DNA sequencing analysis was used to investigate genetic alterations in the rpoB, katG, and inhA regulatory region and embB in 66 Mycobacterium tuberculosis isolates recovered from Central China. Of the 36 multidrug-resistant isolates, 33 (92%) had mutations in the amplified region of rpoB. The most frequent mutation (58%, 19/36) was S531L (TCG→TTG). At least one mutation was found in the katG and inhA regulatory region in 83% (30/36) of the multidrug-resistant isolates, and mutations at katG codon 315 were identified in 78% (28/36). Alterations at embB306 may not confer resistance to EMB, and embB306 mutants were more frequently accompanied by rpoB mutations (100%, 16/16) than by katG 315 mutations (75%, 12/16). Our results show that geographic variation in the molecular genetic mechanism is responsible for drug resistance in multidrug-resistant M. tuberculosis. This observation will facilitate the development of a rapid molecular drug resistance screening approach for drug-resistant M. tuberculosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Tuberculosis (TB) is the most common cause of death due to a single infectious agent—it is responsible for approximately two million deaths annually around the world. The global burden of tuberculosis remains enormous, especially in South and East Asia, sub-Saharan Africa, and eastern Europe (Dye et al., 1999). Moreover, 7.6% of the new cases are resistant to multiple drugs, or MDR-TB, which is caused by M. tuberculosis isolates that are resistant to, at least, rifampin (RIF) and isoniazid (INH). Although most of the MDR-TB is curable, it requires long-term chemotherapy. Furthermore, some patients have persistently active disease that is refractory to multidrug therapy. Some studies show cure rates for MDR-TB that remain well below those for drug-susceptible TB (Goble et al., 1993; Suarez et al., 2002). Inadequate chemotherapeutic regimens and poor compliance are proposed to be the major factors in the emergence of MDR-TB. Thus, control of tuberculosis caused by drug-resistant M. tuberculosis has become an urgent public health problem in many regions of the world, particularly in developing countries.
Rapid and reliable diagnosis is essential in the management of MDR-TB, not only to optimize treatment but also to prevent transmission. Traditional drug resistance testing for pulmonary TB, however, is still based on isolation of strains on culture media, which takes 4 to 6 weeks, because M. tuberculosis is a slow-growing organism. Thus, clinical treatment is prescribed empirically. Understanding the molecular genetic basis of drug resistance might help in the establishment of a novel method for rapid detection of drug-resistant M. tuberculosis strains. In contrast to most bacteria, M. tuberculosis does not acquire drug resistance through horizontal transfer of resistance-bearing genetic elements. Rather, resistance originates from mutations (caused by nucleotide substitutions, insertions, or deletions) in specific resistance-determining regions of the genetic targets (or their promoters) or by activating enzymes of anti-TB chemotherapeutic agents (Ramaswamy and Musser, 1998). In nearly 95% of rifampin-resistant strains, some mutations can be detected by analyzing a certain part of the RNA polymerase β-subunit gene, rpoB (Ramaswamy and Musser, 1998). In contrast, resistance to INH is associated with a variety of mutations affecting one or more genes. INH is a pro-drug that requires activation by the catalase-peroxidase KatG. The resulting active principle, still unknown, inhibits the activity of the InhA enzyme that belongs to a type II fatty acid elongation system, together with KasA/KasB and MabA. Several studies have revealed that resistance to INH is due to mutations in the catalase-peroxidase gene (katG) or inhA. Mutations in katG are responsible for 60–70% of INH-resistant strains (Ramaswamy and Musser, 1998; Zhang et al., 2000; Herrera et al., 2004), and 20 to 35% of INH-resistant strains contain mutations in the inhA regulatory region (Banerjee et al., 1994). Ethambutol (EMB) is also a first-line anti-TB drug. embB Met306 is located in a cytoplasmic loop that forms an EMB resistance determining region (Telenti et al., 1997). Nearly 50% of EMB resistant strains are involved in mutations at embB codon 306 (Rinder et al., 2001).
The previous studies have supplied a database of molecular mutations of multidrug-resistant M. tuberculosis isolates from different countries or areas. Disequilibrium has been reported, however, in the distribution of resistance mutations from different regions, or different genotype strains isolated from TB patients (Rinder et al., 1997; Hillemann et al., 2005). The aim of this study was to identify resistance-related mutations in the MDR-TB isolates recovered from the World Health Organization (WHO) drug resistance surveillance program in Henan, Central China. All MDR-TB strains and statistics were obtained according to the principles recommended in the guidelines of the WHO and the International Union Against TB and Lung Disease. Thus, information gained by genotypic analysis of drug-resistant isolates helps not only to identify genetic markers in M. tuberculosis strains unique to a particular geographic niche but also to develop some novel molecular screening methods for rapid detection of MDR-TB isolates.
