Abstract
The establishment of predatory mites on protected crops is affected by the availability of shelter and alternative food. We suggest that a pollen covered twine, coined here “pollen on-twine”, may provide these necessities when attached to the plant. We evaluated the effect of twine types and two pollen species on the establishment of Euseius scutalis (Athias-Henriot) and Amblyseius swirskii Athias-Henriot on pre-flowering pepper plants in a growth chamber. For both phytoseiids, rayon jute twine was found more beneficial while corn and oak pollen did not differ in their effect. Though populations of both predators were best promoted when twine and pollen were applied separately, E. scutalis population increased by more than tenfold and A. swirskii doubled when plants were applied with pollen on-twine. We propose that after further refining, pollen on-twine can serve as a feasible solution for predatory mite establishment on protected crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much attention has been given to conservation of generalist predatory mites belonging to the family Phytoseiidae (Acari), as a preventive biological solution to pest outbreaks on protected crops (Nomikou et al. 2010; Ramakers and Voet 1996; Weintraub et al. 2009). Several factors may affect the establishment of these predatory mites, including availability of alternative food (McMurtry and Croft 1997; van Rijn and Sabelis 1990), oviposition sites and shelter (Duso 1992; Kreiter et al. 2002). These factors may be provided by the plant itself: pollen and nectar as alternative food (Wäckers 2005), and leaf hairs and domatia as oviposition sites and shelter (Romero and Benson 2005; Walter 1996). Nevertheless, numerous crops lack at least one of these attributes in all or in some stages of their growth, making it difficult to maintain predatory mites on the crop when prey is scarce.
Conservation (e.g. van Rijn and Sabelis 1990) and efficacy (e.g. McMurtry and Scriven 1966; Tuovinen and Lindqvist 2010; van Rijn et al. 1999; Wäckers et al. 2007) of predatory mites can be improved by the provision of alternative food, particularly pollen. There are several methods of pollen application on protected crops, but none of them are widely used in commercial systems. This is because the application of pollen by dusting or placing the pollen on leaves (e.g. Nomikou et al. 2002; Tuovinen and Lindqvist 2010; van Rijn et al. 2002) and hanging small containers of pollen on the petioles (Nomikou et al. 2010), is labor intensive. While applying pollen with electrostatic pollen applicators was more labor efficient (Gan-Mor et al. 2003; Weintraub et al. 2009), it still required large amounts of pollen to dust the foliage of greenhouse crops. Thus, not surprisingly, all of these methods reported above were used in small scale greenhouse experiments only. Recently the new predatory mite product Dyna-Mite®G-System utilizing the phytoseiid mite Euseius gallicus Kreiter and Tixier (Tixier et al. 2010), coupled with the blower application of the pollen based food Nutrimite™ has been introduced for the control of various pests in roses. As this product is completely new (to be launched in 2014) it is still premature to evaluate its cost effectiveness (Biobest News and press releases 2013).
Establishing a predatory mite population may also be limited by the availability of oviposition sites and shelters. The abundance of phytoseiids is associated with foliage hairiness (Duso 1992; Kreiter et al. 2002; Loughner et al. 2008) that provide shelter from predators (Roda et al. 2000). It is well known that domatia, a cluster of hairs usually on the main vein axils, is utilized by predatory mites as shelter and as oviposition sites as it protects both eggs and motiles and reduces their predation (Faraji et al. 2002; Ferreira et al. 2011; Norton et al. 2001; Walter 1996). Attempts to mimic hairs and to create artificial domatia on the crop were made in previous studies (Roda et al. 2001; Seelmann et al. 2007). Loughner et al. (2011) added cotton fiber patches and chopped acrylic yarn fibers to beans and Impatiens plants. In this study, populations of the predatory mite Amblyseius swirskii Athias-Henriot increased more on plants when both fibers and pollen were provisioned than when either pollen or fibers were provided alone. Combinations of supplemental food, shelter and oviposition sites for the continuous releasing of predatory mites are commercially available. For instance, the open rearing systems called “sachets” are hanged on plants and contain a substrate with a food source, a prey population that feed on the food source, and a predatory mite population that feed on the prey (e.g. Jacobson et al. 2010). Another example is the banker plant system (Frank 2010; Huang et al. 2011; Ramakers and Voet 1996) in which natural enemies commute from plants, for example castor bean, planted or placed within the rows of the crop, providing food and oviposition sites to predatory mites. However, in those systems the conditions on the crop plant itself are unchanged and the predators will leave the plant if conditions are not suitable (Loughner et al. 2010; Ramakers and Voet 1995).
