Abstract
An integrated management strategy involving fungal (Trichoderma harzianum) and bacterial (Pseudomonas fluorescens and Bacillus species) antagonists, rhizobacterium and a fungicide was developed for the management of chickpea wilt caused by Fusarium oxysporum f. sp. ciceris (Foc). PGPR strain P. fluorescens 80 (Pf 80) and Bacillus species (Bskm 5) caused the highest mycelial growth inhibition. Pf 80 was found to be compatible with T. harzianum and Mesorhizobium ciceri. The fungicides Vitavax, Topsin M, Thiram, Ridomil MZ 72, Captaf and Indofil M 45 inhibited growth of Foc and were found to be compatible with T. harzianum. Pf 80 and M. ciceri were insensitive to the fungicides including Vitavax power. The combination of seed dressing formulation Pusa 5SD developed from T. harzianum, Pf 80, M. ciceri and Vitavax power provided maximum protection to emerging seedlings. The seeds treated with Pusa 5SD + Pf 80 + M. ciceri + Vitavax power provided the highest germination, grain yield and the lowest wilt incidence in pot and field experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea (Cicer arietinum L.) is one of the most important pulse crops of both tropical and temperate regions. In India, chickpea is grown in 9.21 M ha with a total production of 8.22 M tonnes and an average productivity of 896 kg ha−1 (Agriculture Statistics at a Glance 2011). Susceptibility to disease is one of the major causes of yield lose of chickpea. In general, estimates of yield losses by insects and diseases range from 5 to 10 % in temperate regions and 50–100 % in tropical regions (van Emden et al. 1988). Among the diseases affecting chickpea, wilt caused by Fusarium oxysporum Schlechtend Fr. f. sp. ciceris (Padwick) Matuo & K. Sato (Foc) is considered one of the factors limiting productivity (Haware and Nene 1982). The disease is widespread in the chickpea growing areas of the world (Nene et al. 1996). In India, it has been reported from all the chickpea growing states causing an annual loss of 10 % (Singh and Dahiya 1973). The losses varied according to the stages of the crop affected. Wilting at seedling stage of the crop growth causes 77–94 % losses while late wilting causes 24–65 % loss (Haware and Nene 1980). Its incidence varied from 14.1 to 32.0 % in different states of India (Dubey et al. 2010).
Use of bio-agents in combination with reduced doses of chemical fungicide has recently been emphasized for sustainable agriculture (Someya et al. 2007; Andrabi et al. 2011). Cultural methods of disease management are not sufficiently effective for the pathogens like Fusarium having prolonged saprophytic survival ability. Use of resistant varieties is the best option but availability and durability of resistant varieties are major bottlenecks. Under such conditions, a bio-agent based management offers great promise. Biological control of a disease primarily requires the identification and deployment of highly effective strains. The uses of Trichoderma as a bio-agent have attracted attention because of its effectiveness against various plant pathogens and for its growth promoting action (Harman et al. 2004). The Trichoderma species evaluated against the wilt pathogen exhibited great potential in managing chickpea wilt under glasshouse and field conditions (Kaur and Mukhopadhayay 1992; Dubey et al. 2007). Selected isolates of Pseudomonas fluorescens were found to be effective in reducing the wilt incidence and increasing the plant growth as well as grain yield of chickpea (Liu et al. 2007).
The combination of bio-agents along with lower dose of fungicides has been successfully used for the control of several diseases (Kumar and Dubey 2001; Moradi et al. 2012). A novel bio-formulation Pusa 5SD developed from a potential strain of Trichoderma harzianum was effective against the wilt of chickpea (Dubey et al. 2007, 2012). To enhance the efficacy of Pusa 5SD, it may be combined with a compatible strain of bacterial antagonist and new fungicide. Integrating bio-agents with chemicals or two different bio-agents has become an acceptable strategy for managing many diseases but it has not been fully explored at the field level for Fusarium wilt of chickpea. The benefits of this approach include improving plant growth and quality, reducing the amount of chemical application, reducing the possibility of developing resistance in the pathogens and potential environmental hazards. Therefore, the present study aimed to determine the compatibility of Pusa 5SD with a bacterial bio-agent, a fungicide and Mesorhizobium and their combined as well as individual efficacies for the suppression of wilt and enhancement of chickpea yield.
