Abstract
Supplemental food sources to support natural enemies in crops are increasingly being tested and used. This is particularly interesting for generalist predators that can reproduce on these food sources. However, a potential risk for pest control could occur when herbivores also benefit from supplemental food sources. In order to optimize biological control, it may be important to select food sources that support predator populations more than herbivore populations. In this study we evaluated the nutritional quality of four types of supplemental food for the generalist predatory mites Amblyseius swirskii Athias-Henriot and Amblydromalus (Typhlodromalus) limonicus (Garman and McGregor), both important thrips predators, and for the herbivore western flower thrips Frankliniella occidentalis Pergande, by assessing oviposition rates. These tests showed that application of corn pollen, cattail pollen or sterilized eggs of Ephestia kuehniella Zeller to chrysanthemum leaves resulted in three times higher oviposition rates of thrips compared to leaves without additional food. None of the tested food sources promoted predatory mites or western flower thrips exclusively. Decapsulated cysts of Artemia franciscana Kellogg were not suitable, whereas cattail pollen was very suitable for both predatory mites and western flower thrips. In addition, we found that the rate of thrips predation by A. swirskii can be reduced by 50 %, when pollen is present. Nevertheless, application of pollen or Ephestia eggs to a chrysanthemum crop still strongly enhanced the biological control of thrips with A. swirskii, both at low and high release densities of predatory mites through the strong numerical response of the predators. Despite these positive results, application in a crop should be approached with caution, as the results may strongly depend on the initial predator–prey ratio, the nutritional quality of the supplemental food source, the species of predatory mites, the distribution of the food in the crop and the type of crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The addition of supplementary or alternative food sources to crops to support natural enemies has received increasing attention in biological control (Wade et al. 2008; Lundgren 2009). This is particularly relevant for the case of generalist predators that are able to reproduce on these food sources, enabling them to establish a population even before pests occur (Ramakers and Rabasse 1995; Messelink et al. 2012). Another advantage associated with supplemental food is the increased retention of natural enemies in a crop in case prey densities are low (Kunkel and Cottrell 2007). Well-studied alternative food sources suitable for generalist predators are eggs of the flour moth Ephestia kuehniella Zeller and decapsulated cysts of the brine shrimp Artemia franciscana Kellogg (Cocuzza et al. 1997; Arijs and De Clercq 2001; Vantornhout et al. 2004; Castañé et al. 2006; Nguyen et al. 2014; Vangansbeke et al. 2014). Currently, these two food sources are increasingly used to boost densities of the predatory bug Macrolophus pygmaeus Rambur in tomato and sweet pepper crops (Messelink et al. 2014). In addition to factitious prey, the use of pollen has also received much attention as an alternative food source for predatory mites and predatory bugs (McMurtry and Scriven 1964; Calvert and Huffaker 1974; Cocuzza et al. 1997; Vandekerkhove and De Clercq 2010). Application of pollen in crops can strongly enhance pest control, as shown for the control of thrips and whiteflies by predatory mites in cucumber (van Rijn et al. 2002; Nomikou et al. 2010).
However, the use of alternative food could involve risks to pest control. Firstly, a surplus of alternative food may result in predator satiation or distraction from the target pest species in the short-term. Many studies show that such short-term effects occur (Symondson et al. 2002). For example, pollen provided to predatory mites and predatory bugs reduced their predation of thrips in short-term experiments (Skirvin et al. 2007). However, theory predicts that supplying alternative food may have negative short-term effects, but positive long-term effects on biological control through the increase of equilibrium predator densities (apparent competition, Holt 1977). Hence, whether or not alternative food negatively affects biological control strongly depends on the duration of the cropping cycle relative to the reproduction rate and the dynamics of the pests and the predators (Abrams et al. 1998).
