Abstract
Parasitic insects use herbivore induced plant volatiles as signals for host location. However, their responses to these volatiles in the background of natural habitat odours need further evaluation for developing successful biological control strategies. Field elms (Ulmus minor Miller (Ulmaceae)) release a blend of volatiles in response to oviposition of the elm leaf beetle, Xanthogaleruca luteola Müller (Coleoptera: Chrysomelidae), a major urban and forest pest in the USA and Australia. This induced blend attracts the beneficial egg parasitoid Oomyzus gallerucae Fonscolombe (Hymenoptera: Eulophidae). Our olfactory assays showed that an odorous background of non-attractive host plant volatiles from feeding damaged elms or (Z)-3-hexenyl acetate masks the attractive effect of the host-induced (E)-β-caryophyllene to O. gallerucae. Quantitative GC–MS analyses revealed decreased concentrations of (Z)-3-hexenyl acetate accompanied by highly increased concentrations of sesquiterpenes in oviposition and feeding treated elms compared to undamaged elms. This finding hints to how the parasitoid might distinguish between different odorous backgrounds. It is corroborated by the outcome of our field study in natural elm stands, where the egg parasitoid parasitized more host egg masses due to an artificially induced blend of elm terpenoids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insect parasitoids and predators are an alternative to the use of pesticides to control pest organisms in forest, agriculture, or wildland (e.g. van Driesche and Bellows 1996; Hajek 2004; van Driesche 2012). Parasitic and predatory arthropods use herbivore induced plant volatiles as olfactory cues when searching for their herbivorous hosts (e.g. Dicke et al.1990; Arimura et al. 2005). Induced plant defence reactions and the role of induced plant compounds in mediating attraction at the third trophic level have been intensively studied in numerous plant–insect systems for more than 20 years (Dicke and Sabelis 1988; De Moraes et al. 1998; Bruinsma and Dicke 2008; Hilker and Meiners 2011; McCormick et al. 2012). Despite the enormous variety of existing plant volatiles available, parasitoids use relatively few ubiquitous compounds for help in locating their hosts, including terpenoids and green leaf volatiles (GLV), the latter consisting of C6-aldehydes, -alcohols, and their esters (Pichersky and Gershenzon 2002; Arimura et al. 2009). The blend emitted by herbivore infested plants often qualitatively and quantitatively differs from that of uninfested plants (Vet and Dicke 1992; Arimura et al. 2009). Especially in homogeneous tree stands and in agricultural monocultures, parasitoids and predators are faced with the situation that infested host plants stand close to uninfested ones or to host plants infested by non-hosts or by an unsuitable developmental stage of the host. Little is known about how odours from differently infested and uninfested host plants of the same species in close proximity affect the orientation of parasitoids towards the specific blend of induced plant volatiles indicating its host.
Parasitoids are able to recognise host-specific cues present in the highly variable odorous backgrounds generated from plants in the surrounding habitat, as well as from the many abiotic and biotic factors that influence the odour profile and the perception of olfactory signals (Takabayashi et al. 1994; Hilker and McNeil 2008). Habitat odour can overlay the host-indicating odour, but may also serve as a necessary cue to elicit behavioural responses from parasitoids (Schroeder and Hilker 2008; Beyaert et al. 2010). Recent studies trying to elucidate the effects of diverse vegetation on parasitoid orientation observed a decrease in the proportions of parasitoid emergence with increasing plant diversity, most likely accompanied by increased odour diversity (reviewed in Randlkofer et al. 2010; Wäschke et al. 2013).
