Abstract
Fusarium wilt caused by Fusarium oxysporum f. sp. ciceris is one of the main threats to chickpea and affects sustainable food production. To combat the phytopathogens, successful measures the so-called “biocontrol” are developed over the years wherein numerous plant growth-promoting rhizobacteria (PGPRs) have been investigated for their capacities to protect plants from pathogens and stimulate plant growth. A putative PGPR qualifies as PGPR when it is able to produce a positive effect on the plant upon inoculation, hence demonstrating good competitive skills over the existing rhizosphere communities. This competence comprises the effective root colonization combined with the ability to survive and proliferate along growing plant roots over a considerable time period. In the present chapter, the author focused on PGPRs as biocontrol agents against F. oxysporum f. sp. ciceris (FOC).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
Chickpea (Cicer arietinum L.) is the world’s fourth most important legume crop after soybean and contributes 3.1 % to the world grain legume production. In developing countries, chickpea is a rich complement to the cereal diet since it has a high nutritive value and is mostly grown for its highly proteinated edible seeds and may be treated for both seed and forage production (Yadav et al. 2011). From ancient period of time, chickpea has been grown in India together with the Middle East and parts of Africa (Upadhyaya et al. 2008). Despite its economic importance, the productivity is low owing to biotic stress wherein many soilborne as well as seed-borne pathogens of which the vascular wilt fungus Fusarium oxysporum f. sp. ciceris (FOC). Fusarium wilt of chickpea caused by FOC is one of the most important and destructive vascular diseases of chickpea whereby losses are estimated with the rate at 10 % in India and Spain, 40 % in Tunisia, and 17 % in Iran (Dileep kumar 1999; Jamali et al. 2004). The most effective control strategy for the containment of FOC is the use of resistant chickpea cultivar wherein numbers of chickpea lines have been reported as resistant to wilt from different countries of the world. A resistant cultivar to be deployed is made on the choice with a particular FOC race that is prevalent in the field. Control of Fusarium wilt through breeding lines has become a difficult struggle due to the existence of several physiological pathogenic races of FOC (Nene et al. 1981). If FOC inoculum establishes in the soil, it is difficult to find out the disease or eliminate the pathogen by employing crop rotation for more than 6 years, and because of this, developing new alternatives is required for more effective disease management (Haware and Nene 1982; Gupta 1991).
The application of chemicals helped in the increase of yields obtained, but two of the major problems with the constant use of chemicals are the resistance induced in target organisms and contamination of the environment with very toxic substances. It is extremely difficult to control soilborne fungi by employing conventional strategies that may include the use of synthetic fungicides. Upon the continuous use of chemicals, fungal spores survive for many years in the soil due to their resistance, and hence, biological control strategies are selected and handled in an eco-friendly way instead of using chemical fungicides (Okigbo 2004). Biological control of plant pathogens using antagonistic bacteria is a promising strategy for plant protection wherein plant growth-promoting bacteria (PGPB) specifically plant growth-promoting rhizobacteria (PGPRs) have been shown to improve plant health and increase yield (Kloepper et al. 1999; Maheshwari 2010).
This chapter examines biological control employing PGPRs and their mechanisms involved. In organization, the chapter opens with a discussion of concepts, viz., Fusarium wilt of chickpea, PGPR, and the use of PGPR for biocontrol of Fusarium wilt of chickpea including comprehensive trials of PGPR. A cohort effect of PGPR occurs by local antagonism to the pathogen or by induction of systemic resistance in chickpea. Several substances produced by antagonistic rhizobacteria have been related to pathogen control and indirect promotion of growth in many plants, such as HCN and antibiotics. The induced systemic resistance (ISR) has recently gained considerable importance in the control of Fusarium wilt of chickpea diseases; result of works presented in this chapter has shown the possibility of exploitation for greenhouses/fields. Throughout this chapter, the author uses data from long-studied research operated in the northwest of Algeria to support perspectives of the biocontrol of Fusarium wilt using native rhizobacteria.
10.2 Fusarium Wilt of Chickpea
It is our consensus that strains of Fusarium species are the major soilborne as well as seed-borne pathogens causing wilt and rot diseases in more than 80 plant species including chickpea that caused up to 100 % yield loss worldwide (Santos et al. 2002). The major limiting factor in chickpea production is Fusarium wilt that has been reported almost all over the world including India and defined by Butler in 1918, and further, its etiology was determined in 1940 by Padwick. McKerral (1923) described that Fusarium wilt is a soilborne disease that belongs to the genus Fusarium. An association of Fusarium sp. and Rhizoctonia sp. may also cause wilted plants (Narasimhan 1929). McRae (1932) as well as Prasad and Padwick (1939) reported FOC to be pathogenic to chickpea crop which is now accepted worldwide as the causal agent of Cicer spp. (Booth 1971; Kaiser et al. 1994). Fusarium wilt of chickpea is caused by Fusarium oxysporum (Schlechtend.:Fr.) f. sp. ciceris (Padwick) Matuo & K. Sato. The fungus was first named Fusarium orthoceras Appel & Wollenw. var. ciceri by Padwick, and later Chattopadhyay and Sen Gupta renamed the pathogen F. oxysporum Schl. f. sp. ciceri (Padwick) Snyder & Hansen. This was accepted as the correct name of the pathogen until revised by Holliday in 1980 (Jalali and Chand 1992; Nene and Reddy 1987). FOC is one of the few formae speciales of monophyletic origin in the F. oxysporum complex of the Gibberella clade, most of which are polyphyletic (O’Donnell et al. 1998; Baayen et al. 2000; Kistler 2001; Jiménez-Gasco et al. 2002; Demers et al. 2014; Jiménez-Díaz et al. 2015).
In the year 2000, around 33 countries of the world have been reported to be affected, causing 10–15 % yield losses annually depending upon the environmental conditions along with losses due to FOC. Fusarium wilt reduces chickpea production by decreasing both seed yield and seed weight (Singh and Dahiya 1973; Navas-Cortés et al. 2000; Nene et al. 1996). The FOC is more prevalent in the Indian subcontinent including the USA, Tunisia, Turkey, Ethiopia, Spain, and Mexico (Westerlund et al. 1974; Nene et al. 1989; Halila and Strange 1996). FOC is a primarily soilborne pathogen; however, it can be transmitted through seeds (Haware et al. 1978). Pathogens survive in soil and seed in the form of chlamydospores for many years. Mycelia enter the epidermal tissues invading through roots, extend to the vascular bundles, and form spores in plants (Chehri et al. 2010). The pathogen causes seed abortion and rot, necrosis, reduction or elimination of germination capacity, as well as plant damage at later stages of plant growth resulting in the development of the disease as systemic or local infection (Khanzada et al. 2002).
Upon pathogen attack, adult plants show typical wilt symptoms that involve drooping of petioles, rachis, and leaflets (Fig. 10.1). The roots of the wilting plants do not show any external rotting, but when split open vertically, dark brown discoloration of internal xylem is seen (Nene et al. 1991). Pods from the wilted plants look normal but seeds are generally smaller, wrinkled, and discolored. Though such seeds can be detected visually, a normal-looking seed harvested from wilted plants may also harbor the wilt pathogen.