Secondly, alternative food can also serve as a food source for some omnivorous pest species such as thrips. A study on western flower thrips, Frankliniella occidentalis Pergande, showed that application of pollen to cucumber leaves strongly increased the population growth rate, depending on the type of pollen (Hulshof et al. 2003). Thus, the impact of providing pollen to predators that control thrips depends not only on the benefit of pollen to the predator, but also on the benefit of pollen to thrips. The benefit for thrips is a sum of increased population growth rate and reduced consumption by the predators. Whether the overall effect on the plant is positive or negative depends on how pollen influences the predator–prey ratio and the predator’s numerical response to herbivore densities on the plant. Van Rijn et al. (2002) evaluated such dynamics in a model for western flower thrips, the predatory mite Iphiseius degenerans (Berlese) and pollen of Typha latifolia L. on cucumber. This model indeed shows, in the case of transient predator–prey dynamics, an initial predator density below which the mean herbivore density will be higher rather than lower in the presence of pollen. This is caused by the increased reproduction rates of thrips and the reduced predation rates by predatory mites. Hence, in the short-term, there is always a risk that supplemental food may increase plant damage by herbivores. This depends on the timing of predator releases and initial predator densities. This risk will also depend on the extent to which the supplemental food source promotes population growth of the predators compared to the pest species. It is known from earlier studies that the suitability of pollen for predatory mites can differ (van Rijn and Tanigoshi 1999; Matsuo et al. 2003; Goleva and Zebitz 2013) from that for thrips (Hulshof et al. 2003). In order to optimize biological control with supplemental food, it may be important to select food sources that support predator populations more than herbivore populations. This may be particularly relevant in ornamental crops with a short cropping cycle and low pest tolerance.
In this study we evaluated the nutritional quality of four types of supplemental food for the generalist predatory mite Amblyseius swirskii Athias-Henriot, which is a commonly used and important predator of western flower thrips (Messelink et al. 2006; van Lenteren 2012). This was done by assessing the effects of food on the daily oviposition rates. The food sources were selected based on their commercial availability or potential suitability for application in greenhouse crops. The same food sources were also tested for western flower thrips to see whether there are differences in suitability between thrips and the predators. In addition we tested how the initial density ratios of predatory mites and thrips in presence of alternative food sources affects the control of thrips. Based on earlier model predictions (van Rijn et al. 2002), we hypothesized that if supplemental food is available, the lower the initial predator-to-thrips ratio, the more thrips escape from predation. Food sources that support thrips more than the predator are likely to increase this effect. The effects of supplemental food may also differ per species of predatory mite and therefore the predatory mite Amblydromalus (Typhlodromalus) limonicus (Garman and McGregor) was included in the oviposition experiments. This predatory mite is also an effective thrips predator, but less commonly used (Messelink et al. 2006).
The model crop in our study was chrysanthemum, a crop with a relatively short growing period. The results of these experiments may help us to design some guidelines for the use of supplemental food in biological pest control.
Materials and methods
Cultures of predatory mites and thrips
The predatory mites A. swirskii and A. limonicus were obtained from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands) and subsequently reared on a diet of cattail pollen (T. latifolia). Amblyseius swirskii was reared on plastic arenas (6 × 12 cm) placed on water-saturated cotton wool in plastic trays. Strips of wet filter paper were placed along the sides of the arena to provide the mites with water (according to van Rijn and Tanigoshi 1999). The same method was used for A. limonicus, except that the plastic arenas were replaced with sweet pepper leaves placed upside down on water-saturated cotton wool. This species probably needs to feed on plant material in addition to pollen (Messelink et al. 2006). The predatory mite cultures were placed in climate rooms at 25 °C and 70 % RH and with 16 h of artificial illumination (three lamps of 34 W, OSRAM FL40ss w/37) per day. The oviposition experiments were carried out with predatory mites taken from these cultures, but predatory mites for the greenhouse experiments were provided by Koppert (reared on Carpoglyphus lactis (L.) with bran as a substrate).
Western flower thrips, F. occidentalis, were reared on flowering chrysanthemum plants (Dendranthema grandiflora Tzvelev cv. Miramar) in a separate greenhouse compartment. In order to produce female thrips of the same age for the laboratory experiment, thrips females were collected from the culture on chrysanthemum and offered fresh bean pods as oviposition substrate, in glass jars that were closed with lids equipped with a mesh (size 80 µm) to allow ventilation. After 1 day the adult thrips were removed and the larvae that emerged from the eggs were grown on the same pods until they reached the adult stage. Thrips on bean pods were reared under the same climatic conditions as the predatory mites.