MATERIALS AND METHODS
We randomly selected 36 multidrug-resistant M. tuberculosis isolates and 30 susceptible M. tuberculosis isolates from WHO drug resistance surveillance in Henan, China. All 66 resistant isolates and susceptible strains were identified by biochemical tests. Drug susceptibility was tested using the proportion method with INH (0.2 μg mL−1), RIF (40 μg mL−1), and EMB (2 μg mL−1). Resistance to any of the four drugs tested was defined as 1% or more bacterial growth on drug-containing medium compared to a control medium.
Genomic DNA was extracted as previously described by Peng et al. (2000). Sequences of rpoB (resistance determining hot spot region), katG (codon 315), inhA (regulatory region), and embB were obtained from GenBank (Table I). Four pairs of primers were designed using Primer Premier 5.0 and Oligo 6 software. The uniqueness of the sequences of the primers designed was analyzed with the Blast search (http://www.ncbi.nlm.nih.gov).
PCR amplification was performed with a Perkin-Elmer model 9600 thermal cycler. Ten microliters of extracted DNA was added to a 50 μL reaction mixture containing 50 mM KCl, 10 mM Tris (pH 8.3), 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, 0.6 μM (each) primer, 200 μM (each) deoxynucleotide triphosphates, and 1.5 U Taq polymerase (Promega, Shanghai), which then underwent an initial denaturation step at 95°C for 5 min before 35 cycles at 95°C for 1 min, 56°C for 1 min, and 72°C for 1 min, and then a final step at 72°C for 5 min. Efficient PCR amplifications were confirmed by electrophoresis on a 1.5% (w/v) agarose gel, followed by ethidium bromide staining.
PCR products from the rpoB, katG, inhA regulatory region, and embB in 36 multidrug-resistant M. tuberculosis isolates and 30 susceptible M. tuberculosis isolates were sequenced. The amplicons were purified over a QIAquick PCR purification kit (Qiagen, Fremont, CA) according to the manufacturer’s protocol. Sequence analysis was determined on both strands by direct sequencing of the PCR products on an automated model 377 DNA sequencer (Applied Biosystems, Foster City, CA) with fluorescence-labeled dideoxynucleotide terminators (ABI Prism Big Dye terminator cycle sequencing ready reaction kit; Applied Biosystems).
RESULTS
The drug resistance phenotype for each of the 36 multidrug-resistant M. tuberculosis isolates is shown in Table II. All of the strains were resistant to at least RIF and INH, and the drug resistance information for ethambutol EMB is also presented. The 30 susceptible M. tuberculosis isolates were susceptible to all three drugs, including RIF, INH, and EMB.
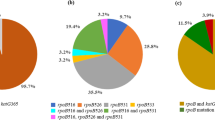
DNA sequencing of the 30 susceptible strains detected no mutations in the rpoB, katG, inhA regulatory region, and embB. Of the 36 MDR-TB isolates studied, 33 (92%) had mutations in the amplified region of rpoB (Table II). Three isolates (8%) carried no mutations in the amplified region. All of the 33 mutated isolates carried mutations in the hot-spot region. Overall, 12 different missense mutations, involving 13 kinds of amino acids, were identified. The most frequent mutation was S531L (TCG→TTG), found in 19 of 33 mutated isolates (58%). Another kind of codon 531 mutation was S531W (TCG→TGG), identified in one multidrug-resistant isolate (3%). Mutations affecting codon 526 (5 isolates, 15%) contained H526Y (CAC→TAC), H526D (CAC→GAC), and H526R (CAC→CGC). Three isolates (9%) exhibited codon 516 mutations, including D516V (GAC→GTC), D516G (GAC→GGC), and D516Y (GAC→TAC). Two isolates (6%) showed the codon 533 mutation L533P (CTG→CCG). One (3%) had the codon 513 mutation Q513K (CAA→AAA). In addition, two (6%) were found to carry double mutations: one carried L511P (CTG→CCG) and H526Q (CAC→CAG); the other carried L511R (CTG→CGG) and H516Y (GAC→TAC). Mutations in katG codon 315 were detected in 28 of the 36 MDR-TB strains (78%). There were 25 strains with the S315T (AGC→ACC) mutation, the most common base substitution mutation; two carried the S315N (AGC→AAC) mutation, and one carried the S315T (AGC→ACA) mutation. Sequencing analysis of the inhA regulatory region revealed base substitutions (C→T) at nucleotide position −15 in three resistant isolates (8%). Of these, two had mutations only in the inhA regulatory region, and one had an accompanying katG mutation at codon 315. Of the MDR-TB isolates, 83% had at least one mutation in the katG and inhA regulatory region. Mutations in embB were detected in 13 of 22 EMB-resistant isolates (59%), and three of the embB306 mutants were EMB susceptible. The observed mutations included M306V, M306I, and M306L (Table II).