Symptoms of the disease develop at any stage of plant growth, and affected plants may be grouped in patches or appear spread across a field (Trapero-Casas and Jimènez-Díaz 1985; Nene and Reddy 1987; Haware 1990). In molecular analysis, namely, random amplified polymorphic DNA (RAPD), DNA banding patterns allowed the identification of markers which differentiate among wilting and yellowing pathotypes (Kelly et al. 1994; Gupta et al. 2009). Upon occurrence, FOC exhibited two pathotypes and eight pathogenic races on chickpea; the yellowing pathotype induces progressive foliar yellowing and vascular discoloration with plant death within 40 days, whereas the wilt-causing pathotype induces severe and fast chlorosis, flaccidity, and vascular discoloration with plant death within 20 days after inoculation (Haware and Nene 1982; Jiménez-Diaz et al. 1993; Jorge et al. 2005). The eight races of FOC were identified as 0, 1A, 1B/1C, 2, 3, 4, 5, and 6, by reaction on a set of differential chickpea cultivars (Jiménez-Gasco and Jiménez-Diaz 2003). Besides, races 0 and 1B/1C cause yellowing, whereas races 1A, 2, 3, 4, 5, and 6 induce wilting (Jiménez-Gasco et al. 2001). Out of these, races 2, 3, and 4 have been reported only in India, while races 0, 1B/1C, and 5 have been found mainly in the Mediterranean region and in California, USA (Jimènez-Gasco and Jimènez-Diaz 2003). Race 1A has been reported from India, California, Morocco, and Spain, while race 6 has been found in California, Spain, Israel, and Morocco (Jimènez-Gasco et al. 2001).
10.3 Plant Growth-Promoting Rhizobacteria (PGPRs)
The German agronomist Hiltner first defined the rhizosphere, in 1904, wherein microbial activity was higher around the roots of legumes. This zone harbors a multitude of microorganisms that are affected by both abiotic and biotic stresses. Among these are the dominant bacteria that prefer living in close vicinity to the root or on its surface and play a crucial role in soil health and plant growth. These benign bacteria inhabiting the rhizosphere termed PGPR (Kloepper et al. 1989) were introduced in 1978 by the same author in the Proceedings of the Fourth International Congress of Bacterial Plant Pathogens, conducted in France (Ramos Solano et al. 2008).
It is well established that only 1–2 % of bacteria promote plant growth in the rhizosphere, and among them, strains from genera Pseudomonas, Azospirillum, Burkholderia, Bacillus, Enterobacter, Rhizobium, Erwinia, Serratia, Alcaligenes, Arthrobacter, Acinetobacter, and Flavobacterium have reported to enhance plant growth (Glick 1995; Antoun and Kloepper 2001). The mechanism by which PGPRs promote growth of plants can be either direct mechanism (biofertilizer and biostimulator activity) or indirect mechanism (biocontrol activity). The direct promotion of plant growth by PGPR entails either providing the plant with a compound that is synthesized by the bacterium or facilitating the availability of a nutrient and its uptake from environment (Glick 1995). The rhizobacteria produce the secondary metabolites, which are directly utilized by the plants thus promoting plant growth (Glick et al. 1999).
There are several ways the PGPR may directly facilitate the proliferation of their plant hosts:
-
Solubilize minerals like phosphates in a form that can be used by the plant
-
Synthesize phytohormones like auxins that trigger plant cell growth and proliferation
-
The ability to produce or change the concentration of plant growth regulators like indole acetic acid
-
Synthesize enzymes that can modulate plant hormone levels
-
Fix atmospheric nitrogen and supply it to the plant
The indirect promotion of plant growth occurs when PGPRs lessen/prevent the deleterious effects of phytopathogenic organisms through antibiosis and can be either due to the depletion of a scarce resource, required by the pathogen, or to the production and release of a compound that impedes the growth of the phytopathogenic organism (Glick 1995; Smitha et al. 2015).
The list of indirect mechanisms used by PGPR is substantial:
-
Synthesis of enzymes able to hydrolyze fungal cell walls
-
Synthesis of hydrocyanic acid (HCN) which suppresses growth of fungal pathogens
-
Production of antibiotics that kill the phytopathogen fungus
-
Induction of systemic resistance (ISR)
-
Antagonism against phytopathogenic microorganisms by production of siderophores
A detailed discussion of the first four mechanisms listed above follows.
10.4 Comprehensive Trials of PGPR for Biocontrol of Fusarium Wilt of Chickpea
The first step in obtaining PGPR is the isolation of rhizospheric bacteria from the soil volume close to the roots. After isolation of the maximum number of bacteria to avoid the loss of bacterial variability, different tests were performed to reduce the various types of bacteria chosen, so that only the beneficial ones remain. For identification of successful PGPR, standard methodologies for isolation, screening, and mode of action have been well documented (Landa et al. 1997a, b; Swain and Ray 2007; Idris et al. 2007). Several protocols have been developed for the identification of this PGPR, which can be broadly classified as in vitro and greenhouse and field tests.
10.4.1 In Vitro Antagonistic Activity Trials
Antibiosis is an important mechanism used by biocontrol agents to suppress diseased plants by producing volatile and nonvolatile antibiotics which disrupt the cell contents of pathogenic microorganisms before coming in contact with the biocontrol agent. The in vitro trials have been successfully used with all groups of biocontrol agents such as PGPRs. These trials were performed in vitro to check biochemical activities that correspond with potential PGPR characteristic. Some of the frequently used methods are briefly described here.
10.4.1.1 Dual Culture Assay
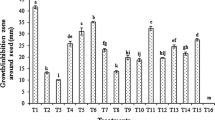
This technique known as biculture/paired culture has been extensively used for preliminary screening of large populations of rhizobacteria. In principle, the pathogen and the rhizobacteria should be allowed to interact in a petri dish under optimum conditions for both the pathogen and the rhizobacteria. The inhibition is recorded in the form of the inhibition zone produced by the antagonistic rhizobacteria (Fig. 10.2). The antagonistic effects are scored and the interface region was observed under light microscope (Zaim et al. 2013). The antagonistic potential of Bacillus spp. is well documented (Johri et al. 2003; Saharan and Nehra 2011). Thus, this phenomenon has often been used as a means for in vitro screening of biocontrol agents (Zaim et al. 2013).
In our study, the Bacillus isolates Rb29, Rb6, Rb12, Rb4, and Rb15 caused a modification in the mycelium appearance (Fig. 10.3). These modifications were changes in mycelia color from white to red, reddish brown, or darker brown. With these isolates, a coagulation of fungal cytoplasm that can be observed up to the hyphae was detected, resulting in the presence of small vesicles and the appearance of big vacuoles. In this case, the destructive effect of FOC by rhizobacteria was high, resulting in serious damage of the hyphae, associated with a series of degradation events (Zaim et al. 2013).