Oviposition of predatory mites and thrips on four types of food
The nutritional quality of four types of supplemental food for the predatory mites A. swirskii and A. limonicus and for western flower thrips was assessed in the laboratory by measuring oviposition rates on these diets. Four types of supplemental food were tested: (1) cattail pollen (T. latifolia), dried and stored at −20 °C for 3 months; (2) sweet corn pollen (Zea mays L. convar. Saccharata cv. Conqueror) dried and stored at −20 °C for 10 months; (3) eggs of E. kuehniella, provided by Biobest (Westerlo, Belgium) and stored at −20 °C; and (4) freeze dried decapsulated cysts of A. franciscana, obtained from Smulders wholesale (Artemia quick HS aqua, Ulestraten, The Netherlands) and stored at room temperature. The cysts were rehydrated 1 h before use by adding water.
Oviposition of predatory mites was measured on leaf discs of sweet pepper (Capsicum annuum L.) with a diameter of 2.5 cm for A. swirskii and 4 cm for A. limonicus. The leaf discs for A. limonicus were larger because this mite tends to escape more from leaf discs than A. swirskii. Each disc was excised from the leaf axils and had a domatium for hiding and oviposition (Faraji et al. 2002). The presence of these domatia was also the reason to choose for sweet pepper leaf discs and not chrysanthemum, as these domatia lower the number of mites that escape. The discs were placed upside down on water-saturated cotton wool in plastic containers and provided with ample amounts of one of the four food sources (5–6 mg/disc/day). Young predatory mite females that just started to oviposit were individually placed on the discs with a small brush. Each treatment was repeated 20 times. Oviposition was measured daily for 3 days and all eggs were removed daily to prevent cannibalism. Because oviposition rates are affected by the previous food source of the adult predatory mites (Sabelis 1990), we omitted data from the first day of the experiment.
Oviposition of female thrips was measured on chrysanthemum leaves of cv. Euro White. Single leaves were embedded upside-down in water agar (1 % agar) in plastic boxes (5 cm high, 6 cm diameter), making the abaxial side of the leaf available to the thrips. The boxes were closed with lids equipped with a mesh (size 80 µm) to allow ventilation. One young thrips female was placed in each box where the same food as in the test with predatory mites was offered to them in ample amounts (5–6 mg/leaf/day). Every 3 days, females were transferred to new boxes with fresh food. This was repeated five times, so that oviposition was measured for 15 days in total. The number of offspring per box was determined by counting the emerged larvae, 4 and 7 days after removal of the adults. The experiment was carried out in a climate chamber with the same settings as those used for rearing the predatory mites. Each treatment was repeated 12 times.
Effects of pollen on thrips predation
A separate laboratory experiment was carried out to assess the effects of pollen on thrips predation by the predatory mite A. swirskii. Small plastic cups of 25 ml were filled with 10 ml water agar. Leaf discs (3 cm diameter) from chrysanthemum cv. Euro White were embedded upside down in the agar, making the abaxial side of the discs available to the predatory mites and thrips. The leaf discs were provided with either eight first instars of F. occidentalis, 4 mg of cattail pollen (similar source as previous experiment) or the combination of eight thrips larvae and 4 mg of cattail pollen. The thrips larvae were collected from chrysanthemum leaf discs on water agar that were offered as an oviposition site for thrips females 1 week before. Young predatory mite females that just started to oviposit were individually placed on the discs with a small brush. Each treatment was repeated ten times. The cups were closed with lids equipped with a mesh (size 80 µm) to allow ventilation and kept in a climate chamber with 16 h of artificial illumination per day, at 25 °C and 70 % RH. Thrips predation and predatory mite oviposition was measured daily for 3 days. Each day, the predatory mites were transferred to a new leaf disc with new thrips larvae and pollen. All data from the first day of the experiment were omitted to reduce effects of pre-experimental conditions (Sabelis 1990).