DICUSSION
Mutations leading to RIF resistance were first elucidated by studies on the DNA-dependent RNA polymerase of Escherichia coli, and mutations conferring resistance to RIF were found to be located exclusively in the core region of rpoB (Jin and Gross, 1988). RIF acts by binding the β-subunit of the RNA polymerase, thus interfering with transcription and RNA elongation. Subsequent studies revealed that ca. 95% of RIF-resistant MTB strains carried mutations within an 81-bp region determining rifampin resistance (RRDR) of rpoB (Chaves et al., 2000). Later studies showed targeted molecular analysis of rpoB to be effective for detecting RIF resistance in over 90% of RIF-resistant strains from diverse geographic regions (Kapur et al., 1994; Caugant et al., 1995; Qian et al., 2002). Moreover, disequilibrium has been reported in the distribution of resistance mutations among different regions or different genotype strains isolated from TB patients (Caugant et al., 1995; Hillemann et al., 2005). In this study, we report the presence of mutations in the core region of rpoB in 92% (33/36) of M. tuberculosis strains that exhibited RIF resistance. The most common mutation was S531L (TCG→TTG), identified in 19 of the 33 mutated isolates (58%). One multidrug-resistant isolate presented another kind of codon 531 mutation, S531W (TCG→TGG). The mutations at codon position 531 amounted to 61% (20/33). Other mutations were detected at rpoB codons 526, 516, 533, and 513. Two double mutations, both affecting codon 511, were found. The double mutation of L511R (CTG→CGG) and H516Y (GAC→TAC) has not been reported previously. None of the 30 susceptible control strains carried any mutation in rpoB.
The molecular mechanisms of INH resistance are highly complex and affect several genes that are involved in mycolic acid biosynthesis or are overexpressed as a response to the buildup of cellular toxicity of INH (Ramaswamy and Musser, 1998; Herrera et al., 2004). Frequencies of the katG 315 mutations from different geographic regions show obvious discrepancies (Herrera et al., 2004; Coll et al., 2005). Our study detected mutations of katG codon 315 in 28 of the 36 MDR-TB strains (78%), with the most common nucleotide change being from AGC to ACC (S315T). Additionally, three (8%) had mutations in the putative inhA regulatory region. It is thought that the mutation in the putative inhA regulatory sequences may cause overexpression of the InhA protein, which could elevate levels of the drug target for INH, resulting in a more resistant phenotype (Banerjee et al., 1994). As a whole, 83% (30/36) of MDR-TB isolates had at least one mutation in the katG and inhA regulatory region.
Several lines of evidence suggest that EMB exerts its toxic effect on mycobacteria by inhibiting the embABC-encoded proteins and finally inhibits the growth of M. tuberculosis by blocking the synthesis of arabinogalactan (Khoo et al., 1996, 2001). Associations between EMB resistance and mutations in embB have been reported in clinical strains of M. tuberculosis (Telenti et al., 1997; Rinder et al., 2001). Mokrousov et al. (2002) and Hazbon et al. (2005) recently reported, however, that some clinical M. tuberculosis isolates from different regions were susceptible to EMB yet had mutations in embB306. These results strongly suggest that embB306 mutations do not cause EMB resistance in M. tuberculosis. But embB306 mutations may predispose M. tuberculosis to become resistant to any antibiotic and to become multidrug resistant. Our results revealed 13 (59%) embB306 mutants in the 22 EMB-resistant MDR isolates and 3 embB306 mutants in EMB-susceptible MDR isolates. No mutations in embB were detected in the 30 strains susceptible to INH, RIF, and EMB. Although recent studies demonstrated that EMB-resistant isolates with embB306 mutations were also resistant to isoniazid (Lee et al., 2004; Parsons et al., 2005), our results primarily revealed that embB306 mutants were more frequently accompanied by rpoB mutations (100%, 16/16) than by katG315 mutations (75%, 12/16). Alterations at embB306 may not confer resistance to EMB and may be only polymorphisms, which increase the likelihood that any type of drug resistance will develop (Hazbon et al., 2005). Obviously, further investigations are imperative in order to understand the involvement of these drug-resistant genes in the molecular genetic mechanism of EMB resistance.
