao Z, Shi L, Yuan W (2015) Tissue microRNA-126 expression level predicts outcome in human osteosarcoma. Diagn Pathol 21(10):116
Mohan S, Thiagarajan K, Chandrasekaran R, Arul J (2014) In vitro protection of biological macromolecules against oxidative stress and in vivo toxicity evaluation of Acacia nilotica (L.) and ethyl gallate in rats. BMC Complement Altern Med 14:257
Namløs HM, Meza-zepeda LA, Barøy T et al (2012) Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS ONE 7(10):e48086
Papurica M, Rogobete AF, Sandesc D et al (2015) Redox changes induced by general anesthesia in critically ill patients with multiple traumas. Mol Biol Int 2015:238586
Papurica M, Rogobete AF, Sandesc D et al (2016) The expression of nuclear transcription factor kappa B (NF-κB) in the case of critically Ill polytrauma patients with sepsis and its interactions with microRNAs. Biochem Genet 54(4):337–347
Poulsen RC, Knowles HJ, Carr AJ, Hulley PA (2014) Cell differentiation versus cell death: extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis 5:e1074
Pu Y, Zhao F, Cai W, Meng X (2016) MiR-193a-3p and miR-193a-5p suppress the metastasis of human osteosarcoma cells by down-regulating Rab27B and SRR, respectively. Clin Exp Metastasis 33:359–372
Rippo MR, Olivieri F, Monsurrò V et al (2014) MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol 56:154–163
Rogobete AF, Bedreag OH, Popovici SE et al (2016) Detection of myocardial injury using miRNAs expression as genetic biomarkers in acute cardiac care. J Cardiovasc Emerg 2(4):169–172
Schnetzke U, Spies-Weisshart B, Yomade O et al (2015) Polymorphisms of Toll-like receptors (TLR2 and TLR4) are associated with the risk of infectious complications in acute myeloid leukemia. Genes Immun 16:83–88
Song B, Wang Y, Xi Y et al (2009) Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 28(46):4065–4074
Sun B, Yang M, Li M, Wang F (2015a) Science direct The microRNA-217 functions as a tumor suppressor and is frequently downregulated in human osteosarcoma. Biomed Pharmacother 71:58–63
Sun T, Kalionis B, Lv G et al (2015b) Role of exosomal noncoding RNAs in lung carcinogenesis. Biomed Res Int 2015:125807
Sun M, Zhou X, Chen L et al (2016) The regulatory roles of microRNAs in bone remodeling and perspectives as biomarkers in osteoporosis. Biomed Res Int 2016:1652417
Tang J, Zhao H, Cai H, Wu H (2015) Science direct diagnostic and prognostic potentials of microRNA-27a in osteosarcoma. Biomed Pharmacother 71:222–226
Ticlea M, Melania L, Bodog F, Horea O (2016) The use of exosomes as biomarkers for evaluating and monitoring critically Ill polytrauma patients with sepsis. Biochem Genet 55(1):1–9
Van Driel M, Van Leeuwen JPTM (2014) Cancer and bone: a complex complex. Arch Biochem Biophys 561:159–166
Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N (2013) Regulation of breast cancer and bone metastasis by MicroRNAs. Dis Mark 35(5):369–387
Wang Y, Yue B, Yu X et al (2013) SLUG is activated by nuclear factor kappa B and confers human alveolar epithelial A549 cells resistance to tumor necrosis factor-alpha-induced apoptosis. World J Surg Oncol 11:12
Wang Z, Cai H, Lin L et al (2014) Upregulated expression of microRNA-214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatr Blood Cancer 61:206–210
Xu S, Yang Y, Han S, Wu Z (2014) MicroRNA-9 expression is a prognostic biomarker in patients with osteosarcoma. World J Surg Oncol 12:195
Yang Z, Zhang Y, Zhang X et al (2015) Science direct serum microRNA-221 functions as a potential diagnostic and prognostic marker for patients with osteosarcoma. Biomed Pharmacother 75:153–158
Zhang HAO, Wang Y, Xu T et al (2016) Increased expression of microRNA-148a in osteosarcoma promotes cancer cell growth by targeting PTEN. Oncol Lett 12(5):3208–3214
Zoni E, Van Der Pluijm G (2016) The role of microRNAs in bone metastasis. J Bone Oncol 5:104–108
Zou P, Ding J, Fu S (2016) Elevated expression of microRNA-19a predicts a poor prognosis in patients with osteosarcoma. Pathol Res Pract. doi:10.1016/j.prp.2016.12.020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Hutanu, D., Popescu, R., Stefanescu, H. et al. The Molecular Genetic Expression as a Novel Biomarker in the Evaluation and Monitoring of Patients With Osteosarcoma-Subtype Bone Cancer Disease. Biochem Genet 55, 291–299 (2017). https://doi.org/10.1007/s10528-017-9801-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-017-9801-1