Population experiment
The effects of supplemental food on biological control of western flower thrips in chrysanthemum was tested in a greenhouse trial in a compartment (24 m2) on three tables (7 m2). The windows of this compartment were provided with insect gauze (mesh size 0.40 × 0.45 mm) to exclude contamination by other organisms. The experimental unit consisted of a 36-cm-diameter pot filled with peat and six cuttings of chrysanthemums cv. Euro White, supplied by Dekkers Chrysanten (Hensbroek, The Netherlands). Each pot was covered with a cylindrical Plexiglas cage (30 cm diameter, 40 cm high). Ventilation was achieved through six holes in the sides of the cage, each with a diameter of 10 cm and covered with insect gauze (mesh size 80 µm). The top of each cage was also covered with this insect gauze. The plants were provided with a standard nutrient solution for chrysanthemum plants with an ebb-and-flow irrigation system for 10 min per day or every 2 days. The selected food sources for the treatments were similar to the oviposition tests: (1) cattail pollen, (2) sweet corn pollen, and (3) eggs of E. kuehniella, but Artemia cysts were excluded because of the low oviposition rates of predatory mites on this food source. The three selected food treatments were combined with releases of A. swirskii and compared with releases of predatory mites without supplemental food and a treatment without predators and supplemental food. Each treatment with predatory mites was carried out with a low and high release density of adult female predatory mites per plant (10 and 50, respectively). This resulted in a total of nine treatments, with four replicates of each treatment. The experiment was set-up as a randomized block design with four blocks which were distributed over three tables.
All treatments were inoculated two times with five adult female thrips with an interval of 1 week. These thrips were collected from the thrips culture on chrysanthemum plants with an aspirator. Predatory mites were released once, on the same day as the second thrips release. For this release we selected female predatory mites using a small brush and these were collected and placed in groups of 10 per leaf disc of chrysanthemum (4 cm diameter). These leaf discs were placed on the chrysanthemum plants. The various food sources were added shortly after the release of the predatory mites. From each food source, 0.125 g was added per plant by dusting it with a brush all over the plant. The application of the food sources was repeated after 2 weeks, as earlier experiments indicated that pollen needs to be refreshed after this time period (Messelink et al. 2009). Because of the relatively short cropping cycle of chrysanthemum (10–12 weeks), it was decided to apply the food sources only twice.
Densities of thrips and predatory mites were assessed weekly for 8 weeks. The first observation was 1 week after the introduction of the predatory mites to the plants. Twelve leaves from each plant were gently removed and transported to the laboratory in closed plastic containers. All mites and thrips present on each leaf were counted using a binocular microscope (40× magnification). After examination the leaves were put back into the cages. Temperature and relative humidity in the greenhouse compartment were recorded every 5 min with a climate recorder (Hoogendoorn Growth Management, Vlaardingen, The Netherlands) throughout the experiment. The average temperature was 20.2 °C (range 17.6–22.9 °C) and the average relative humidity 68 % (range 64–79 %).
Statistical analysis
Daily oviposition rates of thrips were analysed through time by using a generalized linear mixed model (GLMM) with a Poisson error distribution of the data. Treatment was the fixed factor and time was included as a random factor to correct for pseudo-replication (Bolker et al. 2009). Differences among treatments were determined with a Wald test followed by Fisher’s Least Significant Difference (LSD) test at the 5 % level of significance. The results of the oviposition and predation tests for predatory mites were analysed per day with a generalised linear model (GLM) with a Poisson data distribution. Differences between treatments were tested with pairwise t tests (α = 0.05). Population dynamics of thrips and predatory mites in the greenhouse experiment were also analysed with a GLMM with a Poisson error distribution of the pest and predator densities. This time, both block and time were included as random factors. Differences among treatments were determined with a Wald test followed by Fisher’s LSD test. All statistical analyses were performed using the statistical package GenStat Release 16.1 (Payne et al. 2010).
Results
Oviposition of predatory mites and thrips on four types of food
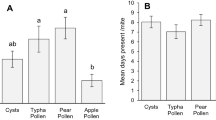
The oviposition rate of western flower thrips females was significantly higher on chrysanthemum leaves with supplemental food than on leaves without food, except for the treatment with Artemia cysts (F4,291 = 26.96, p < 0.001; Fig. 1). The predatory mite A. swirskii also reproduced well on cattail pollen, corn pollen and Ephestia eggs, but not on Artemia cysts (Fig. 2a). The predatory mite A. limonicus reproduced well on both pollen species, but not on Ephestia eggs and Artemia cysts (Fig. 2b). Oviposition rates were significantly different among treatments on day 2 (F3,43 = 11.94) and day 3 (F3,43 = 11.71) for A. swirskii and also on day 2 (F3,43 = 11.73) and day 3 (F3,43 = 28.72, all p < 0.001) for A. limonicus.