Many studies on the role of specific herbivore-induced plant volatiles (HIPVs) in natural enemy attraction have ignored the biology and ecology of the involved organisms and whether these have developed in co-evolved multitrophic interactions. Here we focus on the olfactory orientation of Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid that has developed its interactions with its host and the host plant in an entirely natural tritrophic system in the areas around the Mediterranean basin. This wasp is highly specialised to parasitize the eggs of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae) (Meiners et al. 2000). X. luteola in turn is highly specialised on the European field elm Ulmus minor (Ulmaceae), which occurs throughout cultivation in the whole temperate world (Richens 1983). This herbivore can defoliate entire trees and is recognised as a major urban and forest pest in the USA and Australia, which can be controlled by the release of the parasitoid O. gallerucae (Kielbaso and Kennedy 1983; Kwong and Field 1994).
Oviposition by the elm leaf beetle on leaves of elms induces the emission of volatiles that attract O. gallerucae. Oviposition is usually accompanied by feeding damage on leaves by the adults. However, odours from only feeding damaged leaves or from undamaged elm leaves are not attractive to the parasitic wasp (Meiners and Hilker 1997; 2000). Parasitoids like O. gallerucae whose hosts live upon large woody plants (trees) may encounter much higher amounts of background odour compared to parasitoids whose hosts are present on small herbaceous host plants. Therefore the elm system is a very appropriate one to investigate the importance of background odour on parasitoids.
For developing biological control strategies via induced volatiles it is important to know which oviposition-induced volatiles mediate the egg parasitoid’s host location and how this process is affected by the background odour in the elm habitat. However, herbivore-induced volatiles might not attract parasitoids in the same way in the field as observed in the lab (Bernasconi Ockroy et al. 2001). Laboratory studies alone are not sufficient to evaluate the impact of applying parasitoids against herbivores for plant protection in the field (Hunter 2002). In the few natural tree species studied, as well as in crop plants (reviewed in Hilker and Meiners 2011), oviposition-induced blends of volatiles are characterised by increased amounts of terpenoids: (E)-β-farnesene in pine (Mumm et al. 2003; Beyaert et al. 2010), (E)-ß-caryophyllene (EBC) in bean and cabbage (Colazza et al. 2004a, b; Conti et al. 2008), (E)-ß-ocimene in cabbage (Fatouros et al. 2012), and inter alia by (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) in maize varieties (Tamiru et al. 2011). Previous findings demonstrate that O. gallerucae perceives the presence of host eggs by elm terpenoids including EBC, which are induced by egg-deposition and adult feeding. EBC can by itself attract the parasitoids (Büchel et al. 2011). The oviposition-induced attraction of parasitoids can be mimicked by applying jasmonic acid (JA) (via roots) or methyl jasmonate (MeJA) (on leaves) to elms, which leads almost exclusively to the emission of terpenes (in particular sesquiterpenes like EBC and the homoterpene DMNT) (Meiners and Hilker 2000; Meiners unpublished data; Wegener et al. 2001). Elm leaf beetles usually feed on leaves of the same twig where they oviposit, although not necessarily on the same leaf. Thus feeding and oviposition can only be separated experimentally (Meiners and Hilker 2000). It is already known from a quantitative study that the blend of volatiles emitted by elms following egg deposition and feeding consists mainly of GLVs including (Z)-3-hexenyl acetate (Z3HexAc) and terpenoids like (E,E)-α-farnesene and EBC (Büchel et al. 2011). However, the terpenoids that attract the parasitoid of elm leaf beetle eggs are ubiquitous volatile compounds that do not occur singly in nature. The parasitoid will encounter them in mixtures with other compounds emitted from the same or neighbouring trees.
In natural monocultural tree stands (here called “habitat”), parasitoids will often encounter infested host plants in close proximity to uninfested ones, or host plants infested by an unsuitable developmental stage of the host. A major aim of our study was to elucidate how background odours of the elm habitat affect the orientation of O. gallerucae towards oviposition-induced attractive terpenoids. To reach this aim, we conducted the following tests and analyses:
-
1.
We tested the attractiveness of EBC in a laboratory olfactometer in combination with odours from elm uninfested and infested by elm leaf beetles as background odours.
-
2.