10.4.1.2 Production of Volatile Inhibitory Compounds
Many biocontrol microorganisms produce chemicals that are inhibitory to the pathogens. These chemicals can either be volatile or released into the medium (nonvolatile). Dennis and Webster (Dennis and Webster 1971) have developed methods for studying the production of volatile inhibitory compounds by the biocontrol agents. While testing for the production of volatiles, the pathogen and the rhizobacteria are inoculated on individual petri dishes. Inoculated petri dish with the test fungus was inverted and placed over the rhizobacterial culture. The two plates were sealed together with Parafilm to prevent gas diffusion, and then they were incubated under optimum conditions. This incubation ensured that both organisms were growing in the same conditions though they were physically separated. Any radial growth increase of the test fungus was recorded. PGPR strains release a blend of volatile organic compounds (2, 3-butanediol and acetone) that promote growth and induce resistance against pathogen (Ryu et al. 2004). In our study, volatile metabolite activity was observed in all 29 isolates where the target pathogen FOC1 was inhibited from 14.11 to 44.68 % (Zaim et al. 2013). Bacillus subtilis G8 isolated from soil in China produced antifungal volatile organic compounds. These volatile organic compounds detected include alkyls, alcohols, esters, ketones, acids, amines, phenols, and heterocyclic compounds (Liu et al. 2008).
10.4.1.3 In Vitro Detection of Plant Growth-Promoting Traits
Among the functional tests used to find efficient PGPR traits, the most common are the following: (1) test for enzymes (chitinase and β-1, 3-glucanase) that can degrade pathogenic fungi cell walls preventing plant diseases, (2) test for antibiotic, (3) test for antifungal metabolites such as HCN which suppress growth of fungal pathogens, (4) phosphate solubilization test, (5) test for plant growth regulator production, and (6) test for bacteria capable of producing biochemical compounds associated with host defense. PGPR may use more than one of these mechanisms as experimental evidence suggests that biocontrol of plant pathogens is the net result of multiple mechanisms that may be activated simultaneously.
In addition to the above-described plant growth-promoting features, the PGPRs protect the chickpea from FOC by several mechanisms. The mechanisms include the production of antibiotic, production of lytic enzymes that can lyse the cell wall of pathogenic fungi, production of antifungal metabolites such as hydrogen cyanide which suppress growth of fungal pathogens, production of phytohormones like IAA (indole-3-acetic acid), production of antibiotic metabolites, and induction of systemic resistance in plants (Hammerschmidt 1999; Raju et al. 2008; Moradi et al. 2012; Karimi et al. 2012; Kandoliya and Vakharia 2013; Patil et al. 2015; Smitha et al. 2015). Karimi et al. (2012) found that 232 bacteria isolated from the rhizosphere and root of chickpea showed substantial inhibition zones against FOC in vitro. Twelve out of 232 bacterial strains identified as Pseudomonas and Bacillus genera that exhibited high antifungal activity against pathogens were selected, and several biochemical activity indicators for putative PGPR abilities were tested. The indicators tested were the production of protease, siderophore, cyanide hydrogen, indole acetic acid, antifungal volatile, and extracellular compound. Moreover, Bacillus strains were tested for volatiles, cyanide production, and solubilization of phosphorus because of the potential implication of such traits in promoting plant growth (Bakker and Schippers 1987; Glick 1995).
10.4.1.3.1 Production of Lytic Enzymes That Can Lyse Fungal Cell Wall
The hydrolytic enzymes have received considerable attention because they play a role in controlling diseases by excreting cell wall hydrolases (Chernin and Chet 2002). Testing for production of hydrolases and antibiotics helps in the characterization of PGPR and thus deploys them in a systematic way.
Chitin and β-glucan are the main components of fungal cell wall of filamentous fungi. Chitin is a linear polysaccharide composed of β-1, 4-N-acetylglucosamine units and is found in nature as α- and β-chitin, whereas laminarin is a polymer of D-glucose in β-1, 3 configurations arranged in helical coils, from which minor polymers of β-1, 4 D-glucose branch. Fungal cell walls contain more than 60 % of laminarin which is hydrolyzed mainly by β-1, 3 glucanases (Cohen-Kupiec et al. 1999). Chitinases and glucanases have many roles in a wide range of different biological systems. These enzymes are usually extracellular, and they may be produced in multiple forms that differ in charge, size, regulation, stability, and ability to degrade cell walls (Koga et al. 1999). In in vitro trials, chitinases are inducible enzymes secreted only in the presence of chitin; hence, colloidal chitin was used as sole carbon source in the production medium. In the same context, glucanases are inducible enzymes secreted in the presence of cellulose. The fungal wall components such as chitin, β-1, 3-glucan, mannan, cellulose, and proteins may induce the lytic enzymes, thus showing antagonistic activities (Adams 2004).
Chitinolytic enzymes have been considered important in the biological control of plant pathogens because of their ability to degrade fungal cell walls (Hoster et al. 2005). Chitinases producing microorganisms have been reported as biocontrol agents for different kinds of fungal diseases of plants. There are effective tools for complete degradation of mycelia and conidial walls of phytopathogenic fungi (Kobayashi et al. 2002). Several rhizobacteria, including genera of Bacillus and Pseudomonas, are known to produce a battery of hydrolases such as chitinase and glucanase, which help in the maceration of cell walls of those plant pathogens (Lim et al. 1991; Singh et al. 1999; Huang et al. 2004; Bogas et al. 2007; Aktuganov et al. 2007). Singh et al. (2013) reported that chitinase-producing strain Lysinibacillus fusiformis B-CM18, isolated from chickpea rhizosphere, exhibited in vitro antifungal activity against a wide range of fungal plant pathogens, among them F. oxysporum f. sp. ciceris. This strain B-CM18 was also found to produce several PGPR activities that make these rhizobacteria an ideal candidate for biological control of chickpea pathogens. Patil et al. (2015) reported that two rhizobacterial strains isolated from chickpea, Paenibacillus polymyxa CTS-B19 and Bacillus subtilis CTS-G24, produced chitinase, and β-1, 3-glucanase may act synergistically in degrading fungal cell wall thus achieving biocontrol of pathogenic fungi F. oxysporum f. sp. ciceris.
10.4.1.3.2 Production of Antibiotics
One of the effective means of control of soilborne pathogens in a natural ecosystem is by means of production of antibiotics (Raaijmakers and Weller 1998). Production of antibiotics has been described as the potent mode of action in disease suppression by which development and/or activity of the pathogen is believed to be directly inhibited. The antibiotics produced in vitro were generally assumed to be the compounds responsible for biocontrol in vivo (Leifert et al. 1995). The most common antibiotics produced by Pseudomonas are phenazines, 2,4-diacetylphloroglucinol (DAPG), pyrrolnitrin (Prn), pyoluteorin (Plt), and others (Raaijmakers et al. 2002; Mavrodi et al. 2010). Handelsman and Stabb (1996) reported that a significant quantitative relationship existed between the disease suppression and the antibiotic production by the bacilli species. The beneficial rhizobacterium Bacillus subtilis is one of the best biocontrol agents that produced lipopeptides, viz., fengycin, iturin, and surfactin, which displayed multifaceted biocontrol activity against plant pathogens (Ongena and Jacques 2008). The antifungal activity of plant growth-promoting rhizobacterium B. amyloliquefaciens FZB42 has been attributed mainly to bacillomycin D production, and this has been shown to suppress the plant pathogenic fungus F. oxysporum (Koumoutsi et al. 2004). B. cereus strain UW85 is known to produce both zwittermicin (Silo-Suh et al. 1994) and kanosamine (Milner et al. 1996). Mycosubtilin is another variant of the iturin family and is produced by strains of B. subtilis (Leclere et al. 2005). Overproduction of mycosubtilin by a recombinant B. subtilis strain BBG100 has been found to show significant antagonistic properties against various fungal pathogens including F. oxysporum (Leclere et al. 2005).