Materials and methods
Collection and maintenance of bio-agents, Fusarium oxysporum f. sp. ciceris, cultures and seeds
The cultures of T. harzianum (IARI P4; MTCC No. 5371) and Delhi isolate of F. oxysporum f. sp. ciceris (Foc 53) available in the Pulse Laboratory of the Division of Plant Pathology, IARI, New Delhi, India on 2 % potato dextrose agar medium were used in the present study. The isolate of T. harzianum had been proved inhibitory to Foc in vitro (Dubey et al. 2007) and Pusa 5SD formulation developed from this isolate was used for seed treatment (Dubey et al. 2009). The cultures of Pseudomonas fluorescens, namely, P. fluorescens 59 (Pf 59), P. fluorescens 62 (Pf 62) and P. fluorescens 80 (Pf 80) obtained from the Bacteriology Laboratory of the Division of Plant Pathology, IARI, New Delhi, India and the cultures of Bacillus species, namely, B. lechniformis (Bl) and Bacillus species (Bskm 5) obtained from the Division of Chemicals, IARI, New Delhi, India were obtained and maintained on nutrient agar medium. The culture of Mesorhizobium ciceri 66 (CP 66) obtained from the Division of Microbiology, IARI, New Delhi, India was maintained on yeast extract mannitol agar medium. Seeds of chickpea variety Pusa 362 were taken from Pulse Laboratory, Division of Plant Pathology, IARI, New Delhi, India.
In vitro efficacy of bacterial antagonists against F. oxysporum f. sp. ciceris
Three isolates of P. fluorescens, Pf 59, Pf 62 and Pf 80 and two isolates of Bacillus, Bl and Bskm 5 were evaluated against Foc representing two different races of the pathogen isolated from Delhi, northern India (Foc 53; race 4) and Hyderabad, southern India (Foc 118; race 1) by the dual culture technique using completely randomized design (CRD)-factorial in five replications. Nutrient agar medium (15 ml) was poured in each Petri dish (90 mm). Seven day old inoculum (5 mm disc) of Foc and three days old inoculum of bacteria (10 mm streak) were used. The pathogen was placed in the centre and two streaks of bacterium were made at both sides of the inoculum of the pathogen. The control Petri dishes were inoculated only with the pathogen. Growth diameter of the pathogen in each Petri dish was measured 14 days after incubation at 25 ± 1 °C in BOD incubator (Colton, NSW-152, NSW, India) and percent growth inhibition was calculated (Dubey et al. 2007) to determine the efficacy of each isolates of the bacterial antagonist.
Compatibility test among T. harzianum, bacterial antagonists and M. ciceri
The compatibility of the most effective antagonists, Pf 80 and Bskm 5, T. harzianum and M. ciceri were tested amongst themselves. The overlapping growth to each other was determined as compatible interaction whereas incompatible interaction showed inhibition zone between the paired microorganisms. Petri dishes (90 mm) were poured with sterilized PDA medium (15 ml) and inoculated by placing 5 mm disc of T. harzianum and a 10 mm piece of blotting paper pre-inoculated with bacterial antagonists and M. ciceri separately in different combinations, and each combination was replicated thrice.
Determination of tolerance in F. oxysporum f. sp. ciceris, T. harzianum, P. fluorescens, and M. ciceri to fungicides
Two set of experiments were conducted to evaluate the fungicides against Foc 53 and T. harzianum in vitro using the poisoned food technique (Nene and Thapliyal 1993). Twelve fungicides, namely, carboxin 37.5 % + TMTD 37.5 % WS (Vitavax power™), metalaxyl 8 % + mancozeb 64 % WP (Ridomil MZ72™), captan 50 % WP (Captaf™), iprodione 25 % + carbendazim 25 % WP (Quintal™), carbendazim 50 % WP (Bavistin™), mancozeb 75 % WP (Indofil M45™), tetramethyl thiuram disulphide (TMTD) 75 % WS (Thiram™), copper oxychloride 50 % WP (Blitox50™), carboxin 75 % WP (Vitavax™), metalaxyl 35 % WS (Ridoxyl™), thiophanate methyl 70 % WP (Topsin M™) and propiconazole 25 % EC (Result™) at three concentrations (0.05, 0.1 and 0.2 %) were evaluated using completely randomized design (CRD)-factorial in three replications. The requisite quantity of fungicides was incorporated into a sterile non-solidified PDA medium, shaken well to make it homogenous and poured (15 ml) into 90 mm Petri dishes in three replications. The control was maintained without fungicide. Each Petri dish was inoculated by placing a 5 mm mycelial disc in centre and incubated at 25 ± 1 °C. The relative efficacies of the fungicides were determined by measuring the growth diameter of the mycelium and the percent growth inhibition over the control was calculated for each treatment (Dubey et al. 2007).