We tested how Z3HexAc, the main GLV compound emitted by herbivore-infested elms, influences the parasitoid’s orientation towards the attractive terpenoid EBC.
-
3.
Quantitative volatile analyses of uninfested and infested elms were performed to identify different background odours.
-
4.
To elucidate whether the parasitoids respond to enhanced amounts of terpenoids in the 125 field in the presence of background elm odour we induced enhanced terpenoid emission by 126 spraying elms with MeJA and exposed them with test (sentinel) eggs in natural elm stands.
Methods
Insects and plants
Parasitoids
We collected elm leaves with parasitised beetle eggs in May in the years 2008–2011 in the environs of Montpellier, Perpignan (France), and Parlavà (Spain), since we did not succeed in rearing beetles and parasitoids in sufficient number and quality (size, longevity) during winter. O. gallerucae Fonscolombe (Hymenoptera: Eulophidae) enclosing from the eggs were kept in Petri dishes with a moistened filter paper in a climate chamber (10 °C, 70 % r.h., L16:D8) and fed with diluted honey. A few days prior to testing, the parasitoids were transferred to warmer conditions in a climate chamber (22 °C, 70 % r.h., L16:D8).
Beetles
Adults of the elm leaf beetles X. luteola Müller (Coleoptera: Chrysomelidae) were also collected in the spring in France and Spain. Beetles and larvae were subsequently reared for experiments in the laboratory in cages (40 × 40 × 70 cm) on elm plants in the greenhouse (20–40 °C, 40–50 % r.h., 150 μmol m−2 s−1 PAR, L16:D8). Pupae were transferred in transparent plastic boxes (20 × 20 × 6 cm) for hatching in the climate chamber (see above).
Plants
Shoots of the European field elm, Ulmus minor Miller (Ulmaceae) were grown in tissue culture throughout the year. All plants originated from a shoot culture of a single genotype of the field elm, referred to as U. minor cv. ‘Dahlem’. To rear mature plants, shoots were transferred individually into plastic pots (11 × 11 × 12 cm) filled with potting soil (type T, Kausek GmbH, Germany) and grown in a climate chamber (20 °C, 55 % r.h., 200 μmol m−2 s−1 PAR, L16:D8) (detailed description is provided by Büchel et al. 2011). Head space collection and olfactory assays were conducted with two-month-old elm plants with approximately 15 leaves. The height of the plants used for experiments was approximately 30 cm. The field assay was conducted with two-year-old elm plants of 70–110 cm height (K. Appel GmbH, Germany), grown in plastic pots (20 cm Ø) filled with potting soil (N2 type 1, Klasman-Dellmann GmbH, Germany) and grown in the greenhouse (15–30 °C, 60–80 % r.h., 150 μmol m−2 s−1 PAR, L16:D8).
Plant infestation by beetles
Elm plants were infested with X. luteola adults for 48 h, since elms are known to respond to elm leaf beetle infestation by releasing synomones attractive to egg parasitoids after that period (e.g. Büchel et al. 2011). For the “feeding only” treatment only male beetles were allowed to feed on the plants and for the treatment “egg deposition and feeding” only female beetles were allowed to lay eggs and to feed on the plant. Seven to 15 beetles (depending on their feeding or egg laying activity ascertained from the rearing at that time) were kept within micro perforate plastic bags (180 × 350 mm, Weber Packaging GmbH, Germany) on each treated elm plant. Feeding damage was measured (see Meiners et al. 2005 for details) and the extent of damage (10–15 % of the total leaf area) did not differ between feeding and egg+feeding treatments. During treatments all plants were kept in a climate chamber (Sneijder, UK) (23 °C, 55 % r.h., 200 μmol m−2 s−1 PAR, L16:D8).