Presently there are no commercial solutions that include pollen application, provision of shelter and oviposition sites for predatory mites on the plants. Here, we examine the hypothesis that a pollen coated twine, coined here “pollen on-twine” (Gan-Mor et al. 2011), will efficiently provide food and oviposition sites and will improve the conditions for predatory mites on the plants, thereby improving predatory mite establishment. We examine the effect of pollen on-twine on two Israeli indigenous phytoseiid species, representatives of two different life style groups (McMurtry and Croft 1997): the commercially available generalist predator A. swirskii and the pollen specialist Euseius scutalis (Athias-Henriot), a predator with plant cell piercing abilities (McMurtry et al. 2013). Both phytoseiid species are known to be effective biocontrol agents of whiteflies (Nomikou et al. 2001), more recently A. swirskii was also reported to control broad mite Polyphagotarsonemus latus (Banks) (van Maanen et al. 2010; Weintraub et al. 2009). Pollen on-twine is assembled from two components, pollen and twine. Twine fibers may affect population increase (Loughner et al. 2011). Size and adhesiveness of pollen grains might influence the attachment of these grains to the substrate and the ability of the mites to feed on it. Accordingly, in this study we evaluated the effect of two twine types, coated with pollen of Zea mays (corn) or Quercus ithaburensis (Tabor oak) on the population of the two phytoseiid species, on young pepper plants prior to flowering, and hence lacking domatia. Then, we assessed the contribution of each of the components (pollen and twine) separately on the establishment of these phytoseiids.
Materials and methods
General methods
All experiments were conducted at the Newe Ya’ar Research Center, Agricultural Research Organization, Israel. Tabor oak male flowers were pruned in the spring at the Newe Ya’ar Research Center and allowed to dry for two days at ambient temperatures. To prevent clumping, pollen was passed through a sieve of 180 μ and stored at −20 °C with anhydrous calcium chloride as a desiccant. Corn pollen was collected from the commercial sweet corn cultivar ‘Royalty’ (Eden Seeds, Israel) grown in the summer months in the Hula Valley, north of Israel and processed and stored as described above for oak pollen.
Euseius scutalis collected from avocado leaves, were reared on potted plants of Solanum nigrum (black nightshade) in a growth chamber maintained at 24 ± 4 °C, 60 ± 13 % RH, 16L:8D. Oak pollen was applied to leaves of potted plants twice a week as a food source. Amblyseius swirskii, originally collected from citrus foliage and reared on Carpoglyphus lactis L. (Acari: Carpoglyphidae), were received from Bio-Bee Biological Systems, Sdeh Eliyahu, Israel.
Young potted pepper plants Capsicum annuum (cultivar Red-rock 7180, Hishtil, Israel), prior to flowering, were used to evaluate the effects of pollen on-twine on E. scutalis and A. swirskii. Experiments with different mite species were conducted separately and therefore analyzed and described separately. Statistical analysis was performed using JMP 7.0 and SPSS 21 software. Normality of data was determined using the Shapiro–Wilk Goodness of fit test. When data was not normally distributed, transformations were used to attain normality. Tukey HSD multiple range test was used to achieve mean separations. In the cases that normality could not be achieved non-parametric tests were applied.
Effect of horticultural twine types and pollen species on predator establishment
The effects of twine and pollen types, were tested in growth chambers with two month old potted pepper plants cut and standardized to six leaves per plant as follows : two twine types and three pollen treatments (see below) yielding six treatment combinations replicated five times. Hence, 30 plants randomly assigned to treatments, were used for each phytoseiid species. Twine was either polypropylene (Tama Plastic Industry, Israel) or rayon (80 %) -jute (20 %) (Paskal, Israel). Pollen treatments were: 1. not coated, 2. coated with Tabor oak pollen, 3. coated with corn pollen. To avoid predator contamination, the species were evaluated in sequential experiments, first E. scutalis and then A. swirskii, the growth chambers being thoroughly cleaned between trials. In both experiments, twine segments (15 cm long) were tied up in a ring and coated by shaking them in a plastic 400 ml container with 40–50 ml pollen. Once coated, the twine ring was held with a tweezers and gently shaken (in the container above the pollen) to remove excess pollen. A ring of twine was then hanged over the top two leaves of each of the pepper plants. Mature female predators, delicately vacuumed into 200 μl micropipette tips (Argov et al. 2002), twenty per tip, were released by hanging the partially cut opened tips on the lowest leaf of each potted plant. After 15 days, motiles and eggs on the plant and on the twine were counted separately under a stereomicroscope, by gently removing the twine, leaves and stem for examination.