Effects of added food sources to chrysanthemum leaves on oviposition rates of young females of western flower thrips (Frankliniella occidentalis). The food supplements were cattail pollen (Typha latifolia), sweet corn pollen (Zea mays convar. Saccharata), sterilized eggs of Ephestia kuehniella, and freeze-dried cysts of Artemia franciscana. Shown are average (±SE) numbers of offspring per female through time. Different letters among treatments through time indicate significant differences (Fisher’s LSD test: p < 0.05)
Oviposition rates of young adult females of a Amblyseius swirskii and b Amblydromalus limonicus on four types of food source added to a sweet pepper leaf disc: cattail pollen (Typha latifolia), sweet corn pollen (Zea mays convar. Saccharata), sterilized eggs of Ephestia kuehniella, and freeze-dried cysts of Artemia franciscana. Shown are average numbers of eggs (±SE) per female per day assessed after 48 (day 2) and 72 h (day 3). Different letters above bars within a panel indicate significant differences in oviposition rates on day 2 (uppercase) and day 3 (lowercase) (pairwise t tests: p < 0.05)
Effects of pollen on thrips predation
Predation of thrips was not significantly different between treatments on day 2 (F1,18 = 1.23, p = 0.28), but in the presence of pollen it was significantly lower than without pollen on day 3 (F1,17 = 7.92, p = 0.01) (Fig. 3). Oviposition of A. swirskii was not significantly different among the three diets on both day 2 (F2,27 = 0.52, p = 0.60) and day 3 (F2,26 = 1.36, p = 0.28) (Fig. 4).
Daily predation rate by Amblyseius swirskii females on first larval stages of western flower thrips (Frankliniella occidentalis) on chrysanthemum leaf discs in the presence or absence of cattail pollen (Typha latifolia). Shown are average numbers of thrips larvae (±SE) consumed per day measured on day 2 (48 h) and day 3 (72 h). The asterisk (*) indicates a significant effect of the presence of pollen on predation rate (pairwise t test: p < 0.05)
Oviposition rates of young adult females of Amblyseius swirskii with either first larval stages of western flower thrips (Frankliniella occidentalis), cattail pollen (Typha latifolia), or both, added to a chrysanthemum leaf disc. Shown are average numbers of eggs (±SE) per female per day measured after 48 (day 2) and 72 h (day 3). Differences among treatments were not significant (pairwise t test: p > 0.05)
Population experiment
Thrips populations showed three fluctuations: (1) an initial decline in week 1–2, (2) a rise and fall in week 2–5, and (3) another rise and fall in week 6–8 (Fig. 5). Although differences among treatments in thrips densities in the first week were not significantly different (F8,24 = 1.50, p = 0.21), there seemed to be a trend towards higher thrips densities in the treatments where Ephestia eggs or cattail pollen had been added to the low release density of A. swirskii compared to the control treatment (Fig. 5a; Table 1). In the following weeks, there were significant differences among treatments, both in the period of week 2–5 (F8,120 = 7.11) and week 6–8 (F8,96 = 15.10, both p < 0.001) (Table 1). However, in week 2–5, there was no significant reduction of thrips in any treatment with low release densities of thrips compared to the control treatment without predatory mites (Fig. 5a; Table 1), but at the high release densities of predatory mites, all treatments with food application reduced thrips to significantly lower levels compared to the control treatment without predatory mites (Fig. 5b; Table 1). In the last 3 weeks of the experiment, thrips control was significantly better for all treatments with supplemental food at low release densities of predatory mites, but at high release densities of predatory mites, only the addition of cattail pollen or Ephestia eggs was beneficial for thrips control. When predatory mites were added without supplemental food, they reduced thrips densities to significantly lower levels than the control treatment only during the last 3 weeks of the experiment when released at high density (Fig. 5b; Table 1). The lower thrips densities in the treatments with food application were associated with the higher densities of predatory mites in these treatments (Figs. 5, 6). Overall densities of predatory mites were significantly different among treatments (F8,248 = 18.06, p < 0.001). The highest predator densities were achieved in the treatments with cattail pollen, followed by corn pollen and Ephestia eggs, both at low and high release densities of predatory mites (Fig. 6).