Olfactometer assays
Parasitoid behaviour was tested in a four-arm airflow olfactometer (details described in Büchel et al. 2011). The olfactometer arena (30 × 30 × 1.2 cm) in which the parasitoids could walk around was illuminated from above by a neon lamp screened with glassine paper and providing 16 μmol m−2 s−1 PAR. The glass cylinders (250 mL) with the odour sources were illuminated from above with neon tubes of type “daylight” (40 μmol m−2 s−1 PAR). Charcoal-filtered, humidified air flowed at 150 ml min−1 through four glass flasks containing the odour sources and then into the arena from the four edges establishing four distinct odour fields. Two opposite odour test fields were supplied with air from synthetic reference compounds either alone or in combination with treated plants, whereas the other two opposite fields of the olfactometer served as control fields and were supplied with control air after the solvent (n-hexane, Promochem, Germany) had evaporated. The plants as odour sources for test and control fields were renewed after testing the responses of six parasitoids. Thus, in total, 5–7 plants were used per treatment. There were no statistical differences in the parasitoids’ responses between the individual trees (Kruskal–Wallis tests, data not shown). The individual reference compounds were renewed after each tested parasitoid. A range of 23–32 parasitoids were tested per test odour and used as individual data points.
When a plant was to be used as test odour source, a potted elm plant was placed in a glass cylinder (250 ml) with an open bottom. The bottom was enclosed around the plant stem with help of a polyvinyl acetate oven bag to exclude the pot and soil. When testing synthetic reference compounds alone, the filter paper was placed in a conical glass flask (250 ml). The synthetic reference compounds EBC (Sigma-Aldrich; 80 % purity) and Z3HexAc (Sigma-Aldrich; 98 % purity) were spotted (10 ng in 10 μl hexane each) singly or in combination (final volume 20 μl) on a filter paper (94 mm Ø, Melitta, Minden, Germany) that was placed after solvent evaporation (20 s) into the glass flask together with the plant. Emission rates from these filter papers were within the range of the average rates of emission of the respective volatile compounds from elm plants, rates to which parasitoids responded (Büchel et al. 2011). The experiments were performed at 22–25 °C and 70–80 % r.h.
Bioassays were initiated by placing a female parasitoid into the centre of the olfactometer arena. For a period of 300 s, we recorded the parasitoid’s residence time in each of the four olfactometer fields using the Observer programme 3.0 (Noldus, Wageningen, Netherlands).
Plant volatile collection
Volatiles emitted by U. minor after feeding, feeding and egg deposition or control treatment were collected using an open loop dynamic headspace technique sampling device located in a growth chamber (20 °C, 55 % r.h., 350 μmol m−2 s−1). In total, five (control, C), 13 (feeding, F) and 17 (egg and feeding, EF) repetitions were used. 48 h after the start of the treatments, each potted elm plant (30 cm) was placed individually in an 800 ml glass vessel (Schott, Germany, 12 cm Ø), with the soil in the pot being excluded by two parts of a Teflon® disk (with a hole for the stem). The air inlets consisted of two Teflon® valves on the top of the vessel allowing humidified and dry air (1:1) previously purified through activated charcoal to enter the vessel. The air outlet consisted of one Teflon® valve on the lower side of the vessel that allowed the air to pass through a volatile collection filter filled with 5 mg charcoal (Gränicher and Quartero, Daumazan, France). The incoming air flux was adjusted to 1.5 l min−1 and the outgoing air flux to 1.2 l min−1 so that the slight overpressure in the system prevented contamination with unfiltered air entering from outside. The flow through the glass vessels was controlled by flowmeters (Supelco, Bellefonte, PA, USA). All collections were performed within a 6-h-timeframe from 9 am to 3 pm to reduce differences due to possible diurnal rhythm of volatile emission. Plant volatiles were extracted from the charcoal filter with 12.5 μl dichloromethane (Roth) containing 25 ng μl−1 n-tridecane (Sigma Aldrich) as an internal standard.