The experiments with the two phytoseiid species were separated by time, but otherwise were similarly performed according to the following experimental setup. Due to space limitation, the plants were randomly divided between two adjacent similar walk-in growth chambers, considered as blocks. For E. scutalis (March 2012) two replicates of each treatment were placed in room 1 (block 1) (23 ± 2 °C, 50 ± 6 % RH, 16L:8D) and the remaining three replicates in room 2 (block 2) (24 ± 1 °C, 51 ± 9 % RH, 16L:8D). Pepper plants were randomly positioned in each room. The same setup was used for A. swirskii (June 2012), with similar climatic conditions, in room 1 (24 ± 2 °C, 62 ± 8 % RH, 16L:8D) and in room 2 (24 ± 2 °C, 64 ± 6 % RH, 16L:8D). At the end of the experiment, numbers of A. swirskii motiles (55.3 ± 3.69, mean ± SE) and eggs (10.4 ± 1.32) per plant were very low compared with E. scutalis. Because A. swirskii are naturally found in the humid coastal plain we assumed that low ambient humidity could have been a limiting factor for A. swirskii establishment. Subsequently we conducted an additional experiment in August–September 2012 in one walk-in growth chamber equipped with a cold vapor humidifier (25 ± 1 °C, 74 ± 4 % RH, 16L:8D). The experiment was replicated five times, with all plants positioned randomly. Despite the increased humidity level, numbers of A. swirskii remained similar to the first trial. Statistics described in the results are from the latter trial.
Two-way ANOVA was used to analyze the total numbers of motiles and eggs, the two factors being twine type (two levels) and pollen type (two levels). The control treatment without pollen yielded almost nil motiles and eggs with both predators. Such results with multiple zero values could not fit the ANOVA pre-requirement and therefore were excluded from the analyses, leaving two pollen factors to be analyzed: corn and oak. Though omitted from the analyses, the control data (mostly zeroes) are presented in Fig. 1. Data of A. swirskii motiles underwent rank transformation. The following two-way ANOVA analysis is equivalent to the analogous non-parametric test (Conover and Iman 1981). Prior to the E. scutalis analysis, total numbers of eggs and motiles per plant were examined for differences between the two blocks (two separate rooms). Two-way ANOVA was performed twice, first on the factors room and pollen type, then on room and twine type, to rule out possible effects of the room on the data, taking into account the main factors. Since no differences were found in any analysis, the subsequent ANOVA analyses were performed without the block factor.
Rayon-jute twine as artificial domatia and a tool for pollen provisioning
The roles of the rayon-jute twine (found in our previous experiments to better enhance predator populations) in food provisioning and oviposition of E. scutalis and A. swirskii were examined on pre-flowering pepper plants (five weeks old, each plant serving as one replicate) in three treatments: (1) Pollen applied to the twine and leaves remained clean of pollen (L–T+, L for leaves and T for twine), (2) Pollen applied on three leaves and twine placed without pollen (L+ T−), (3) Pollen applied on three leaves and no twine added (L+). All treatments, replicated eight times, received equal amounts of oak pollen either on the twine or on the leaves. Rayon-jute twine segments of 15 cm long were tied into rings and coated with oak pollen as described above. To determine the amount of pollen to be provisioned on foliage, for each experiment eight twine rings (all pollen coated rings used in each experiment) were weighed together before and after the pollen coating. Accordingly treatments received 0.178 and 0.175 g of pollen per plant for the E. scutalis and A. swirskii experiments, respectively. Experiments with E. scutalis and A. swirskii were conducted simultaneously, each species in a separate identical walk-in growth chamber (E. scutalis: 25 ± 3 °C, 44 ± 9 % RH, 16L:8D; A. swirskii: 25 ± 4 °C, 53 ± 9 % RH; 16L:8D). To prevent predator movement between chambers plants were irrigated in each chamber on different days. Twenty-four plants (eight replicates of three treatments) were randomly positioned in each room. Twenty mature female predators per plant were released as described in the above experiment, assessing the effects of horticultural twine and pollen on predator establishment. After 20 days, motiles and eggs were counted separately on the plant, and on the twine, using a stereomicroscope at a maximum magnification of 60×. Total predator populations per plant were calculated by adding the number of mites (motiles and eggs separately) found on the plant to the number found on the twine. The percentages of motiles and eggs on the twine were calculated out of total predator population on plant and twine.
To evaluate the effects of treatments on E. scutalis motiles, a log transformation was conducted followed by ANOVA. For the effect on eggs ANOVA was performed without transformation. To evaluate the effect of the modes of pollen provisioning (on the twine or on the plant) on the percentage of E. scutalis motiles and eggs on the twine two t-tests were conducted.
Amblyseius swirskii motile data were log (1 + x) transformed followed by ANOVA. Kruskal–Wallis χ 2 approximation for non parametric data was used to evaluate the effect of treatments on the total number of eggs. Student’s t-test, and Wilcoxon χ 2 approximation were used to evaluate the effect of food provisioning modes on the percentage of A. swirskii motiles and eggs, respectively, on twine.