Population dynamics of western flower thrips (Frankliniella occidentalis) on chrysanthemum plants with a low and b high release rates (10 vs. 50/plant) of the predatory mite Amblyseius swirskii in the presence or absence of alternative food sources compared to plants without food and predatory mites (untreated). Food [cattail pollen (Typha latifolia), sweet corn pollen (Zea mays convar. Saccharata), and sterilized eggs of Ephestia kuehniella] was added twice, at the start and after 2 weeks (indicated by arrows). Shown are average densities (±SE) of larvae and adults of thrips per week. Differences among treatments in week 1, week 2–5 and week 6–8 are presented in Table 1
Population dynamics of the predatory mite Amblyseius swirskii on chrysanthemum plants with a low and b high predator release rates (10 vs. 50/plant) in the presence of western flower thrips (Frankliniella occidentalis) and three types of alternative food: cattail pollen (Typha latifolia), sweet corn pollen (Zea mays convar. Saccharata), and sterilized eggs of Ephestia kuehniella. Food was added twice, at the start and after 2 weeks (indicated by arrows). Shown are average densities (±SE) of motile predatory mites per week. Treatments associated with different letters within a panel are significantly different through time (Fisher’s LSD test: p < 0.05)
Discussion
Our study clearly shows that supplemental food that was intended to support population growth of predatory mites can also promote the population growth of the target pest, in this case the western flower thrips. Both the corn and cattail pollen and the sterilized eggs of E. kuehniella increased the oviposition rate of western flower thrips in the laboratory. None of the tested food sources promoted predatory mites or western flower thrips exclusively. Decapsulated cysts of A. franciscana were not suitable for predatory mites, nor for western flower thrips. Cattail pollen appeared to be one of the best food sources for predatory mites and was also among the best food sources for western flower thrips. Hence, we did not succeed in selecting a food source that is suitable for predatory mites but not for thrips. Our study also shows that predation of thrips can be reduced by half in the presence of pollen (Fig. 3), as was found earlier for the predatory mite Neoseiulus cucumeris (Oudemans) in the presence of castor bean pollen (Skirvin et al. 2007). This suggests that pollen could distract the predatory mites from preying on thrips and reduce short-term thrips control. However, these potential risks of promoting reproduction and reducing predation of thrips with supplemental food did not negatively affect the control of thrips in our greenhouse experiment. To the contrary, the added food sources strongly enhanced the control of thrips. In the short-term, the application of supplemental food indeed seemed to stimulate thrips reproduction, resulting in slightly higher thrips densities compared to treatments without food, but this effect was offset after 1 week already through the strong numerical response of the predatory mites to the pollen. Although there is a risk of increasing thrips densities through satiation of predatory mites, this effect seems to be short-term. We hypothesized that the short-term negative effect of supplemental food on thrips control depends strongly on the initial predator–prey ratios. In our experiments, food application enhanced thrips control under each of the two predator–prey ratios tested (Fig. 5). However, lower initial predator densities compared to initial thrips densities could have extended the period of short-term positive effects on thrips. In practice, this could occur in chrysanthemum crops, because young plants often experience a high invasion of thrips adults from neighbouring plants that are harvested (companies with year-round production systems), whereas predatory mite densities are often low at the start of a new cropping cycle.
The results of the oviposition measurements in the laboratory were consistent with the results of the greenhouse experiment; the highest predatory mite densities were achieved in the treatment with cattail pollen. Thus, although this pollen species is one of the most suitable sources for thrips reproduction, it also gave in combination with predatory mites the best control of thrips. This suggests that the reproduction rate of predatory mites on a supplemental food source is the most important criterion for selecting food sources that can support biological control. It seems that any possible negative effect through predator satiation or increased thrips reproduction will soon be compensated by the strong numerical response of the predatory mites.
The results of our study confirm what has been found by van Rijn et al. (2002), where providing cattail pollen to populations of the predatory mite I. degenerans on cucumber also enhanced thrips control. However, in addition to the duration of the experiment and the initial predator–prey ratios, the results may also depend on the species of predatory mite, the type of food source, the method of food application and the species of plant. Different species of predatory mite may respond differently to the alternative food source. For example, pollen of red alder and hazel were not suitable for oviposition by N. cucumeris, but very suitable for I. degenerans (van Rijn and Tanigoshi 1999). In our study, eggs of E. kuehniella were not suitable for oviposition of A. limonicus, whereas A. swirskii reproduced well on this food source. Providing this food source to A. limonicus in the presence of thrips may decrease the control of thrips, or at least increase the risk of thrips escaping from predation in the short-term, because the reproduction of thrips is also promoted by this food. In contrast to our study, Vangansbeke et al. (2014) found high oviposition rates of A. limonicus on Ephestia eggs. This indicates that the nutritional quality of Ephestia eggs can be variable. The same may apply for Artemia cysts: the cysts used in our study did not increase reproduction by predatory mites, whereas in other studies they did (Nguyen et al. 2014; Vangansbeke et al. 2014).