Chemical analysis of plant volatiles
Volatiles were identified with a gas chromatograph (Agilent Hewlett–Packard 6890, Agilent Technologies) coupled to a mass spectrometer (Agilent Hewlett–Packard 5973, Agilent Technologies) with a quadrupole mass selective detector (ionization potential, 70 eV, scan range of m/z 50–350). We used splitless injection (injection temperature: 220 °C, injection volume: 1 μl). The volatiles were separated on a DB-5MS column (DB-5MS 30 m × 0.25 mm × 0.25 μm film, J and W Scientific, Folsom, USA), with the carrier gas helium (2 ml min−1). The temperature program started at 40 °C (3 min hold), and temperature increased to 300 °C (2 min hold) with a first gradient of 5 °C min−1–210 °C and a second gradient of 60 °C min−1–300 °C. Individual volatile compounds were quantified by calculating the peak area of the compound relative to the peak area for the internal standard. The volatile amounts were standardised by calculation in ng gFW−1 (fresh weight of the leaves). For identification, the mass spectra and linear retention index (van Den Dool and Kratz 1963) of the individual compounds were compared with commercially available standards (sabinene [Roth, ≥96 % purity], α-copaene (≥90 %), caryophyllene oxide (≥99 %), α–terpinolene (≥95 %), α-terpineol (≥97 %), (E)-nerolidol, α–humulene (≥96 %) [Fluka] and phenylacetonitrile [Sigma-Aldrich, ≥98 %], or with the mass spectra libraries (Wiley 6.1 and NIST 98.1) using GC/MSD software ChemStation (Agilent). DMNT was a gift (synthesised and kindly provided by Stefan Schulz, Technical University Braunschweig, Germany). Germacrene D was not available as an authentic compound and was identified using the mass spectral libraries. Only compounds that were detected in at least 50 % of the replicates of egg- and feeding-induced elm plants were included in the statistical analysis (other compounds were marked as “traces” in Table 1).
Field experiment
To test whether the attractiveness of elm odour towards O. gallerucae in the field after terpenoid emission was enhanced by MeJA treatment, elm plants were treated in two steps. First, plants with undamaged leaves were sprayed on the lower half of the plant with 30 ml (each plant) of an aqueous solution of MeJA (1 μmol/ml−1; Sigma, Germany; 95 % pure). The induction was conducted in the lower half, because it is known that the defence reaction (odour induction) is systemic and proceeds upwards. MeJA treated plants and untreated control plants (15 repetitions respectively) were placed for 40 h in separate climate chambers (23 °C, 70 % r.h., L16:D8). Afterwards, ten female beetles each were allowed to lay eggs and to feed for 24 h in the upper half of the MeJA treated plants and the formerly untreated control plants by containing them with frost protection fleece. Leaves with egg clutches were marked for later collection by small metal clips.
MeJA-induced plants and control plants with eggs were transferred to four naturally grown, X. luteola-infested, 2–4 m high elm stands near Montpellier (Southern France) that were located in close distance (2–4 km) to each other outside Prades-le-Lèz (43.69386°N, 3.87177°E), outside Assas (43.67982°N, 3.89995°E), and outside (43.69381°N, 3.92911°E) and in (43.67364°N, 3.91566°E) Teyran. This proximity allowed to expose and to collect the experimental trees on time as well as to obtain similar abiotic conditions. The field experiment was conducted once within one week July/August with no precipitation, 1010–1017 hPa atmospheric pressure, 16–22 °C lower temperature limit, 26–32 °C upper temperature limit, 36–72 % r.h., and L14.5:D9.5. One to five of the 15 test and 14 control trees each were distributed between the four locations according to stand size and positioned randomly at 0.5 m distance to X. luteola-infested elm trees and uninfested trees to provide a natural background odour for O. gallerucae. The distance between the experimental plants was at least 4 m. After 48 h leaves with egg clutches of the experimental plants were collected, eggs were dissected under a stereomicroscope, and the parasitisation rate was determined.