Results
Effect of horticultural twine types and pollen species on predator establishment
Euseius scutalis
Rayon jute twine treatments had significantly more motiles (Fig. 1a; F(1,16) = 10.21, P = 0.006) and eggs (Fig. 1b; F(1,16) = 24.28, P < 0.0001) than polypropylene treatments. There were no significant differences in numbers of motiles (Fig. 1a; F(1,16) = 2.59, P = 0.127) and eggs (Fig. 1b; F(1,16) = 2.1, P = 0.17) between plants that received either corn or oak pollen. Numbers of E. scutalis motiles and eggs in the treatments that received no pollen were very close to nil on both twine types (2.6 and 0.2 respectively), and therefore were excluded from the main analysis. In general, when pollen was provisioned, motiles increased by more than an order of magnitude (from 20 to 362.5 motiles per plant) in both pollen species and twine types. There were no interactions between twine type and pollen species in numbers of both motiles (Fig. 1a; F(1,16) = 0.192, P = 0.67) and eggs (Fig. 1b; F(1,16) = 0.013, P = 0.91).
Amblyseius swirskii
Rayon jute twine treatments had significantly more eggs of A. swirskii (Fig. 1d; F(1,16) = 12.92, P = 0.002) than polypropylene treatments. Motile numbers did not significantly differ between these treatments (Fig. 1c; F(1,16) = 2.06, P = 0.17) although slightly trended in favor of rayon jute twine. There were no significant differences between plants that received either corn or oak pollen in numbers of motiles (Fig. 1c; F(1,16) = 0.57, P = 0.46) or eggs (Fig. 1d; F(1,16) = 2.69, P = 0.12). Numbers of motiles and eggs (1.2 and 0 respectively) in the treatments that received no pollen were close to nil on both twine types, and therefore were excluded from the main analysis. On plants with pollen coated twines, numbers of motiles only doubled over the two weeks period (20–50 motiles per plant) and numbers of eggs were minimal (on average ten eggs per plant). There were no interactions between twine type and pollen species in numbers of both motiles (Fig. 1c; F(1,16) = 0.37, P = 0.55) and eggs (Fig. 1d; F(1,16) = 0.19, P = 0.66).
Rayon-jute twine as artificial domatia and a tool for pollen provisioning
Euseius scutalis
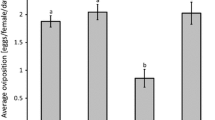
Numbers of E. scutalis motiles increased by more than an order of magnitude (on average 506 motiles per plant) without significant differences among all treatments (Fig. 2a; F(2,21) = 3.28, P = 0.057). Though not significant, motile population levels on plants that received uncoated twine and pollen applied to their leaves (L+ T−) were higher than in the two remaining treatments, where pollen was either provisioned on the twine (L− T+), or on the plant without twine (L+). In the numbers of eggs per plant, however, the same differences were significant (Fig. 2b; F(2,21) = 10.17, P < 0.001). While there were no significant differences between the two modes of pollen application (pollen coated twine [L− T+] versus pollen provisioned on leaves [L+ T−]) in the proportions of motiles (Fig. 3a; t = 0.78, df = 14, P = 0.45) and eggs (Fig. 3b; t = −1.25, df = 14, P = 0.23) on twine, the mean proportion of motiles (22 %) was substantially lower than the mean proportion of eggs (93 %).
Effects of rayon-jute twine and pollen provisioning modes on populations of Euseius scutalis (a, b) and Amblyseius swirskii (c, d). Mean (±SE) number of total motiles per plant (a, c). Mean (±SE) number of total eggs per plant (b, d). Treatments include pollen coated twine and leaves remain clean (L− T+), pollen provisioned on leaves and an uncoated twine (L+ T−) and pollen provisioned on leaves with no twine (L+). Different letters indicate significant differences among treatments (α = 0.05, n = 8)
Effects of pollen application mode (pollen coated twine [L− T+] vs. pollen provisioned on leaves [L+ T−]), on the proportion of Euseius scutalis (a, b) and Amblyseius swirskii (c, d) on rayon-jute twine. Mean (±SE) percentage of motiles per plant on twine (a, c). Mean (±SE) percentage of eggs per plant on twine (b, d). Asterisks indicate significant differences between treatments (α = 0.05, n = 8)
Amblyseius swirskii
There were significant differences in numbers of A. swirskii motiles (Fig. 2c; F(2,21) = 4.08, P = 0.03) and eggs (Fig. 2d; χ 22 = 13.04, P < 0.0001) among treatments, though the overall number of motiles did not increase compared to the initial predator numbers (20–21 motiles per plant) and the overall number of eggs was exceedingly low (on average eight eggs per plant). Population levels (motiles and eggs) were higher on plants with pollen on their leaves and uncoated twine (L+ T−) than on plants with pollen on their leaves without twine (L+). Population levels (motiles and eggs) on plants that were treated with pollen on twine and not on leaves (L− T+) did not significantly differ from the two other above mentioned treatments. Proportion of motiles (Fig. 3c; t = 4.21, df = 14, P < 0.0001) and eggs (Fig. 3d; χ 21 = 6.96, P < 0.01) on twine were significantly higher on plants with pollen on the leaves and uncoated twine (L+ T−) than on plants with pollen on the twine (L− T+).