10.4.1.3.3 Production of Antifungal Metabolites Such as HCN
PGPR produces a wide range of low-molecular-weight metabolites with antifungal activity wherein hydrocyanic acid (HCN) plays an important role that inhibits the electron transport and the energy supply to the cell leading to death of the organisms; it inhibits the proper functioning of enzymes and natural receptor’s reversible mechanism of inhibition, and it is also known to inhibit the action of cytochrome oxidase (Dowling and O’Gara 1994). HCN is produced by many rhizobacteria which have antifungal properties and is postulated to play a role in biological control of pathogens. In in vitro trials, production of HCN is detected qualitatively using nutrient agar medium amended with 4.4 g glycine L1 (Lorck 2004). A Whatman filter paper no. 1 soaked in 2 % sodium carbonate solution and 0.5 % picric acid solution was placed on the top of the plates. Plates were sealed with Parafilm. Upon incubation of the rhizobacteria on the solid plates, color changes from yellow to pink/red color that indicated HCN production. Toyoda and Utsumi (1991) reported that P. solanacearum were able to produce HCN and hydrolyze the compound, fusaric acid. Fusaric acid is the causative agent of the damage to plant that occurs upon Fusarium infection. As a consequence of the ability to hydrolyze fusaric acid, the bacterial strains can prevent the damage that is caused by various species of the fungus Fusarium (Toyoda and Utsumi 1991).
10.4.1.3.4 Phosphate-Solubilizing PGPR
The use of rock phosphate as a phosphate fertilizer with its solubilization by microbes (Kang et al. 2002), through the production of organic acids (Maliha et al. 2004), has become a valid alternative to chemical fertilizers. Several studies have shown that phosphate-solubilizing microorganisms solubilize the fixed P in the soil resulting in higher crop yields (Gull et al. 2004). Most predominant phosphorus-solubilizing bacteria (PSB) belong to the genera Bacillus and Pseudomonas (Richardson 2001). Rhizobacteria solubilizing the phosphate can be isolated using serial dilutions or enrichment culture techniques on/in Pikovskaya medium supplemented with bromophenol (Pikovskaya 1948) from rhizosphere soils. Upon incubation of the organisms on the solid plates containing insoluble phosphate, phosphate-solubilizing PGPRs are detected by the formation of clear halos around their colonies. Finally, the selected efficient phosphate-solubilizing cultures are used for making the inoculants, and their performance under pot or field conditions is tested against various crops such as chickpea. Wani et al. (2007) showed that multiple inoculation with Mesorhizobium ciceri and phosphate-solubilizing rhizobacteria increased the nodule number and biomass per plant. Similar results were obtained by Rokhzadi and Toashih (2011) which showed that inoculation treatments contain Azospirillum and Azotobacter strains in their combinations, suggesting that Azospirillum and Azotobacter jointly may have a role in promoting phosphorus uptake by chickpea. Similarly, PGPRs have been shown to solubilize precipitated phosphates and enhance phosphate availability to chickpea that represent a possible mechanism of plant growth promotion under field conditions (Verma et al. 2001, 2010). The use of PGPRs as inoculant biofertilizers is an efficient approach to replace chemical fertilizers and pesticides for sustainable chickpea cultivation.
10.4.1.3.5 Plant Growth Regulator Production Such as IAA (Indole-3-Acetic Acid)
The ability of bacteria to produce IAA in the rhizosphere depends on the availability of precursors and uptake of microbial IAA by plant (Glick 1995). Plant growth regulators participate in the growth and development of cells, tissues, organs, and in fact the entire plant. These compounds are active in plants in very minute amounts and their synthesis is extremely regulated. Plants not only produce phytohormones but also numerous plant-associated bacteria that are both beneficial, and they produce one or more of these substances (Dobbelaere et al. 2003). Phytohormones that are produced by plant-associated bacteria, including indole-3-acetic acid (IAA), cytokinins, and gibberellins, can frequently stimulate germination, growth, and reproduction and protect plants against both biotic and abiotic stress (Taghavi et al. 2009). As the most studied phytohormones, IAA produced in the plant shoot and transported basipetally to the root tips associated with cell elongation and cell division (Rashotte et al. 2000) contributes to plant growth and plant defense system development (Navarro et al. 2006). In general, root elongation changes qualitatively based on the IAA level; therefore, the amount of released IAA could have an important role in modulating the plant–microbe interaction. Many rhizosphere bacteria produce IAA in culture media especially in the presence of tryptophan (Yadav et al. 2010; Patil et al. 2015). Yadav et al. (2010) reported that the bacterial strain Pseudomonas putida BHUPSB04 showed maximum significant concentration of IAA 25.65 μg ml−1 followed by Pseudomonas aeruginosa BHUPSB02 (21.35 μg ml−1), Bacillus subtilis BHUPSB13 (16.23 μg ml−1), Paenibacillus polymyxa BHUPSB17 (15.79 μg ml−1), and Bacillus boroniphilus BHUPSB19 (11 μg ml−1). In a similar study, the PGPR isolates significantly affected the length of chickpea seedlings. Results reveal that the shoot length increased in PGPR-treated plants over uninoculated control. The highest shoot length 15.6 cm plant−1 was recorded in treatment of P. putida BHUPSB04 isolate followed by statistically at par values due to isolates P. aeruginosa BHUPSB02 (14.5 cm plant−1). B. subtilis BHUPSB13, P. polymyxa BHUPSB17, and B. boroniphilus BHUPSB19 which showed significantly higher shoot length over control (Yadav et al. 2010).
10.4.1.3.6 Induction of Systemic Resistance
When physical contact of the pathogen and the protecting microorganism is required, the process is known as biocontrol (Bloemberg and Lugtenberg 2001; Compant et al. 2005). As already mentioned, the existence of microorganisms capable of preventing diseases in plants without the plants’ participation is known. This occurs by systems such as niche exclusion or pathogen-inhibiting substance production. Apart from the direct action against plant pathogens, many PGPRs induce resistance in the plant system by signaling host defense mechanisms. The plant and bacterial interactions in the rhizosphere are important for plant health and resistance to disease. PGPRs are known to rapidly colonize the rhizosphere and enhance plant resistance, which is termed induced systemic resistance (ISR), while pathogen-induced resistance is called systemic acquired resistance (SAR) (Hammerschmidt 1999). Recently, several studies have reported the importance of strains of PGPR in enhancing plant resistance (Kloepper 1993; Martin and Loper 1998; Silva et al. 2004; Moradi et al. 2012; Altinok et al. 2013).
They are both related with the induction of pathogenesis-related (PR) proteins. Moradi et al. (2012) showed an increase in the induction of resistance to Fusarium wilt in chickpea by B. subtilis. They also demonstrated that PGPR resulted in the accumulation of PR proteins via increased synthesis of chitinase and β-1, 3-glucanase. In this case, Raju et al. (2008) claimed that induction of proteins and accumulation of phenolics might have contributed to restrict the invasion of FOC, in resistant cultivar ICCV10. Their investigation showed that Hashem cultivar contained higher levels of soluble protein content and β-1, 3-glucanase activity than Pirooz cultivar after inoculation with a biocontrol agent such as Bacillus subtilis which is apparently associated with the establishment of a higher level of resistance to Fusarium wilt of chickpea (Moradi et al. 2012).