Another two sets of experiments were conducted for the evaluation of fungicides against Pf 80 and M. ciceri in vitro using the paper disc plate method (Nene and Thapliyal 1993). Twelve fungicides mentioned earlier were evaluated at three concentrations. The Petri dish (90 mm) was poured with 10 ml of nutrient agar medium and allowed to solidify. Five ml warm nutrient agar medium (40 °C) containing bacterial suspension (106 cells ml−1) was spread in the plate to ensure even coverage. Sterilized blotting paper discs of 10 mm were dipped in the required concentration of fungicide solution and four such pieces were placed in each Petri dish on the surface of the medium preseeded with bacterial solution in three replicates. The control was maintained without any fungicide. The Petri dishes were incubated at 25 ± 1 °C. Inhibition zone around paper discs was recorded.
The mycelial discs showing no growth in fungicide amended medium were transferred to plates containing PDA medium without fungicide to determine the fungistatic and fungicidal effect of the fungicide used (Soliman and Badeaa 2002). Median effective concentration (EC50) was calculated by using software EC50––calculator 2001 (CSIRO, Australia).
Evaluation of various seed treatment in vitro
The seed dressing formulation Pusa 5SD developed from T. harzianum (IARI P4; MTCC No. 5371) proved effective (Dubey et al. 2007, 2009) and was selected for evaluation along with P. fluorescens, M. ciceri and fungicide Vitavax power. These treatments were compatible among themselves and evaluated alone and in combinations as seed treatment against the pathogen. The experiment was conducted in CRD consisting of 16 treatments, namely, Pusa 5SD (T. harzianum), talc formulation of Pf 80, talc formulation of M. ciceri, Vitavax power, Pusa 5SD + Pf 80, Pusa 5SD + M. ciceri, Pusa 5SD + Vitavax power, Pf 80 + M. ciceri, Pf 80 + Vitavax power, M. ciceri + Vitavax power, Pusa 5SD + Pf 80 + M. ciceri, Pusa 5SD + Pf 80 + Vitavax power, Pusa 5SD + M. ciceri + Vitavax power, Pf 80 + M. ciceri + Vitavax power, Pusa 5SD + Pf 80 + M. ciceri + Vitavax power and control (untreated seeds). Seeds were treated with the fungicide at 2 g kg−1 seed while Pusa 5SD and the talc based formulations of P. fluorescens and M. ciceri were used at 4 g kg−1 of seed (108 cfu g−1) separately and for integrated treatment with half doses of the fungicide (1 g kg−1) followed by bio-formulations. Talc based formulations of P. fluorescens and M. ciceri was prepared by adding the bacterial suspension multiplied on KB broth medium (100 ml) for 72 h at 28 ± 1 °C (150 rpm) in sterilized talk powder (1:15 v/w) so as to obtain 108 cfu g−1. Seeds of susceptible chickpea cultivar Pusa 362 were treated with the requisite quantity of bio-agent and fungicides, alone and in combinations. The seeds for each treatment were placed in 250 ml conical flasks and the requisite quantity of bio-agent formulation/fungicide was added. The flasks were shaken vigorously for 2–3 min for uniform coating on the seeds. The Petri dish (90 mm) was poured with 10 ml PDA medium. Solidified medium in plate was seeded with 5 ml warm PDA medium (40 °C) containing spores of Foc 53 (105 conidia ml−1). Three treated seeds were placed in each Petri dish with the help of sterilized forceps in three replicates, and control was made by placing untreated seeds. The Petri dishes were incubated at 25 ± 1 °C and inhibition zone or growth of antagonist around the seeds was recorded.
Evaluation of seed treatments in pot experiments
The pot experiments were conducted in a CRD during 2009–2010 and 2010–2011 with the selected 12 treatments based on the results of the in vitro experiment. Pusa 5SD, talc formulation of Pf 80, Vitavax power, Pusa 5SD + Pf 80, Pusa 5SD + M. ciceri, Pusa 5SD + Vitavax power, Pusa 5SD + Pf 80 + M. ciceri, Pusa 5SD + Pf 80 + Vitavax power, Pusa 5SD + M. ciceri + Vitavax power, Pf 80 + M. ciceri + Vitavax power and Pusa 5SD + Pf 80 + M. ciceri + Vitavax power were evaluated in three replications. Seeds of susceptible chickpea cultivar Pusa 362 were treated as per the procedure described earlier. Ten treated seeds were sown in 15 cm diameter surface sterilized plastic pots (0.1 % mercuric chloride) filled with 2 kg sterilized soil (1 % formalin for 15 days) and inoculated with a 12-day old inoculum of the pathogen (Foc 53) multiplied on sorghum grains (10 g kg−1 soil) seven days before sowing (Dubey et al. 2009). The pots sown with untreated seeds were also maintained as controls. Seed germination was recorded 15 days after sowing (DAS). Wilt incidence was recorded at 20 day intervals up to the maturity of the crop. Mean of two years data were presented.