Discussion
Our study suggests a feasible solution combining supplements of food and oviposition sites into one “pollen on-twine” treatment. We found that supplemental pollen on-twine increases E. scutalis populations by an order of magnitude in 2–3 weeks and A. swirskii populations are doubled. In nature, phytoseiids are more abundant on plants with pubescent leaves because the leaf hairs retains more airborne pollen and thus provide alternative food (Roda et al. 2003), and also because they shelter the acarine predators from their predators (Roda et al. 2000; Seelmann et al. 2007). Similarly pollen on-twine attached to the plant can serve as an artificial “pollen reservoir” in enhancing populations of predatory mites, and may serve also as an artificial shelter for them.
Twine type and pollen effect
Rayon-jute twine was found as a more favorable oviposition site than the polypropylene twine to both E. scutalis and A. swirskii, possibly because it better mimics natural domatia and leaf hairs. These are known to reduce egg predation for example by thrips (e.g. Faraji et al. 2002; Roda et al. 2000), intraguild predation of immatures (Ferreira et al. 2011) and cannibalism (Ferreira et al. 2008). Artificial oviposition sites can similarly protect phytoseiid eggs from predation as was demonstrated by Roda et al. (2000) with cotton fibers on bean leaf discs. Although predators of phytoseiids were absent in the current study, we hypothesize that the distinct preference of both predators to lay eggs on the twine served to protect eggs from predation. The preference to lay eggs on the twine could hardly have resulted from an improvement in abiotic conditions, as the humidity needed for egg development was probably lower on the twine than on the leaf boundary layer where the humidity level is higher and more stable (Boulard et al. 2004). Loughner et al. (2011) compared jute with other fibers in maintaining A. swirskii on plants with cattail pollen. He found that cotton patches and paper pulp supplements increased and maintained population levels. In contrast, in their jute treatment the population level of A. swirskii decreased, and in the celluflo and no fibers treatments most of the mites left the plants. The authors also stressed that the form of application of the fibers was crucial to the success of the supplements in enhancing predator populations. In our study where we used a combination of rayon (80 %) and jute fibers (20 %) the twine had a beneficial effect on A. swirskii populations. Yet, while counting the number of eggs on the twine we observed that most of the laid eggs were on the thin rayon fibers and not on the thick jute fibers, in concurrence with the results of Loughner et al. (2011).
The pollen of Tabor oak was found suitable for the development and reproduction of E. scutalis and other phytoseiids (Adar et al. 2012; Argov et al. 2006), but is relatively hard to collect because of its brief flowering period. Corn pollen can be easily obtained but in some cases was found less optimal for A. swirskii (Goleva and Zebitz 2013; Loughner et al. 2011; Onzo et al. 2011; Swirski et al. 1967) and E. scutalis (Elbadry and Elbenhawy 1968; Swirski et al. 1967). In the current study, pollen origin did not affect mite development. However, corn pollen could be preferred over other pollens as it can be standardized by collecting from a specific cultivar and it can mechanically harvested from corn fields at a relatively low cost.
Rayon-jute twine as artificial domatia and a tool for pollen provisioning
Loughner et al. (2011) reported an additive effect when pollen and acrylic yarn fibers were applied separately on the same plant compared to each treatment on different plants. Similarly, in the present study, while pollen on-twine enhanced populations of E. scutalis and A. swirskii, the treatment that promoted the highest population increase was a combination of uncoated twine and pollen applied on the leaves (Fig. 2a–d). For A. swirskii (with generally low populations), this outcome may result from predator avoidance of pollen coated twine in comparison with uncoated twine, as on the uncoated twine we found a higher proportion of A. swirskii motiles (Fig. 3c) and eggs (Fig. 3d). In contrast, population levels of E. scutalis were exceedingly high and proportions of E. scutalis motiles (Fig. 3a) and eggs (Fig. 3b) on the coated and uncoated twine did not differ. The most obvious explanation for these differences is that the generalist A. swirskii, a type III phytoseiid is less suited for feeding only on pollen than the type IV pollen specialist feeder E. scutalis (McMurtry et al. 2013). In contrast, Faraji et al. (2002) suggested that the phytoseiid Iphiseius degenerans (Berlese) prefers to lay eggs in a secure location away from their food abundant in the flowers (pollen, nectar and thrips) to reduce egg predation by flower-visiting thrips. In Israel A. swirskii found on citrus, usually co-occurs with other phytoseiid species (Palevsky et al. 2003) and other arthropod predators (Palevsky unpublished data). In contrast E. scutalis naturally occurring on avocado, is abundant in the presence of pollen without prey and other predator species (Maoz et al. 2011). These different ecosystems could explain inherent species differences in oviposition behavior, where A. swirskii prefers to lay its eggs away from the pollen feeding sites of potential intraguild predators even in their absence, whereas E. scutalis oviposits in domatia regardless of the presence of pollen. With the latter species we suggest that food and oviposition sites were limiting factors. Evidently when these resources were separated, populations could increase substantially (Fig. 2a, b), possibly due to increased accessibility to feeding and oviposition sites. Further research evaluating the application of coated and uncoated twine on the same plant, could test this hypothesis.