Jiang et al. (2015) demonstrated that in the interactions with invading pathogens, plants frequently activate defense-related genes that lead to the expression of pathogenesis-related (PR) proteins. Among the studied PGPRs are some Rhizobium spp. which have been shown to induce a defense response in chickpea infected with FOC. Arfaoui et al. (2005) suggested that treatment of germinated seeds with Rhizobium induced the expression of compounds involved in plant defense such as peroxidases and polyphenol oxidases and increased levels of phenolic compounds. It has been reported that volatile organic compounds may play a key role in the induced systemic resistance. In this case, volatiles secreted by B. subtilis GBO3 were able to activate an ISR pathway in Arabidopsis seedlings challenged with the soft-rot pathogen Erwinia carotovora subsp. carotovora (Compant et al. 2005), and the same isolate was found to suppress Fusarium oxysporum f. sp. ciceris (Hervas et al. 1998).
Various studies reported the importance of the phytoalexins medicarpin and maackiain in the overall defense response of chickpea (Stevenson et al. 1997). Peroxidases and hydrolases, particularly chitinases and glucanases, also play a major role in the defense mechanisms of this plant. PGPRs also induce ISR by triggering jasmonic acid (JA) and ethylene synthesis (Pieterse et al. 1998). ISR is dependent on colonization of the root system by sufficient numbers of PGPR, and this has been achieved by coating seed with high numbers of bacteria or by adding bacterial suspensions to soil before sowing or at transplanting (Kloepper 1996).
10.4.1.4 Greenhouse and Field Testing
Root colonization is a necessary requirement for the bacteria to exert its effect (Germida and Walley 1996). Unfortunately, the PGPR inoculation in distinct plant species sometimes produces erratic results. The good results obtained in vitro cannot always be dependably reproduced under field conditions. The variability in the performance of PGPR may be due to various environmental factors that may affect their growth and exert their effects on plant (Joseph et al. 2007). Further, after the screening process, the PGPR potential shown in vitro should be tested to ensure that the same effect occurs in the plant and so the evaluation of the isolates exhibiting multiple plant growth-promoting traits on the soil–plant system is needed to uncover their efficacy as effective PGPR. Inoculant bacteria are often applied to seeds or root of the plant for rapid colonization. After sowing, the inoculant bacteria must be able to establish in the rhizosphere at population densities sufficient to produce a beneficial effect. Therefore, efficient inoculant bacteria should survive in the rhizosphere, make use of nutrients exuded by the plant root, be able to efficiently colonize the entire root system, and compete with indigenous microorganisms. After being implicit in the colonization process, these rhizobacteria have the ability to survive on seeds, can multiply in spermosphere in response to seed exudates, and can attach to the surface of the root system and colonize. The use of inoculation with a beneficial, biological control organism that will colonize the rhizosphere shows some promise as a means to suppress plant disease (Cook 1993).
The first successful application and commercial production of PGPR is by a B. subtilis strain A13. B. subtilis A13 was isolated more than 25 years ago in Australia based on in vitro inhibitory activity to all of nine pathogens tested and was subsequently shown to promote plant growth. Since 1990, Bacillus spp. have been developed as fungal disease control agents in the form of a commercial product, namely, Serenade, EcoGuard, Kodiak, Yield Shield, and Bio Yield (Idris et al. 2008). Pseudomonas and Bacillus strains have great potential in control of Fusarium wilt disease of chickpea (Hervas et al. 1997; Landa et al. 1997a, b; Anjajah et al. 2003; Inam-ul-Haq et al. 2003). Some species of Bacillus were isolated from the rhizosphere of chickpea and demonstrated to inhibit conidial germination and hyphal growth of F. oxysporum f. sp. ciceris (Landa et al. 1997b) and suppress Fusarium wilt development (Landa et al. 1997a).
In our study, the test in pots showed that the susceptible cultivar ILC 482 reacts to FOC1 with a high incidence of Fusarium wilt. Nevertheless, 6 weeks after sowing, there was 100 % more disease on wilted plants. However, bacterized seeds with five rhizobacteria Rb29, Rb6, Rb12, Rb4, and Rb15 isolated from rhizosphere soils of healthy chickpea plants significantly reduced the percentage of wilted plants, from 99 to 60 % (Zaim et al. 2013). Karimi et al. (2012) used six isolates of Pseudomonas and six isolates of Bacillus genera that were tested for biocontrol of Fusarium wilt and promotion of chickpea growth. In the same study, the isolates of P. aeruginosa and B. subtilis protected chickpea against Fusarium wilt with 15.8–44.8 % in seed treatment and soil inoculation. Therefore, growth parameters (plant height, fresh and dry weight of plants) were significantly increased. The influence of PGPR on chickpea yield under field conditions has been thoroughly studied. Studies have shown that a combined inoculation of Azospirillum spp., A. chroococcum 5, Mesorhizobium ciceri SWR17, and P. fluorescens P21 improved nodulation and increased dry matter accumulation in roots and shoots, grain yields, biomass, and protein yield of chickpea by a significant margin. This can be attributed to the cumulative effects of an enhanced supply of nutrients, mainly nitrogen and phosphorus, and the production of growth-promoting substances (Rokhzadi et al. 2008).
10.5 Conclusion and Perspective
The rhizosphere is a highly dynamic system with a vast number of fungi and bacteria interacting simultaneously; the difficulty of excluding endemic PGPR may preclude clear conclusions from inoculation experiments in the field. In order to increase our understanding of the role of various root-associated organisms as PGPRs in plant growth and health as well as make use of their potential beneficial features in plant production, more information is urgently needed on the interactions among plants and rhizosphere microorganisms. Selection of biocontrol agents for controlling diseases such as Fusarium wilt of chickpea has emphasized the use of individual agents. However, it would seem logical that increasing the number of biological control agents as a mixture may result in treatments that could persist longer in the rhizosphere, provide a wider array of biocontrol mechanisms, and/or function under a broader range of environmental conditions, especially if these mixtures were of different species. The ability of rhizobacterial mechanisms to suppress F. oxysporum f. sp. ciceris could be of significant agronomic importance. These mechanisms have essential functions in the microbial antagonism, on the one hand, but also are able to elicit induced resistance, on the other hand. Resistance-inducing and antagonistic rhizobacteria might be useful in formulating new inoculants, offering an attractive alternative of environmentally friendly biological control of Fusarium wilt of chickpea and improving the cropping systems into which it can be most profitably applied.