This is the first report on simultaneous genotypic analysis of the rpoB, katG, inhA regulatory region, and embB in multidrug-resistant M. tuberculosis isolates recovered from the WHO drug resistance surveillance program in Central China. Our results show geographic variations in the molecular mechanism responsible for RIF and INH resistance. The relatively high prevalence of mutations within the rpoB core region, katG codon 315, and inhA regulatory region is favorable for developing a rapid and reliable molecular drug susceptibility test for multidrug-resistant M. tuberculosis. Our results primarily reveal the association between embB306 mutants and rpoB mutations and katG315 mutations in multidrug-resistant M. tuberculosis isolates. Further genotypic analysis of multidrug-resistant M. tuberculosis isolates from diverse geographic origins would facilitate research on molecular mechanisms of drug resistance in M. tuberculosis and the development of a rapid approach to molecular screening for drug resistant M. tuberculosis, which is necessary for the effective control and prevention of tuberculosis and MDR-TB.
REFERENCES
Banerjee, A., Dubnau, E., Quemard, A., Balasubramanian, V., Um, K. S., Wilson, T., Collins, D., de Lisle, G., and Jacobs W. R., Jr. (1994). inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227–230.
Caugant, D. A., Sandven, P., Eng, J., Jeque, J. T., and Tonjum, T. (1995). Detection of rifampin resistance among isolates of Mycobacterium tuberculosis from Mozambique. Microb. Drug Resist. 1:321–326.
Chaves, F., Alonso-Sanz, M., Rebollo, M. J., Tercero, J. C., Jimenez, M. S., and Noriega, A. R. (2000). rpoB mutations as an epidemiologic marker in rifampin-resistant Mycobacterium tuberculosis. Int. J. Tuberc. Lung. Dis. 4:765–770.
Coll, P., Aragon, L. M., Alcaide, F., Espasa, M., Garrigo, M., Gonzalez, J., Manterola, J. M., Orus, P., and Salvado, M. (2005). Molecular analysis of isoniazid and rifampin resistance in Mycobacterium tuberculosis isolates recovered from Barcelona. Microb. Drug Resist. 11:107–114.
Dye, C., Scheele, S., Dolin, P., Pathania, V., and Raviglione, M. C. (1999). Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA 282:677–686.
Goble, M., Iseman, M. D., Madsen, L. A., Waite, D., Ackerson, L., and Horsburgh C. R., Jr. (1993). Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N. Engl. J. Med. 328:527–532.
Herrera, L., Valverde, A., Saiz, P., Porterob, J. L., Jiménez, M. S. (2004). Molecular characterization of isoniazid-resistant Mycobacterium tuberculosis clinical strains isolated in the Philippines. Intern. J. Antimicrob. Agents 23:572–576.
Hillemann, D., Kubica, T., Rüsch-Gerde, S., Niemann, S. (2005). Disequilibrium in distribution of resistance mutations among Beijing and non-Beijing Mycobacterium tuberculosis strains isolated from patients resident in Germany. Antimicrob. Agents Chemother. 49:1229–1231.
Hazbon, M. H., Bobadilla del Valle, M., Guerrero, M. I., Varma-Basil, M., Filliol, I., Cavatore, M., Colangeli, R., Safi, H., Billman-Jacobe, H., Lavender, C., Fyfe, J., Garcia-Garcia, L., Davidow, A., Brimacombe, M., Leon, C. I., Porras, T., Bose, M., Chaves, F., Eisenach, K. D., Sifuentes-Osornio, J., Ponce de Leon, A., Cave, M. D., and Alland, D. (2005). Role of embB codon 306 mutations in Mycobacterium tuberculosis revisited: A novel association with broad drug resistance and IS6110 clustering rather than ethambutol resistance. Antimicrob. Agents Chemother. 49:3794–3802.
Jin, D. J., and Gross, C. A. (1988). Mapping and sequencing of mutations in the Ecsherichia coli rpoB gene that lead to rifampicin esistance. J. Mol. Biol. 202:45–58.