Evaluation of seed treatments in field experiment
The field experiments were conducted during the winter season of 2011–2012 and 2012–2013 in a randomized block design with seven treatments in three replications in a sick field (infested with Foc and maintained for the last 30 years only for chickpea cultivation) condition at IARI, New Delhi, India. During 2011–2012 the same experiment was repeated in another field being used for chickpea cultivation at the research farm of IARI, New Delhi, India. The treatments consisted of Pusa 5SD, talc formulation of Pf 80, Vitavax power, Pusa 5SD + Pf 80, Pusa 5SD + Pf 80 + M. ciceri + Vitavax power, the most commonly recommended seed treatment consisting of a mixture of Bavistin + Thiram, and control (untreated seeds). Chickpea wilt susceptible cultivar Pusa 362 was sown at 30 cm × 10 cm spacing in 6.1 m2 sizes plot for each replication of a treatment. Chickpea seeds treated with bio-agents and fungicide separately and in combination as per treatment were sown in six rows in each plot (180 seeds). Pusa 5SD and P. fluorescens were used at 4 g kg−1 of seed. The fungicide Vitavax power and the mixture of Bavistin + Thiram (1:1 ratio) were used at 2 g kg−1 of seed while Vitavax power was used at 1 g kg−1 of seed when combined with bio-agents. Seed germination was counted 15 DAS. Wilt incidence was recorded at 20 day intervals up to the maturity of the crop and total wilted plants per plot were given. Grain yield per plot was measured after the harvesting of the crop.
Statistical analysis
For statistical analysis, the data recorded in percentages were transformed into angular values before the analysis. The data pertaining to all the observations were subjected to ANOVA using the SAS Software (SAS Institute, version 9.1, Cary, NC, USA). The in vitro and pot experiments data were analyzed as per the procedure for a completely randomized design, whereas the data of field experiments were subjected to statistical analysis as per the procedure of a randomized block design for the test of significance. The pooled analysis was also conducted for two years pot and field data. Fisher’s protected least significant differences (LSD) was computed only when ANOVA showed significant differences for any particular effect.
Results
In vitro efficacy of bacterial antagonists against F. oxysporum f. sp. ciceris
Amongst the three isolates of P. fluorescens evaluated against two isolates of Foc, PGPR strain Pf 80 caused significantly highest inhibition followed by Pf 62 and Pf 59. Out of the two isolates of Foc, Foc 53 was inhibited more in comparison with Foc 118. Of the interactions of bacterial antagonist and Foc, the interaction of Pf 80 and Foc 53 showed the highest inhibition followed by Pf 80 × Foc 118 (Table 1). Of the two isolates of Bacillus species evaluated, Bskm 5 isolate showed significantly higher growth inhibition of Foc. Delhi isolate of Foc was more susceptible to the bacterial antagonists. Bskm 5 × Foc 118 showed the highest inhibition followed by Bskm 5 × Foc 53 (Table 2).
Compatibility test among potential isolates of T. harzianum, bacterial antagonists and M. ciceri
The compatibility of the most effective bacterial antagonists, namely, Pf 80 and Bskm 5, the most effective isolates of T. harzianum and M. ciceri showed that out of the two bacterial species, only PGPR strain Pf 80 was compatible with T. harzianum and M. ciceri with no inhibition zone. T. harzianum was also found to be compatible with M. ciceri. Bskm 5 proved to be incompatible with M. ciceri (9.0 mm inhibition zone), T. harzianum (4.3 mm inhibition zone) and Pf 80 (5.7 mm inhibition zone).
Determination of tolerance in bacterial and fungal antagonists, M. ciceri and F. oxysporum f. sp. ciceris to fungicides
The results (Table 3) clearly indicated that amongst the fungicides evaluated against Foc, Vitavax power and Quintal, and Bavistin, Thiram, Rodoxyl, Topsin M and Result alone inhibited 100 % growth at all the three concentrations tested. The next most effective fungicide was Vitavax followed by Indofil M 45, Ridomil MZ 72, Captaf and Blitox 50 in the order of their superiority. Amongst the concentrations evaluated, 0.2 % concentration caused the highest inhibition followed by 0.1 and 0.05 % concentrations. Blitox 50 at 0.2 % and Captaf at 0.2 % were the next most inhibitory interactions after that in which 100 % inhibition was recorded. Except Result, all the fungicides that caused 100 % inhibition of Foc 53, proved to be fungicidal against the pathogen. Captaf, Bavistin and Vitavax showed the lowest EC50 values followed by Blitox 50 and Ridomil MZ 72.