References
Adams DJ (2004) Fungal cell wall chitinases and glucanases. Microbiology 150:2029–2035
Aktuganov GE, Galimzyanova NF, Melent’ev AI, Kuz’mina LY (2007) Extracellular hydrolases of strain Bacillus sp.739 and their involvement in the lysis of micromycete cell walls. Mikrobiologiya 76(4):471–479
Altinok HH, Dikilitas M, Yildiz HN (2013) Potential of Pseudomonas and Bacillus isolates as biocontrol agents against Fusarium wilt of eggplant. Biotechnol Biotechnol Equip 27(4):3952–3958
Anjajah V, Cornelis P, Koedam N (2003) Effect of genotype and root colonization in biological control of Fusarium wilt in pigeonpea and chickpea by Pseudomonas aeruginosa PNA1. Can J Microbiol 49:85–91
Antoun H, Kloepper JW (2001) Plant growth promoting rhizobacteria. In: Brenner S, Miller JH (eds) Encyclopedia of genetics. Academic, New York, pp 1477–1480
Arfaoui A, Sifi B, El Hassni M, El Hadrami I, Boudabous A, Chérif M (2005) Biochemical analysis of chickpea protection against Fusarium wilt afforded by two Rhizobium isolates. Plant Pathol J 4(1):35–42
Baayen RP, ODonnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebroeck EJA, Waalwijk C (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing root rot and wilt diseases. Phytopathology 90:891–899
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bogas AC, Watanabe MAE, Barbosa A, Vilas-Boas LA, Bonatto AC, Dekker R, Souza EM, Fungaro MHP (2007) Structural characterization of the bglH gene encoding a beta-glucosidase-like enzyme in an endophytic Bacillus pumilus strain. Genet Mol Biol 30(1):100–104
Booth C (1971) The genus Fusarium. Kew (surrey): common Wealth Mycologica Institute, England. p 137
Chehri K, Abbasi S, Reddy KRN, Salleh B (2010) Occurrence and pathogenicity of various pathogenic fungi on cucurbits from Kermanshah province, Iran. Afr J Microbiol Res 4:1215–1222
Chernin L, Chet I (2002) Microbial enzymes in biocontrol of plant pathogens and pests. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology, and applications. Marcel Dekker, New York, pp 171–225
Cohen-Kupiec R, Broglie K, Friesem D, Broglie R, Chet I (1999) Molecular characterisation of a novel β-1,3-exoglucanase related to mycoparasitism of Trichoderma harzianum. Gene 226:147–154
Compant S, Duffy B, Nowak J, Clement C, Ait Barka E (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Cook RJ (1993) Making greater use of introduced microorganisms for biological control of plant pathogens. Annu Rev Phytopathol 31:53–80
Demers JE, Garzon CD, Jiménez-Gasco MM (2014) Striking genetic similarity between races of Fusarium oxysporum f. sp. ciceris confirms a monophyletic origin and clonal evolution of the chickpea vascular wilt pathogen. Eur J Plant Pathol 139:303–318
Dennis C, Webster J (1971) Antagonistic properties of species group of Trichoderma, production of volatile antibiotics. Trans Br Mycol Soc 157:25–39
Dileep Kumar BS (1999) Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chickpea growing in soils infested with Fusarium oxysporum f. sp. ciceris. Biol Fertil Soils 29(1):87–91
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. CRC Crit Rev Plant Sci 22:107–149
Dowling DN, O’Gara F (1994) Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol 12:133–141
Germida JJ, Walley FL (1996) Plant growth-promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of field-grown spring wheat. Biol Fertil Soils 23(2):113–120
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR, Patten CL, Holgin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London, p 267
Gull FY, Hafeez I, Saleem M, Malik KA (2004) Phosphorus uptake and growth promotion of chickpea by co-inoculation of mineral phosphate solubilizing bacteria and a mixed rhizobial culture. Aust J Exp Agric 44:623–628
Gupta O (1991) Symptomless carriers of chickpea vascular wilt pathogen (Fusarium oxysporum f. sp. ciceris). Legum Res 14:193–194
Gupta S, Chakraborti D, Rangi RK, Basu D, Das S (2009) A molecular insight into the early events of chickpea (Cicer arietinum) and Fusarium oxysporum f. sp. ciceri (race 1) interaction through cDNAAFLP analysis. Phytopathology 99:1245–1257
Halila MH, Strange RN (1996) Identification of the causal agent of wilt of chickpea in Tunisia as F. oxysporum f. sp. ciceris race 0. Phytopathol Mediterr 35:67–74
Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55:77–84
Handelsman J, Stabb EV (1996) Biocontrol of soilborne plant pathogens. Plant Cell 8:1855–1869
Haware MP (1990) Fusarium wilt and other important diseases of chickpea in the Mediterranean area. Options Mediterr Ser Semin 9:163–166
Haware MP, Nene YL (1982) Symptomless carriers of the chickpea wilt Fusarium. Plant Dis 66:809–810
Haware MP, Nene YL, Rajeswari R (1978) Eradication of Fusarium oxysporum f. sp. ciceris transmitted in chickpea seed. Phytopathology 68:1364–1368
Hervas A, Landa BB, Jiménez-Díaz RM (1997) Influence of chickpea genotype and Bacillus sp. on protection from Fusarium wilt by seed treatment with nonpathogenic Fusarium oxysporum. Eur J Plant Pathol 103:631–642
Hervas A, Landa B, Datnoff LE, Jiménez-Diaz RM (1998) Effects of commercial and indigenous microorganisms on Fusarium wilt development in chickpea. Biol Control 13:166–176
Hoster F, Schmitz JE, Daniel R (2005) Enrichment of chitinolytic microorganisms isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel streptomyces strain. Appl Microbiol Biotechnol 66:434–442
Huang CJ, Wang TK, Chung SC, Chen CY (2004) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28–9. J Biochem Mol Biol 38(1):82–88
Idris HA, Labuschagne N, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106
Idris HA, Labuschagne N, Korsten L (2008) Suppression of Pythium ultimum root rot of sorghum by rhizobacterial isolates from Ethiopia and South Africa. Biol Control 45(1):72–84
Inam-ul-Haq M, Javed N, Ahmad R, Rehman A (2003) Evaluation of different strains of Pseudomonas fluorescens for the biocontrol of fusarium wilt of chickpea. Plant Pathol J 2:65–74
Jalali BL, Chand H (1992) Chickpea wilt. In: Singh US, Mukhopadhayay AN, Kumar J, Chaube HS (eds) Plant diseases of international importance, vol I, Diseases of cereals and pulses. Prentice Hall, Englewood Cliffs, pp 429–444
Jamali F, Sharifi-Tehrani A, Okhovvat M, Zakeri Z, Saberi-Riseh R (2004) Biological control of chickpea Fusarium wilt by antagonistic bacteria under greenhouse condition. Commun Agric Appl Biol Sci 69(4):649–651
Jiang L, Wu J, Fan S, Li W, Dong L, Cheng Q, Xu P, Zhang S (2015) Isolation and characterization of a novel pathogenesis-related protein gene (GmPRP) with induced expression in soybean (Glycine max) during infection with Phytophthora sojae. PLoS ONE 10(6):e0129932
Jiménez-Díaz RM, Alcala-Jiménez AR, Hervas A, Trapero-Casas JL (1993) Pathogenic variability and hosts resistance in the Fusarium oxysporum f. sp. ciceris/Cicer arietinum pathosystem. In: Proceedings of the 3rd European seminar Fusarium Mycotoxins, taxonomy, pathogenicity and host resistance. Hodowsla Roslin Aklimatyazacja i Nasiennictwo. Plant Breeding and Acclimatization Institute, Radzikov, Poland, pp 87–94