In the pollen on-twine treatment, population increase did not differ from the treatment with no twine and pollen provisioning on the plant. The latter method was effective in many experimental studies in the past (e.g. Nomikou et al. 2010; Tuovinen and Lindqvist 2010; Weintraub et al. 2009) but was never implemented commercially. Manually applying pollen to plants is labor intensive and applying pollen using a mechanical blower is not cost effective as a substantial proportion of the pollen is lost. The latter because the amount of pollen that actually sticks to the leaves is small, subsequently requiring multiple applications. Additionally, mold development on pollen applied to the leaves is expected to be more rapid than on pollen on the twine, due to higher humidity levels on the former. Nutrimite™ (BioBest, Belgium) expected to appear on the market in 2014, for the application of Typha pollen on roses, seems to be the first attempt to commercially apply pollen to foliage for the enhancement of a phytoseiid predator. How cost effective this product will be is still an open question.
Differential performance of the two predatory mites
In both experiments E. scutalis populations increased much more than those of A. swirskii (Figs. 1, 2). As discussed above, here too the first explanation to the differential performances of the two predators may be their different life style types (McMurtry et al. 2013). However, since populations of the generalist A. swirskii increased substantially when fed only on pollen (Goleva and Zebitz 2013; Ragusa and Swirski 1975; Swirski et al. 1967), we will henceforth try and discuss other explanations. At the initiation of the experiment A. swirskii underwent a diet change from C. lactis to pollen whereas E. scutalis was reared on pollen and thus no diet change occurred. Diet change can substantially affect population dynamics of phytoseiids (Argov et al. 2006; Castagnoli and Simoni 1999) which could partially explain our results. Still we preferred to use mass reared A. swirskii on C. lactis to lab reared individuals on pollen because the former is the commercial product available to growers. Additionally we should point out that establishment of the commercial product A. swirskii on flowering pepper plants is usually excellent (Bolckmans et al. 2005) resulting in as many as tens of eggs in each domatia (S. Steinberg, pers. comm.). Thus it seems that, in this study, diet history and life style type (McMurtry et al. 2013) might have had less impact on population levels of A. swirskii than other factors.
In previous experiments where pollen was provisioned on pepper seedlings prior to flowering, populations of A. swirskii (also produced and provided by BioBee) never reached substantially higher numbers than in the current study (Weintraub et al. 2009, Adar, unpublished data). A possible explanation for these low A. swirskii population levels is that young seedlings lack the leaf domatia of mature pepper plants, typically populated by A. swirskii in commercial greenhouses. Apparently the twine, designed to provide domatia on young plants, was a poor substitute for A. swirskii as it lacked a source of humidity, compared to the relatively humid leaf domatia on mature leaves (Grostal and Odowd 1994). In contrast, E. scutalis being a more arid adapted predator than A. swirskii, was able to take better advantage of the artificial domatia offered by the twine. Additional studies conducted at varying humidity levels are necessary to determine whether humidity is a limiting factor for A. swirskii.
The current method of pollen on-twine on peppers prior to flowering, allowed for a tenfold increase in populations of E. scutalis, over a period of 2–3 weeks (certainly more than sufficient for biological control), and to a lesser extent, in those of A. swirskii. Clearly, further evaluations of pollen on twine need to include thrips as it is a pest that also feeds on pollen. Nonetheless in a previous study, when pollen was concentrated in patches on the plant, in effect similar to what we have done with pollen on twine, thrips levels were reduced, as these pollen patches were ‘monopolized’ by predatory mites (van Rijn et al. 2002). Further studies are also needed to determine optimal timing for the application of pollen on-twine for enhancing phytoseiid species prior to pest establishment in different cropping systems, but the potential of increasing populations of predators by the enrichment of their environment with pollen on-twine seems promising and feasible.