Kapur, V., Li, L. L., Iordanescu, S., Hamrick, M. R., Wanger, A., Kreiswirth, B. N., and Musser, J. M. (1994). Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase ß subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J. Clin. Microbiol. 32:1095–1098.
Khoo, K. H., Douglas E., Azadi P., Inamine J. M., Besra G. S., Mikusova K., Brennan P. J., and Chatterjee D. (1996). Truncated structural variants of lipoarabinomannan in ethambutol drug-resistant strains of Mycobacterium smegmatis: Inhibition of arabinan biosynthesis by ethambutol. J. Biol. Chem. 271:28682–28690.
Khoo, K. H., Tang J. B., and Chatterjee D. (2001). Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J. Biol. Chem. 276:3863–3871.
Lee, A. S., Othman, S. N., Ho, Y. M., and Wong, S. Y. (2004). Novel mutations within the embB gene in ethambutol-susceptible clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:4447–4449.
Mokrousov, I., Otten T., Vyshnevskiy B., and Narvskaya O. (2002). Detection of embB306 mutations in ethambutol-susceptible clinical isolates of Mycobacterium tuberculosis from northwestern Russia: Implications for genotypic resistance testing. J. Clin. Microbiol. 40:3810–3813.
Parsons, L. M., Salfinger, M., Clobridge, A., Dormandy, J., Mirabello, L., Polletta, V. L., Sanic, A., Sinyavskiy, O., Larsen, S. C., Driscoll, J., Zickas, G., and Taber, H. W. (2005). Phenotypic and molecular characterization of Mycobacterium tuberculosis isolates resistant to both isoniazid and ethambutol. Antimicrob. Agents Chemother. 49:2218–2225.
Peng, Y. L., Wang, G. B., Zhang, S. L., Wu, X. Q., Gao, S. Y., Zhang, L., and Sun, Z. Q. (2000). Detection of the rpsL mutation for streptomycin-resistant Mycobacterium uberculosis. Chin. J. Lab. Med. 23:148–149.
Qian, L., Abe, C., Lin, T. P., Yu, M. C., Cho, S. N., Wang, S., and Douglas, J. T. (2002). rpoB genotypes of Mycobacterium tuberculosis Beijing family isolates from East Asian countries. J. Clin. Microbiol. 40:1091–1094.
Ramaswamy, S., and Musser, J. M. (1998). Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3–29.
Rinder, H., Dobner, P., Feldmann, K., Rifai, M., Bretzel, G., Rüsch-Gerdes, S., and Loscher, T. (1997). Disequilibria in the distribution of rpoB alleles in rifampicin-resistant M. tuberculosis isolates from Germany and Sierra Leone. Microb. Drug Resist. 3:195–197.
Rinder, H., Mieskes, K. T., Tortoli, E., Richter, E., Casal, M., Vaquero, M., Cambau, E., Feldmann, K., Loscher, T. (2001). Detection of embB codon 306 mutations in ethambutol resistant Mycobacterium tuberculosis directly from sputum samples: A low-cost, rapid approach. Mol. Cell. Probes. 15:37–42.
Suarez, P. G., Floyd, K., Portocarrero, J., Alarcon, E., Rapiti, E., Ramos, G., Bonilla, C., Sabogal, I., Aranda, I., Dye, C., Raviglione, M., and Espinal, M. A. (2002). Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: A national cohort study in Peru. Lancet 359:1980–1989.
Telenti, A., Honoré, N., Bernasconi, C., March, J., Ortega, A., Heym, B., Takiff, H. E., and Cole, S. T. (1997). Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: A blind study at reference laboratory level. J. Clin. Microbiol. 35:719–723.
Zhang, S. L., Wu, X. Q., and Zu, Y. (2000). Detection of katG mutations in isoniazid-resistant Mycobacterium tuberculosis strains by polymerase chain reaction-single strand conformational polymorphism. Chin. J. Health Lab Tech. 10:185–187.
ACKNOWLEDGMENTS
This study was supported by Grant No. 971200100 from Henan Key Scientific and Technological Project, China. We thank Zhan-Qiang Sun for excellent technical assistance in identification of Mycobacteria, and also thank Bing-Xiang Tang for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, SL., Qi, H., Qiu, DL. et al. Genotypic Analysis of Multidrug-Resistant Mycobacterium tuberculosis Isolates Recovered From Central China. Biochem Genet 45, 281–290 (2007). https://doi.org/10.1007/s10528-006-9074-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-006-9074-6