Jiménez-Díaz RM, Castillo P, Jiménez-Gasco MDM, Landa B, Navas-Cortés JA (2015) Fusarium wilt of chickpeas: biology, ecology and management, crop protection. http://dx.doi.org/10.1016/j.cropro.2015.02.023
Jiménez-Gasco MM, Jiménez-Diaz RM (2003) Development of a specific polymerase chain reaction-based assay for the identification of Fusarium oxysporum f. sp. ciceris and its pathogenic races 0, 1A, 5, and 6. Am Phytopathol Soc 93(2):200–209
Jiménez-Gasco MM, Pérez-Artés E, Jiménez-Díaz RM (2001) Identification of pathogenic races 0, 1B/C, 5, and 6 of Fusarium oxysporum f. sp. ciceris with random amplified polymorphic DNA (RAPD). Eur J Plant Pathol 107:237–248
Jiménez-Gasco MM, Milgroom MG, Jiménez-Díaz RM (2002) Gene genealogies support Fusarium oxysporum f. sp. ciceris as a monophyletic group. Plant Pathol 51:72–77
Johri BN, Sharma A, Virdi JS (2003) Rhizobacterial diversity in India and its influence on soil and plant health. Adv Biochem Eng/Biotechnol 84:49–89
Jorge I, Rosa O, Navas-Cortes JA, Jimenez-Díaz RM, Tena M (2005) Extracellular xylanases from two pathogenic races of F. oxysporum f. sp. ciceris: enzyme production in culture and purification and characterization of a major isoform as an alkaline endo-β-(1,4)-xylanase of low molecular weight. Antonie Van Leeuwenhoek 88(1):48
Joseph B, Patra RR, Lawrence R (2007) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L). Int J Plant Protect 1(2):141–152
Kaiser WJ, Alcala-Jiménez AR, Hervas-Vargas A, Trapero-Casas JL, Jiménez-Díaz RM (1994) Screening of wild Cicer species for resistance to races 0 and 5 of Fusarium oxysporum f. sp. ciceris. Plant Dis 78:962–967
Kandoliya UK, Vakharia DN (2013) Antagonistic effect of Pseudomonas fluorescens against Fusarium oxysporum f.sp. ciceri causing wilt in chickpea. Legum Res 36(6):569–575
Kang SC, Ha CG, Lee TG, Maheshwari DK (2002) Solubilization of insoluble inorganic phosphates by a soil fungus Fomitopsis sp. PS 102. Curr Sci 82:439–442
Karimi K, Amini J, Harighi B, Bahramnejad B (2012) Evaluation of biocontrol potential of Pseudomonas and Bacillus spp. against Fusarium wilt of chickpea. Aust J Crop Sci 6(4):695
Kelly AG, Alcalà-Jiménez AR, Bainbridge BW, Heale JB, Pérez-Artés E, Jiménez-Díaz RM (1994) Use of genetic fingerprinting and random amplified polymorphic DNA to characterize pathotypes of Fusarium oxysporum f. sp. ciceris infecting chickpea. Phytopathology 84:1293–1298
Khanzada KA, Rajput MA, Shah GS, Lodhi AM, Mehbob F (2002) Effect of seed dressing fungicides for the control of seed borne mycoflora of wheat. Asian J Plant Sci 1(4):441–444
Kistler HC (2001) Evolution of host specificity in Fusarium oxysporum. In: Summerell BA, Leslie JF, Beckhouse D, Bryden WL, Burgess LW (eds) Fusarium. Paul E. Nelson Memorial Symposium. APS Press, St Paul, pp 70–82
Kloepper JW (1993) Plant growth-promoting rhizobacteria as biological control agents. In: Metting B (ed) Soil microbial technologies. Marcel Dekker, New York, pp 255–274
Kloepper JW (1996) Host specificity in microbe-microbe interactions. BioScience 46:406–409
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–44
Kloepper JW, Rodrıguez-K’abana R, Zehnder GW, Murphy JF, Sikora E, Fernandez C (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australias Plant Pathol 28:21–26
Kobayashi DY, Reedy RM, Bick JA, Oudemans PV (2002) Characterization of a chitinase gene from Stenotrophomonas maltophilia strain 34S1 and its involvement in biological control. Appl Environ Microbiol 68:1047–1054
Koga D, Mitsutomi M, Kono M, Matsumiya M (1999) Biochemistry of chitinases. In: Jollès P, Muzzarelli RAA (eds) Chitin and chitinases. Birkhäuser Verlag, Berlin, pp 111–123
Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P et al (2004) Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol 186:1084–1096. doi:10.1128/JB.186.4.1084-1096
Landa BB, Hervas A, Bethiol W, Jiménez-Diaz RM (1997a) Antagonistic activity of bacteria from the chickpea rhizosphere against Fusarium oxysporum f. sp. ciceris. Phytoparasitica 25(4):305–318
Landa BB, Hervas A, Jimenez-Diaz RM (1997a) Effect of Bacillus spp. cell-free culture filtrates and of different bacterial delivery system against Fusarium oxysporum f. sp. ciceris. In: Anonymous (ed) Proceedings 10th congress of the Mediterranean Phytopathological Union, 1–5 June, Montpellier-Le Corum, Societe Francaise de Phytopathologie, ORSTOM, France, pp 723–726
Leclere V, Bechet M, Adam A, Guez JS, Wathelet B, Ongena M et al (2005) Mycosubtilin overproduction by bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71(8):4577–4584
Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HAS, Harbour A (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78:97–108
Lim HS, Kim YS, Kim SD (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol 57:510–516
Liu W, Mu W, Zhu B, Liu F (2008) Antifungal activities and components of VOCs produced by Bacillus subtilis G8. Curr Res Bacteriol 1:28–34
Lorck H (2004) Production of hydrocyanic acid by bacteria. Plant Physiol 1:142–146
Maheshwari DK (ed) (2010) Plant growth and health promoting bacteria, microbiology monographs. Springer, Berlin, p 18. doi:10.1007/978-3-642-13612-2_2
Maliha R, Samina K, Najma A, Sadia A, Farooq L (2004) Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro conditions. Pak J Biol Sci 7:187–196
Martin FN, Loper JE (1998) Soil-borne plant diseases caused by Pythium spp.: ecology, epidemiology and prospects for biological control. Crit Rev Plant Sci 18:111–191
Mavrodi DV, Peever TL, Mavrodi OV, Parejko JA, Raaijmakers JM, Lemanceau P, Mazurier S, Heide L, Blankenfeldt W, Weller DM, Thomashow LS (2010) Diversity and evolution of the phenazine biosynthesis pathway. Appl Environ Microbiol 76:866–879
McKerral A (1923) A note on Fusarium wilt of gram in Burma and measures taken to combat it. Indian Agric J 28:608–613
McRae W (1932) Report on Imperial Mycologists Sci Agric Res Inst Pusa: 31–78
Milner JL, Silo-Suh L, Lee JC, He H, Clardy J, Handelsman J (1996) Production of kanosamine by Bacillus cereus UW85. Appl Environ Microbiol 62(8):3061–3065
Moradi H, Bahramnejad B, Amini J, Siosemardeh A, Haji-Allahverdipoor K (2012) Suppression of chickpea (Cicer arietinum L.) Fusarium wilt by Bacillus subtillis and Trichoderma harzianum. Plant Omics J 5(2):68–74
Narasimhan R (1929) A preliminary note on Fusarium parasite on Bengal gram (C. arietinum L.). Madras Agric Dept Year Book, p 5
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M et al (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Navas-Cortés JA, Alcala-Jiménez AR, Hau B, Jiménez-Díaz RM (2000) Influence of inoculum density of races 0 and 5 of Fusarium oxysporum f. sp. ciceris on development of Fusarium wilt in chickpea cultivars. Eur J Plant Pathol 106:135–146