Not only the type of food, but also the way in which the food is distributed on the plants might affect pest control. The predator–prey model of van Rijn et al. (2002) suggests that a uniform supply of supplemental food provides room for the herbivores to enhance their population growth rates and to escape from predator control, whereas a local supply enables the predators to monopolize the supplemental food source. Further research is required to establish whether local application of the supplemental food indeed reduces risks of increased thrips numbers in the short-term. Our study did not show a strong positive effect on thrips densities in the short-term, despite the fact that the food was equally distributed over the plants.
The type of crop in which alternative or supplemental food is applied can also affect the results through the indirect effects of plant metabolites on predation and oviposition rates of predatory mites (Koller et al. 2007). Plant quality may also affect the feeding habits of thrips. They may switch to feeding on the supplemental food source or prey (spider mites, predatory mite eggs) when plant quality is reduced (Agrawal et al. 1999; Janssen et al. 2003). Consequently, thrips that feed on a supplemental food source may have a different nutritional quality for predatory mites than thrips that feed on plant tissue. Our study did not indicate a strong negative effect of chrysanthemum metabolites on the oviposition rate of A. swirskii, the rates were similar to those on a diet of thrips larvae from beans (Buitenhuis et al. 2010), and also the oviposition rate of A. swirskii on a diet of thrips in our study was not significantly lower than on a diet of pollen. In addition, some eggs of the predatory mites were killed by thrips larvae (A. Leman, pers. obs. 2013). Therefore, the real oviposition rates of A. swirskii on a diet of thrips larvae was probably higher than reported here.
Summarizing, we conclude that, although supplemental food sources increase the reproduction of thrips and reduce predation rates by individual predatory mites, they still enhance the biological control of thrips through a strong numerical response of the predators. However, supplying extra food in a crop must be applied with caution, because the results may strongly depend on the initial predator–prey ratio, the nutritional quality of the alternative food source, the species of predatory mites, the distribution of the food in the crop and the type of crop.
References
Abrams PA, Holt RD, Roth JD (1998) Apparent competition or apparent mutualism? Shared predation when populations cycle. Ecology 79:201–212
Agrawal AA, Kobayashi C, Thaler JS (1999) Influence of prey availability and induced host-plant resistance on omnivory by western flower thrips. Ecology 80:518–523
Arijs Y, De Clercq P (2001) Rearing Orius laevigatus on cysts of the brine shrimp Artemia franciscana. Biol Control 21:79–83
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Buitenhuis R, Shipp L, Scott-Dupree C (2010) Intra-guild vs extra-guild prey: effect on predator fitness and preference of Amblyseius swirskii (Athias-Henriot) and Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae). Bull Entomol Res 100:167–173
Calvert DJ, Huffaker CB (1974) Predator (Metaseiulus occidentalis)—prey (Pronematus spp.) interactions under sulfur and cattail pollen applications in a noncommercial vineyard. Entomophaga 19:361–369
Castañé C, Quero R, Riudavets J (2006) The brine shrimp Artemia sp as alternative prey for rearing the predatory bug Macrolophus caliginosus. Biol Control 38:405–412
Cocuzza GE, DeClercq P, VandeVeire M, DeCock A, Degheele D, Vacante V (1997) Reproduction of Orius laevigatus and Orius albidipennis on pollen and Ephestia kuehniella eggs. Entomol Exp Appl 82:101–104
Faraji F, Janssen A, Sabelis MW (2002) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
Goleva I, Zebitz CW (2013) Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp Appl Acarol 61:259–283
Holt RD (1977) Predation, apparent competition and structure of prey communities. Theor Popul Biol 12:197–229
Hulshof J, Ketoja E, Vänninen I (2003) Life history characteristics of Frankliniella occidentalis on cucumber leaves with and without supplemental food. Entomol Exp Appl 108:19–32