References
Adar E, Inbar M, Gal S, Doron N, Zhang ZQ, Palevsky E (2012) Plant-feeding and non-plant feeding phytoseiids: differences in behavior and cheliceral morphology. Exp Appl Acarol 58:341–357
Argov Y, Amitai S, Beattie GAC, Gerson U (2002) Rearing, release and establishment of imported predatory mites to control citrus rust mite in Israel. BioControl 47:399–409
Argov Y, Berkeley M, Domeratzky S, Melamed E, Weintraub P, Palevsky E (2006) Identification of pollens for small scale mass rearing of Neoseiulus californicus and a novel method for quality control. IOBC/WPRS Bull 29(4):127–132
Biobest News & press releases (2013) Biobest introduces Dyna-Mite®: a new predatory mite strategy in rose. Biobest Belgium N.V. http://www.biobest.be/nieuws/289/3/0/. Accessed 14 Jan 2014
Bolckmans K, van Houten Y, Hoogerbrugge H (2005) Biological control of whiteflies and western flower thrips in greenhouse sweet peppers with the phytoseiid predatory mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). In: Hoddle M (ed) Second International Symposium on biological control of arthropods. U.S. Department of Agriculture, Forest Service, Switzerland, pp 555–565
Boulard T, Fatnassi H, Roy JC, Lagier J, Fargues J, Smits N, Rougier M, Jeannequin B (2004) Effect of greenhouse ventilation on humidity of inside air and in leaf boundary-layer. Agricult Forest Meteorol 125:225–239
Castagnoli M, Simoni S (1999) Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 23:217–234
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Statist 35:124–129
Duso C (1992) Role of Amblyseius aberrans (Oud.), Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari, Phytoseiidae) in vineyards. J Appl Entomol 114:455–462
Elbadry EA, Elbenhawy EM (1968) The effect of non-prey food, mainly pollen, on the development, survival, and fecundity of Amblyseius gossipi (Acarina: Phytoseiidae). Entomol Exp Appl 11:269–272
Faraji F, Janssen A, Sabelis MW (2002) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
Ferreira JAM, Eshuis B, Janssen A, Sabelis M (2008) Domatia reduce larval cannibalism in predatory mites. Ecol Entomol 33:374–379
Ferreira JAM, Cunha DFS, Pallini A, Sabelis MW, Janssen A (2011) Leaf domatia reduce intraguild predation among predatory mites. Ecol Entomol 36:435–441
Frank S (2010) Biological control of arthropod pests using banker plant systems: past progress and future directions. Biol Control 52:8–16
Gan-Mor S, Bechar A, Ronen B, Eisikowitch D, Vaknin Y (2003) Electrostatic pollen applicator development and tests for almond, kiwi, date and pistachio—an overview. Appl Eng Agric 19:119–124
Gan-Mor S, Palevsky E, Ronen B (2011) A device and a method for pollen application for enhancing biological control. United States PCT 12/984,462, Jan-04-2011
Goleva I, Zebitz CPW (2013) Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp Appl Acarol 61:259–283
Grostal P, Odowd DJ (1994) Plants, mites and mutualism—leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97:308–315
Huang N, Enkegaard A, Osborne LS, Ramakers PJ, Messelink GJ, Pijnakker J, Murphy G (2011) The banker plant method in biological control. Crit Rev Plant Sci 30:259–278
Jacobson RJ, Croft P, Fenlon J (2010) Suppressing establishment of Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in cucumber crops by prophylactic release of Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae). Biocontrol Sci Technol 11:27–34
Kreiter S, Tixier MS, Croft BA, Auger P, Barret D (2002) Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ Entomol 31:648–660
Loughner R, Goldman K, Loeb G, Nyrop J (2008) Influence of leaf trichomes on predatory mite (Typhlodromus pyri) abundance in grape varieties. Exp Appl Acarol 45:111–122
Loughner R, Wentworth K, Loeb G, Nyrop J (2010) Influence of leaf trichomes on predatory mite density and distribution in plant assemblages and implications for biological control. Biol Control 54:255–262
Loughner R, Nyrop J, Wentworth K, Sanderson J (2011) Effects of supplemental pollen and fibers on canopy abundance of Amblyseius swirskii. IOBC/WPRS Bull 68:105–109
Maoz Y, Gal S, Argov Y, Coll M, Palevsky E (2011) Biocontrol of persea mite, Oligonychus perseae, with an exotic spider mite predator and an indigenous pollen feeder. Biol Control 59:147–157
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
McMurtry JA, Scriven GT (1966) Studies on predator-prey interactions between Amblyseius hibisci and Oligonychus punicae (Acarina: Phytoseiidae, Tetranychidae) under greenhouse conditions. Ann Entomol Soc Am 59:793–800
McMurtry JA, de Moraes GJ, Sourassou NF (2013) Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18:297–320