Nene YL, Reddy MV (1987) Chickpea diseases and their control. In: Saxena MC, Singh KB (eds) The chickpea. CAB International, Wallingford, pp 233–270
Nene YL, Haware MP, Reddy MV (1981) Chickpea diseases: resistance screening techniques, Information bulletin no. 10. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, p 10
Nene YL, Sheila VK, Sharma SB (1989) A world list of chickpea and pigeon pea pathogens. ICRISAT Legume Pathol Prog Rep, Patancheru, p 7
Nene YL, Reddy MV, Haware MP, Ghanekar AM, Amin KS (1991) Field diagnosis of chickpea diseases and their control. In: Information bulletin no. 28. ed. by International Crops Research Institute for the Semi Arid Tropics, Patancheru
Nene YL, Sheila VK, Sharma SB (1996) A world list of chickpea and pigeonpea pathogens, 5th edn. ICRISAT, Patancheru, p 27
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A 95:2044–2049
Okigbo RN (2004) A review of biological control methods for post harvest yams Dioscorea spp. in storage in south eastern Nigeria. KMITL Sci J 4(1):207–215
Ongena M, Jacques P (2008) Lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Patil S, Shivannavar CT, Bheemaraddi MC, Gaddad SM (2015) Antiphytopathogenic and plant growth promoting attributes of Bacillus strains isolated from rhizospheric soil of chickpea. J Agric Sci Tech 17:1365–1377
Pieterse CMC, van Wees SCM, van Pelt JA, Knoester M et al (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10:1571–1580
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with vital capacity of source microbial species. Microbiologiya 17:362–370
Prasad N, Padwick GW (1939) The genus Fusarium 11. A species of Fusarium as a cause of wilt of gram (C. arietinum L.). Indian Agric Sci 9:731
Raaijmakers JM, Weller DM (1998) Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol Plant Microbe Interact 11:144–152
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Anton van Leeuwenhoek 81:537–547
Raju S, Jayalakshmi SK, Sreeramulu K (2008) Comparative study on the induction of defense related enzymes in two different cultivars of chickpea (Cicer arietinum L.) genotypes by salicylic acid, spermine and Fusarium oxysporum f sp ciceri. Aust J Crop Sci 2:121–140
Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122:481–490
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Rokhzadi A, Toashih V (2011) Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plant growth promoting rhizobacteria. Aust J Crop Sci 5(1):44–48
Rokhzadi A, Asgazadeh A, Darvish F, Nour-Mohammed G, Majidi E (2008) Influence of plant growth promoting rhizobacteria on dry matter accumulation and yield of chickpea (Cicer arietinum L.) under field conditions. Am Eurasian J Agric Environ Sci 3:253–257
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. doi:10.1104/pp.103.026583
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med 21:1–30
Santos FMA, Ramos B, Sanchez MAG, Eslava AP, Minguez JMD (2002) A DNA based procedure for in planta detection of Fusarium oxysporum f. sp. phaseoli. Phytopathol 92:237–244
Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microbiol 60(6):2023–2030
Silva HAS, Romeiro RDS, Macagnan D, Halfeld-Vieira BDA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Singh KB, Dahiya BS (1973) Breeding for wilt resistance in chickpea. In: Symposium on wilt problem and breeding for wilt resistance in Bengal Gram. Indian Research Institute, New Delhi, pp 13–14
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilts of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Singh RK, Kumar DP, Solanki MK, Singh P, Srivastva AK, Kumar S, Kashyap PL, Saxena AK, Singhal PK, Arora DK (2013) Optimization of media components for chitinase production by chickpea rhizosphere associated Lysinibacillus fusiformis B-CM18. J Basic Microbiol 53:451–460
Smitha KP, Mohan R, Devadason A, Raguchander T (2015) Exploiting novel rhizosphere Bacillus species to suppress the root rot and wilt pathogens of chickpea. Afr J Microbiol Res 9(15):1098–1104. doi:10.5897/AJMR2015.7445
Ramos Solano BR, Barriuso J, Gutiérrez Mañero FJ (2008) Physiological and molecular mechanisms of Plant Growth Promoting Rhizobacteria (PGPR). In: Iqbal A, John P, Shamsul H (eds) Plant-bacteria interactions. Strategies and techniques to promote plant growth, pp 41–54
Stevenson PC, Turner HC, Haware MP (1997) Phytoalexin accumulation in the roots of chickpea (Cicer arietinum L.) seedlings associated with resistance to Fusarium wilt (Fusarium oxysporum f. sp. ciceris). Physiol Mol Plant Pathol 50:167–178
Swain MR, Ray RC (2007) Biocontrol and other beneficial activities of Bacillus subtilis isolated from cow dung microflora. Microbiol Res 164(2):121–130
Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N et al (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar. Appl Environ Microbiol 75:748–757
Toyoda H, Utsumi R (1991) Method for the prevention of Fusarium diseases and microorganisms used for the same. U.S. Patent #4,988,586
Trapero-Casas A, Jiménez-Díaz RM (1985) Fungal wilt and root rot diseases of chickpea in southern Spain. Phytopathology 75:1146–1151
Upadhyaya HD, Dwivedi SL, Baum M, Varshney RK, Udupa SM, Gowda CLL, Hoisington D, Singh S (2008) Genetic structure, diversity, and allelic richness in composite collection and reference set in chickpea (Cicer arietinum L.). BMC Plant Biol 8:106
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization, ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Verma JP, Yadav J, Tiwari KN (2010) Application of Rhizobium sp. BHURC01 and plant growth promoting rhizobacteria on nodulation, plant biomass and yields of chickpea (Cicer arietinum L.). Int J Agric Res 5:148–156
Wani PA, Khan MS, Zaidi A (2007) Synergistic effects of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on the performance of field-grown chickpea. J Plant Nutr Soil Sci 170(2):283–287
Westerlund FV, Campbell RN, Kimble KA (1974) Fungal root rot and wilt of chickpea in California. Phytopathology 64:432–436
Yadav J, Verma JP, Tiwari KN (2010) Effect of plant growth promoting Rhizobacteria on seed germination and plant growth Chickpea (Cicer arietinum L.) under in vitro conditions. Biol Forum Int J 2(2):15–18
Yadav J, Verma JP, Tiwari KN (2011) Plant growth promoting activities of fungi and their effect on chickpea plant growth. Asian J Biol Sci 4(3):291–299
Zaim S, Belabid L, Bellahcene M (2013) Biocontrol of chickpea Fusarium wilt by Bacillus spp. rhizobacteria. J Plant Prot Res 53(2):177–183
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Zaim, S., Belabid, L., Bayaa, B., Bekkar, A.A. (2016). Biological Control of Chickpea Fusarium Wilts Using Rhizobacteria “PGPR”. In: Choudhary, D.K., Varma, A. (eds) Microbial-mediated Induced Systemic Resistance in Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-0388-2_10
Download citation
DOI: https://doi.org/10.1007/978-981-10-0388-2_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-0387-5
Online ISBN: 978-981-10-0388-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)