The results (Table 4) showed that amongst the fungicides evaluated against T. harzianum, Vitavax power, Quintal, Ridoxyl and Result completely inhibited the growth of T. harzianum. The next treatment in the order of effectiveness was Blitox 50 followed by Topsin M, Thiram, Captaf, Bavistin, Indofil M 45 and Ridomil MZ 72. Irrespective of fungicides, 0.2 % concentration was most inhibitory followed by 0.1 and 0.5 % concentrations. Amongst the interaction of fungicides and their concentrations, Ridomil MZ 72 at 0.5 % and Indofil M 45 at 0.5 and 0.1 % were less inhibitory. The inhibition percentages recorded in the last two were not statistically different. Quintal, Ridoxyl and Result were fungicidal. Ridomil MZ 72 showed the highest EC50 value followed by Indofil M 45, Vitavax, Topsin M, Captaf and Thiram. The results of compatibility between fungicides and bacteria indicated that none of the fungicides inhibited the growth of P. fluorescens and M. ciceri in the plates.
In vitro evaluation of various seed treatments alone and in combinations against the pathogen
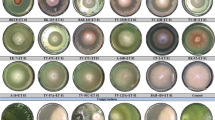
The results indicated that all the treatments provided protection to germinating seeds either by covering them with the growth of Trichoderma or by creating an inhibition zone around the treated seeds (Fig. 1). The treatments that had Pusa 5SD either alone or in combination with others provided the highest protection due to mycelial growth of Trichoderma around the treated seeds. Pusa 5SD followed by Pusa 5SD + M. ciceri, Pusa 5SD + Pf 80 + M. ciceri and Pusa 5SD + Pf 80 showed the best growth of Trichoderma around the treated seeds along with least inhibition zone. The combination of Pusa 5SD + Pf 80 + M. ciceri + Vitavax power provided the highest inhibition zone along with the least growth of Trichoderma around seeds.
Growth/inhibition zone recorded in various seed treatments. T1 Pusa 5SD, T2 Pf 80, T3 M. ciceri, T4 Vitavax power, T5 Pusa 5SD + Pf 80, T6 Pusa 5SD + M. ciceris, T7 Pusa 5SD + Vitavax power, T8 Pf 80 + M. ciceris, T9 Pf 80 + Vitavax power, T10 M. ciceris + Vitavax power, T11 Pusa 5SD + Pf 80 + M. ciceris, T12 Pusa 5SD + Pf 80 + Vitavax power, T13 Pusa 5SD + M. ciceris + Vitavax power, T14 Pf 80 + M. ciceris + Vitavax power, T15 Pusa 5SD + Pf 80 + M. ciceris + Vitavax power and T16 Untreated seeds (control) against F. oxysporum f. sp. ciceris. The values with different letters are significantly different at 5 % level applying Fisher’s least significance difference test for growth/inhibition zone (F15,32 = 147.3, P < 0.05). The error bars are corresponding to SE ±
Evaluation of seed treatments in pot experiments
The results (Fig. 2) indicated that all the treatments significantly (P < 0.05) enhanced the seed germination and reduced the wilt incidence. The seeds treated with a combination of Pusa 5SD + Pf 80 + M. ciceri + Vitavax power provided the highest seed germination and the lowest wilt incidence, followed by Pusa 5SD + Pf 80 + Vitavax power, Pusa 5SD + Vitavax power and Pusa 5SD + M. ciceri + Vitavax power. The seed germination and wilt incidence recorded in Pusa 5SD + Pf 80 + Vitavax power, Pusa 5SD + Vitavax power and Pusa 5SD + M. ciceri + Vitavax power did not differ statistically. However, wilt incidence recorded in Pusa 5SD + Pf 80 + M. ciceri + Vitavax power was statistically similar to these treatments. Pusa 5SD alone was also found to be more effective than Vitavax power and Pf 80 in increasing the seed germination.