Nomikou M, Janssen A, Schraag R, Sabelis MW (2001) Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp Appl Acarol 25:271–291
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Nomikou M, Sabelis MW, Janssen A (2010) Pollen subsidies promote whitefly control through the numerical response of predatory mites. BioControl 55:253–260
Norton AP, English Loeb G, Belden E (2001) Host plant manipulation of natural enemies: leaf domatia protect beneficial mites from insect predators. Oecologia 126:535–542
Onzo A, Houedokoho AF, Hanna R (2011) Potential of the predatory mite, Amblyseius swirskii to suppress the broad mite, Polyphagotarsonemus latus on the gboma eggplant, Solanum macrocarpon. J Insect Sci 12:1–11
Palevsky E, Argov Y, Ben David T, Gerson U (2003) Identification and evaluation of potential predators of citrus rust mite. Syst Appl Acarol 8:39–48
Ragusa S, Swirski E (1975) Feeding habits, development and oviposition of the predacious mite Amblyseius swirskii Athias-Henriot (Acarina: Phytoseiidae) on pollen of various weeds. Israel J Entomol 10:93–103
Ramakers PMJ, Voet SJP (1995) Use of castor bean, Ricinus communis, for the introduction of the thrips predator Amblyseius degenerans on glasshouse-grown sweet peppers. Meded Fac Landbouwk en Toeg Biol Wet Univ Gent 60:885–891
Ramakers PJ, Voet SJP (1996) Introduction of Amblyseius degenerans for thrips control in sweet peppers with potted castor beans as banker plants. IOBC/WPRS Bull 19(1):127–130
Roda A, Nyrop J, Dicke M, English Loeb G (2000) Trichomes and spider-mite webbing protect predatory mite eggs from intraguild predation. Oecologia 125:428–435
Roda A, Nyrop J, English Loeb G, Dicke M (2001) Leaf pubescence and two-spotted spider mite webbing influence phytoseiid behavior and population density. Oecologia 129:551–560
Roda A, Nyrop J, English-Loeb G (2003) Leaf pubescence mediates the abundance of non-prey food and the density of the predatory mite Typhlodromus pyri. Exp Appl Acarol 29:193–211
Romero GQ, Benson WW (2005) Biotic interactions of mites, plants and leaf domatia. Cur Opin Plant Biol 8:436–440
Seelmann L, Auer A, Hoffmann D, Schausberger P (2007) Leaf pubescence mediates intraguild predation between predatory mites. Oikos 116:807–817
Swirski E, Amitai S, Dorzia N (1967) Laboratory studies on the feeding, development and reproduction of the predaceous mites Amblyseius rubini Swirski and Amitai and Amblyseius swirskii Athias (Acarina: Phytoseiidae) on various kinds of food substances. Israel J Agric Res 17:101–119
Tixier MS, Kreiter S, Okassa M, Cheval B (2010) A new species of the genus Euseius Wainstein (Acari: Phytoseiidae) from France. J Nat Hist 44:241–254
Tuovinen T, Lindqvist I (2010) Maintenance of predatory phytoseiid mites for preventive control of strawberry tarsonemid mite Phytonemus pallidus in strawberry plant propagation. Biol Control 54:119–125
van Maanen R, Vila E, Sabelis MW, Janssen A (2010) Biological control of broad mites (Polyphagotarsonemus latus) with the generalist predator Amblyseius swirskii. Exp Appl Acarol 52:29–34
van Rijn PCJ, Sabelis MW (1990) Pollen availability and its effect on the maintenance of populations of Amblyseius cucumeris, a predator of thrips. IOBC/WPRS Bull 13(5):179–184
van Rijn PCJ, van Houten YM, Sabelis MW (1999) Pollen improves thrips control with predatory mites. IOBC/WPRS Bull 22(1):209–212
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Wäckers FL (2005) Suitability of (extra-) floral nectar, pollen and honeydew as insect food sources. In: Wäckers FL, van Rijn PCJ, Bruijn J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, UK, pp 17–74
Wäckers FL, Romeis J, van Rijn PCJ (2007) Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu Rev Entomol 52:301–323
Walter DE (1996) Living on leaves: mites, tomenta, and leaf domatia. Annu Rev Entomol 41:101–114
Weintraub PG, Kleitman S, Mori R, Gan-Mor S, Gannot L, Palevsky E (2009) Novel application of pollen to augment the predator Amblyseius swirskii on greenhouse sweet pepper. IOBC/WPRS Bull 50:119–124
Acknowledgments
We would like to thank Bio-Bee, Israel, and especially Shimon Steinberg, Yehoshua Kaminsky and Arnon Alouche for their cooperation, collaboration and for supplying some of the materials and mites needed for this study. We are grateful to Yonatan Maoz for his technical support and Matan Ben-Ari, Alon Lotan and Prof. Ido Izhaki for their statistical guidance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
About this article
Cite this article
Adar, E., Inbar, M., Gal, S. et al. Pollen on-twine for food provisioning and oviposition of predatory mites in protected crops. BioControl 59, 307–317 (2014). https://doi.org/10.1007/s10526-014-9563-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-014-9563-1