Statistical analysis
The statistics were performed using Statistica (StatSoft Inc. 1999, Tulsa, USA). The Wilcoxon one-sample test evaluated whether the time spent by the parasitoids in the two test odour fields differed significantly from the null hypothesis (150 s (minus time spend in the neutral centre of the arena) for experiments in the four arm-olfactometer assuming equally long residence times in all four olfactometer fields during an observation period of 300 s).
Differences in quantities of individual compounds among the different treatments were compared by Kruskal–Wallis ANOVA and Mann–Whitney U post-hoc tests corrected by Benjamini-Hochberg. Normal distribution and homogeneity of variance were tested by Shapiro–Wilk and by Levene’s test, respectively. Additionally we analysed the chemical dataset by a principal component analysis (PCA) (Wold et al. 1987) using the software program SIMCA-P 10.5 (Umetrics AB, Umea˚, Sweden). The PCA converts the data variables to a lower-dimensional plane (score) formed by the principal components (PCs) and visualises the structure of the investigated data in a score plot. The results of a PCA are usually discussed in terms of the loading plot, which describes the relationships between the variables with regard to the PCs (Eriksson et al. 2001). Raw data (integrated peak areas) of 20 analysed compounds were normalised to the internal standard and corrected for the fresh weight of the aerial parts of the plant (Table 1).
Experimental field parasitisation data were analysed by 2 × 2 χ²–tests. Due to the low number of trees at each stand and the proximity between stands, we pooled the trees for each treatment in the analysis and treated each tree as replicate analysing how many trees of each group carried parasitized eggs.
Results
Plant background odour affects attractiveness of a sesquiterpene to parasitoids
Previous studies demonstrated that the odour of (E)-β-caryophyllene (EBC) and the odour of elms on which X. luteola was allowed to feed and lay eggs (EF) each attract O. gallerucae (Büchel et al. 2011), whereas odours from only feeding damaged (F) or from undamaged (C) elm leaves were not attractive to the parasitic wasp (Meiners and Hilker 1997; 2000). Here we observed that EBC attracted O. gallerucae when presented in combination with odour from EF elms (Z = 2.93, n = 29, p = 0.003) and in combination with odour from C elms (Z = 2.5, n = 30, p = 0.012) (Fig. 1). The combination of EBC and odour from F elm plants was marginally not attractive to the parasitoids (Z = 1.9, n = 32, p = 0.058). The wasps also did not respond to the combination of EBC and Z3HexAc (Z = 1.72, n = 21, p = 0.085), the most abundant GLV released by beetle infested elms (EF+F), and not to the GLV alone (Z = 1.52, n = 23, p = 0.128).
Olfactometer residence times of Oomyzus gallerucae in odour of (E)-ß-caryophyllene (EBC) combined with odours from Ulmus minor plants treated by Xanthogaleruca luteola oviposition and feeding (EF) (n parasitoids = 29), untreated (C) (n = 30) and by X. luteola feeding (F) (n = 32). Odour of (Z)-3-hexenyl acetate (Z3HexAc) was also tested with EBC (n = 21) and alone (n = 23). Dark columns = natural elm odour, light columns = pure compounds, mean expected value (dashed line = 147 s ± 0.5 SE) is shown. Wilcoxon one sample test: * P ≤ 0.05, ** P ≤ 0.01, ns P > 0.05
Volatile analyses reveal differences in the odour of uninfested and beetle infested elms
Background odours from uninfested elms and from elms infested in different ways have varying effects on the orientation of the egg parasitoids to the attractive terpenoid EBC. To reveal any divergence in their volatile profiles, we compared absolute and relative amounts of the volatiles emitted from elms subject to different treatments. In the odour blend of U. minor plants infested with elm leaf beetles we detected in total 28 identifiable and 16 unidentifiable compounds. We present here only detail data for the 20 identifiable compounds that occurred in quantifiable amounts (Table 1), including four aromatics, five GLVs, one homoterpene, ten monoterpenes and eight sesquiterpenes. Plant treatment by F and EF caused an up to six-fold increase in the total amount of volatiles emitted and a significant increase in the absolute amounts of GLVs and terpenes (sesqui- and homoterpenes). Phenylacetonitrile, germacrene D, (Z)-3-hexenyl benzoate and caryophyllene oxide were released from herbivore-treated elm plants (EF and F) and were not present in the odour of untreated elm plants. The quantitative emission of six individual volatile compounds (DMNT, myrcene, EBC, α–humulene, (Z,E)-α-farnesene, and (E,E)-α-farnesene) was much higher (for example, up to 58-fold for EBC) in herbivore-treated elm plants (EF, F) in comparison to control plants (C). However, the differences in the composition of the blends from EF and F-treated plants were very small, even for (E)-2-hexenyl acetate, which was released only from EF plants in significantly higher amounts compared to untreated elm plants (Table 1). Since no separation was possible between EF and F on the basis of absolute amounts, we next analysed the relative amounts of the emitted compounds.