Janssen A, Willemse E, van der Hammen T (2003) Poor host plant quality causes omnivore to consume predator eggs. J Anim Ecol 72:478–483
Koller M, Knapp M, Schausberger P (2007) Direct and indirect adverse effects of tomato on the predatory mite Neoseiulus californicus feeding on the spider mite Tetranychus evansi. Entomol Exp Appl 125:297–305
Kunkel BA, Cottrell TE (2007) Oviposition response of green lacewings (Neuroptera: Chrysopidae) to aphids (Hemiptera: Aphididae) and potential attractants on pecan. Environ Entomol 36:577–583
Lundgren JG (2009) Relationships of natural enemies and non-prey foods. Progress in biological control, vol 7. Springer, New York
Matsuo T, Mochizuki M, Yara K, Mitsunaga T, Mochizuki A (2003) Suitability of pollen as an alternative diet for Amblyseius cucumeris (Oudeman). Jpn J Appl Entomol 47:153–158
McMurtry JA, Scriven GT (1964) Studies on the feeding, reproduction, and development of Amblyseius hibisci (Acarina: Phytoseiidae) on various food substances. Ann Entomol Soc Am 57:649–655
Messelink GJ, Van Steenpaal SEF, Ramakers PMJ (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. Biocontrol 51:753–768
Messelink GJ, Ramakers PMJ, Cortez JA, Janssen A (2009) How to enhance pest control by generalist predatory mites in greenhouse crops. In: Mason PG, Gillespie DR, Vincent C (eds). Proceedings of the 3rd ISBCA. Christchurch, New Zealand, pp 309–318
Messelink GJ, Sabelis MW, Janssen A (2012) Generalist predators, food web complexities and biological pest control in greenhouse crops. In: Larramendy ML, Soloneski S (eds) Integrated pest management and pest control—current and future tactics. InTech, Rijeka, pp 191–214
Messelink GJ, Bennison J, Alomar O, Ingegno BL, Tavella L, Shipp L, Palevsky E, Wäckers FL (2014) Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. Biocontrol 59:377–393
Nguyen DT, Vangansbeke D, De Clercq P (2014) Artificial and factitious foods support the development and reproduction of the predatory mite Amblyseius swirskii. Exp Appl Acarol 62:181–194
Nomikou M, Sabelis MW, Janssen A (2010) Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol 55:253–260
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2010) GenStat for windows (13th edition). Introduction. VSN International, Hemel Hempstead
Ramakers PMJ, Rabasse JM (1995) Integrated pest management in protected cultivation. In: Reuveni R (ed) Novel approaches to integrated pest management. CRC Press, Florida, pp 199–229
Sabelis MW (1990) How to analyze prey preference when prey density varies? A new method to discriminate between effects of gut fullness and prey type composition. Oecologia 82:289–298
Skirvin DJ, Kravar-Garde L, Reynolds K, Jones J, Mead A, Fenlon J (2007) Supplemental food affects thrips predation and movement of Orius laevigatus (Hemiptera: Anthocoridae) and Neoseiulus cucumeris (Acari: Phytoseiidae). Bull Entomol Res 97:309–315
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20
van Rijn PCJ, Tanigoshi LK (1999) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Expl Appl Acarol 23:785–802
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
Vandekerkhove B, De Clercq P (2010) Pollen as an alternative or supplementary food for the mirid predator Macrolophus pygmaeus. Biol Control 53:238–242
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2014) Performance of the predatory mite Amblydromalus limonicus on factitious foods. Biocontrol 59:67–77
Vantornhout I, Minnaert HL, Tirry L, De Clercq P (2004) Effect of pollen, natural prey and factitious prey on the development of Iphiseius degenerans. Biocontrol 49:627–644
Wade MR, Zalucki MP, Wratten SD, Robinson KA (2008) Conservation biological control of arthropods using artificial food sprays: current status and future challenges. Biol Control 45:185–199
Acknowledgments
This study was funded by the Dutch Ministry of Economic Affairs. Comments by two anonymous reviewers substantially improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leman, A., Messelink, G.J. Supplemental food that supports both predator and pest: A risk for biological control?. Exp Appl Acarol 65, 511–524 (2015). https://doi.org/10.1007/s10493-014-9859-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9859-y