Effect of various seed treatment on seed germination and wilt incidence in chickpea. T1 Pusa 5SD, T2 Pf 80, T3 Vitavax power, T4 Pusa 5SD + Pf 80, T5 Pusa 5SD + M. ciceri, T6 Pusa 5SD + Vitavax power, T7 Pusa 5SD + Pf 80 + M. ciceri, T8 Pusa 5SD + Pf 80 + Vitavax power, T9 Pusa 5SD + M. ciceri + Vitavax power, T10 Pf 80 + M. ciceri + Vitavax power, T11 Pusa 5SD + Pf 80 + M. ciceri + Vitavax power and T12 control (untreated seeds). The values with different letters are significantly different at 5 % level applying Fisher’s least significance difference test for seed germination (F11,24 = 101.9, P < 0.05) and wilt incidence (F11,24 = 64.4, P < 0.05) separately. The error bars are corresponding to SE ±
Evaluation of seed treatments under field conditions
The results of field experiments (Table 5) conducted in sick field infested with Foc showed that the treatments evaluated significantly enhanced the seed germination (F6,12 = 26.4, P < 0.05 for 2011–2012 and F6,12 = 164.9, P < 0.05 for 2012–2013) and the grain yield (F6,12 = 138.2, P < 0.05 for 2011–2012 and F6,12 = 102.2, P < 0.05 for 2012–2013) of chickpea and reduced the wilt incidence (F6,12 = 140.1, P < 0.05 for 2011–2012 and F6,12 = 336.6, P < 0.05 for 2012–2013) as compared to those of the control during both the years of experimentation as well as in mean data. A combination of Pusa 5SD + Pf 80 + M. ciceri + Vitavax power provided significantly higher seed germination and grain yield compared to those of other treatments during both the years of experimentation as well as in mean data. The lowest wilt incidence was also recorded in this treatment and the wilt incidence recorded in this treatment did not differ statistically from that of Vitavax power during 2011–2012 and in mean data. The next most effective treatment was Vitavax power for enhancing the seed germination and grain yield, and reducing the wilt incidence followed by Bavistin + Thiram for seed germination and Pusa 5SD + Pf 80 for reducing the wilt incidence and enhancing the grain yield. However, the seed germination recorded in Bavistin + Thiram did not differ significantly with that of Pusa 5SD + Pf 80.
The results (Fig. 3) of field experiment conducted at a different location showed that, except for Pf 80 for seed germination, all treatments significantly enhanced the seed germination (F6,12 = 22, P < 0.05) and grain yield (F6,12 = 84.8, P < 0.05) of chickpea and reduced the wilt incidence (F6,12 = 77.7, P < 0.05) relative to the control. The seeds treated with a combination of Pusa 5SD + Pf 80 + M. ciceri + Vitavax power showed the highest seed germination and grain yield with the lowest wilt incidence. The seed germination recorded in this treatment did not differed statistically from that of Vitavax power at 15 DAS. However, the reduction in wilt incidence and the increase in grain yield were significantly greater in this treatment. The next most effective treatment was Vitavax power which was superior to Bavistin + Thiram for seed germination and grain yield, but did not statistically different for wilt incidence.
Effect of seed treatment T1 Pusa 5SD, T2 Pf 80, T3 Vitavax power, T4 Pusa 5SD + Pf 80, T5 Pusa 5SD + Pf 80 + M. ciceri + Vitavax power, T6 Bavistin + Thiram, T7 control (untreated seeds) on seed germination, wilt incidence and grain yield in chickpea. The values with different letters are significantly different at 5 % level applying Fisher’s least significance difference test for seed germination (F6,12 = 22, P < 0.05), wilt incidence (F6,12 = 84.8, P < 0.05) and grain yield (F6,12 = 77.7, P < 0.05) separately. The error bars are corresponding to SE ±
Discussion
Due to the seed- and soil-borne nature of chickpea wilt, application of chemicals for management is hardly successful in the presence of high level of inoculum and favourable weather conditions. Therefore, a feasible and cost effective approach would be the cultivation of resistant varieties or biological control. The present study was focused on the development of an integrated management module for chickpea wilt. A strain of T. harzianum proved to be effective against Foc isolates representing various races of the pathogen (Dubey et al. 2007) and a novel formulation Pusa 5SD with long shelf life (25 months storage at room conditions) developed (Dubey et al. 2009) and found to be effective against wilt (Dubey et al. 2012) was selected for use as one of the components for integration with others. The seed dressing formulation Pusa 5SD showed novelty in respect of long shelf life (25 months) and efficacy against several soil borne plant pathogens. PGPR strain Pf 80 which was superior to other strains of bacterial antagonists for the inhibition of mycelial growth of the pathogen and proved to be compatible with M. ciceri, and the isolate of T. harzianum (Pusa 5SD) were selected as other components for integration.
Different fungicides commonly available in the market for chickpea seed treatment were evaluated against the pathogen and bio-agents to determine their compatibility. Ridomil MZ 72, Indofil M 45, Vitavax, Captaf, Topsin M, Blitox 50, and Vitavax power proved to be less inhibitory to T. harzianum in comparison to Foc. The fungicide Vitavax power which completely inhibited the mycelial growth of Foc and the growth of T. harzianum was reduced by 70–100 % at all the different concentrations was selected for seed treatment along with bio-agents. All the fungicides at all the concentrations were found to be compatible with Pf 80 strain of PGPR and M. ciceri.
A combined application of Pusa 5SD, a novel formulation of T. harzinaum, Pf 80, M. ciceri and Vitavax power provided the highest seed germination and the lowest wilt incidence under pot conditions. A combination of Pusa 5SD and Vitavax power was equally effective in reducing the wilt incidence besides being the second best combination for enhancing the seed germination. Similarly, under field conditions, the same combination provided the highest seed germination and grain yield, and the lowest wilt incidence. This treatment combination showed similar performance both under the field infested with Foc and the field commonly used for chickpea cultivation, but the level of wilt incidence was more under sick field. Pusa 5SD individually showed superiority over Pf 80 and Bavistin + Thiram in reducing the wilt incidence and increasing the grain yield. An earlier study showed that seed treatment with fungicide (Bavistin) increased the seed germination. Seed coating with the bio-agents (T. viride and T. virens) resulted in the lowest disease incidence. However the highest yield was recorded in the case of fungicide (Bavistin), followed by bio-agent T. viride (Andrabi et al. 2011). In the present study also, the fungicide Vitavax power showed superiority over Pusa 5SD. The present finding is partially supported by the observation made by Amalraj et al. (2012). They recorded the highest seedling emergence in carbendazim treated seeds and it was on a par with a combination of chemical and bio-agents. Seeds treated with B. megatherium var phosphaticum (phosphate solubilising bacterium-PSB) + Rhizobium + T. viride followed by soil application of T. viride + PSB + Rhizobium after 30 DAS mixed with 200 kg of FYM provided the lowest wilt incidence and the highest grain yield. In the present study, the highest germination recorded in a combined application of Pusa 5SD, Pf 80, M. ciceri and Vitavax power might be due to the production of phytohormones and other growth promoting substances in addition to the protection provided by them to the germinating seeds from the pathogen present in the soil (Amalraj et al. 2012). The present findings are supported by the observations made by earlier workers (Ramezani 2009; Shaban and El-Bramawy 2011). Ramezani (2009) reported that among the fungal bio-agents, T. harzianum caused the maximum inhibition zone. There was no significant difference between the inhibition zones produced by P. fluorescens and B. subtillis. Soil application of talc-based formulation of T. harzianum, P. fluorescens and T. virens effectively controlled the wilt of chickpea under field condition. The strain of T. harzianum used in the present study showed superiority over other species of Trichoderma against Foc in in vitro conditions (Dubey et al. 2007). The combined application of fungal and bacterial bio-agents and Mesorhizobium showed higher efficacy in comparison to their individual application, perhaps due to different levels of mycoparasitism and antibiosis. Similarly, Shaban and El-Bramawy (2011) reported that the seeds treated with Rhizobium and T. harzianum controlled the damping-off and root rot diseases in the legume field crops and improved the plant growth parameters and the seed yield.
Of the two species of bacterial antagonists evaluated in the present study, only Pf 80 was found to be compatible with T. harzianum and M. ciceri. The combination of commercial formulations of B. subtillis and T. harzianum effectively controlled the wilt in chickpea but their individual effect did not differ significantly (Moradi et al. 2012). In the present study, Bacillus species was not compatible with T. harzianum and M. ciceri. Therefore, it was not selected for integration. Karimi et al. (2012) observed that P. aeuroginosa and B. subtillis provided better control of wilt in seed treatment and soil-inoculation and improved the growth of chickpea plants. Moradi et al. (2012) reported that the application of B. subtillis and T. harzianum either singly or in combination, in both seed and liquid inoculation methods, suppressed the Fusarium wilt indicating the importance of the application of biocontrol agents. In the present study, Pusa 5SD, a formulation of T. harzianum alone, was also found effective in reducing the wilt and enhancing the grain yield of chickpea. Merkuz and Getachew (2012) also reported the potential of Trichoderma in reducing the wilt incidence and delaying the disease onset.
The present study generated basic information regarding the efficacy and compatibility of fungal and bacterial bio-agents, fungicides and Mesorhizobium. A module consisting of Pusa 5SD (T. harzianum), P. fluorescens, M. ciceri, Vitavax power has been developed for the integrated management of wilt for obtaining high grain yield of chickpea.
References
Agricultural Statistics at a Glance (2011) Directorate of Economics and Statistics, Department of Agriculture, Ministery of Agriculture, Goverment of India. Agricultural Statistics at a Glance, New Delhi
Amalraj ELD, Praveen Kumar G, Mir Hassan Ahmed SK, Desai S (2012) On-farm evaluation of integrated nutrient and pest management in Cicer arietinum L. J Phytol 4:48–51
Andrabi M, Vaid A, Razdan VK (2011) Evaluation of different measures to control wilt causing pathogens in chickpea. J Plant Prot Res 51:55–59
Dubey SC, Suresh M, Singh B (2007) Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biol Control 40:118–127
Dubey SC, Bhavani R, Singh B (2009) Development of Pusa 5SD for seed dressing and Pusa Biopellet 10 G for soil application formulation of Trichoderma harzianum and their evaluation for integrated management of dry root rot of mungbean (Vigna radiata). Biol Control 50:231–242
Dubey SC, Singh SR, Singh B (2010) Morphological and pathogenic variability of Indian isolates of Fusarium oxysporum f. sp. ciceris causing chickpea wilt. Arch Phytopathol Plant Prot 43:174–189
Dubey SC, Tripathi A, Singh B (2012) Integrated management of Fusarium wilt by combined application of soil and seed dressing formulations of Trichoderma species to increase grain yield of chickpea. Int J Pest Manag 59:47–54
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev 2:43–56
Haware MP, Nene YL (1980) Influence of wilt at different growth stages on yield loss of chickpea. Trop Grain Legume Bull 19:38–40
Haware MP, Nene YL (1982) Symptomless carriers of chickpea Fusarium wilt. Plant Dis 66:250–251
Karimi K, Amini J, Harighi B, Bahramnejad B (2012) Evaluation of bio-control potential of Pseudomonas and Bacillus spp. against Fusarium wilt of chickpea. Aust J Crop Sci 6:695–703
Kaur NP, Mukhopadhayay AN (1992) Integrated control of chickpea wilt complex by Trichoderma spp. and chemical methods in India. Trop Pest Manag 38:372–375
Kumar D, Dubey SC (2001) Management of collar rot of pea by the integration of biological and chemical methods. Indian Phytopathol 54:62–66
Liu YF, Chen ZY, Ng TB, Zhang J, Zhou MG, Song FP, Liu YZ (2007) Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtillis strain B-916. Peptides 28:553–559
Merkuz A, Getachew A (2012) Management of chickpea wilt (Fusarium oxysporum f. sp. ciceris) using Trichoderma spp. Int J Curr Res 4:128–134
Moradi H, Bahramnejad B, Amini J, Siosemardeh A, Allahverdipoor K (2012) Suppression of chickpea (Cicer arietinum L.) Fusarium wilt by Bacillus subtillis and Trichoderma harzianum. Plant Omics J 5:68–74
Nene YL, Thapliyal PN (1993) Fungicides in plant disease control. Oxford and IBH Publ Co, New Delhi
Nene YL, Sheila VK, Sharma SB (1996) A world list of chickpea and pigeonopea pathogens. 5th edn. ICRISAT, Patancheru
Ramezani H (2009) Efficacy of some fungal and bacterial bioagents against Fusarium oxysporum f. sp. ciceri on chickpea. Plant Prot J 1:108–113
Shaban WI, El-Bramawy MA (2011) Impact of dual inoculation with Rhizobium and Trichoderma on damping off, root rot diseases and plant growth parameters of some legumes field crop under greenhouse conditions. Int Res J Agric Sci Soil Sci 1:98–108
Singh KB, Dahiya BS (1973) Breeding for wilt resistance in chickpea. Symposium on problem and breeding for wilt resistance in Bengal gram. IARI, New Delhi, pp 13–14
Soliman KM, Badeaa RI (2002) Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol 40:1669–1675
Someya N, Tsuchiya K, Yoshida T, Tsujimoto-noguchi M, Sawada H (2007) Combined application of Pseudomonas fluorescens strain LRB3W1 with a low dosage of benomyl for control of cabbage yellows caused by Fusarium oxysporum f. sp. conglutinans. Biocontrol Sci Technol 17:21–31
van Emden HF, Ball SL, Rao MR (1988) Pest diseases and weed problems in pea lentil and faba bean and chickpea. In: SummerWeld RJ (ed) World crops: cool season food legumes. Kluwer Academic Publishers, Dordrecht, pp 519–534
Acknowledgments
Authors are thankful to Indian Council of Agricultural Research, New Delhi, India for financial support through outreach project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Fouad Daayf.
Rights and permissions
About this article
Cite this article
Dubey, S.C., Singh, V., Priyanka, K. et al. Combined application of fungal and bacterial bio-agents, together with fungicide and Mesorhizobium for integrated management of Fusarium wilt of chickpea. BioControl 60, 413–424 (2015). https://doi.org/10.1007/s10526-015-9653-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9653-8