Considering the relative percentage of each compound in the total blend of emitted volatiles, the sesquiterpenes EBC and (E,E)-α-farnesene together represent up to 70 % of the total emitted from elm plants where elm leaf beetles had been allowed to feed and to lay eggs (EF) (Fig. 2a). Compared to control plants, the largest increase could be observed for EBC, which rose from 2 % in controls to 21 % in EF-treated plants, whereas no significant change was observed for (E,E)-α-farnesene. The strong increase in the emission of EBC was accompanied by a significant reduction in the relative emission of the third most abundant volatile Z3HexAc, which decreased from 13 % in control plants to 5 % after treatments EF and F. This pattern of a shifted ratio between EBC and Z3HexAc is mirrored in the ratio between sesquiterpenes and GLVs (Fig. 2b, c). When looking at the relative emissions of aromatic compounds, no differences can be seen between the different treatments, while for monoterpenes a significant decrease for EF and F in comparison to C can be seen. Again, no difference is detectable between EF and F.
Relative amounts (% of the total emission) of volatile compounds from untreated U. minor plants (C, n = 6), from plants treated with feeding X. luteola (F, n = 13), and from plants treated with X. luteola oviposition and feeding (EF, n = 17). a single compounds; b compound classes; c: compound ratios. Mean (±SE) is given. Different letters above columns indicate significant differences at *P ≤ 0.05 (Mann–Whitney-U-test corrected by Benjamini-Hochberg). (E)-ß-caryophyllene (EBC); (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT); green leaf volatiles (GLV); (Z)-3-hexenyl acetate (Z3HexAc)
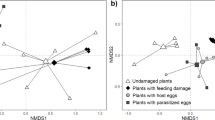
A principal component analysis (PCA) was used to compare the volatile patterns of the differently treated U. minor plants with respect to the relative quantities of the 20 volatile compounds. The results are visualised in a score (scatter) plot and a loading plot (Fig. 3a, b). A total of 56 % of the variance of the data is significantly explained by the first (PC1-36.2 %) and the second (PC2-21.8 %) principal components. The score plot of the relative amount of volatile compounds of the three different treatments showed that untreated elm plants (C) cluster together based on their chemical profiles, whereas feeding-induced plants (F) cannot be separated from oviposition- and feeding treated plants (EF). Control plants mainly separated from the other treatments on the axis of the first principal component (Fig. 3a). In the loading plot, unimportant variables are generally located towards the zero origin and the more important variables are located on the periphery of the plot. The location of EBC on the left side and Z3HexAc on the right side of the PC1 axis indicate important variables which are responsible for the separation of blends from beetle infested plants (EF, F) to that of untreated (C) plants. Other compounds, which increased significantly after beetle infestation in comparison to blends from the control plants are α–humulene, caryophyllene oxide, phenylacetonitrile, germacrene D and (Z)-3-hexenyl benzoate. These are located on the left side in the periphery of the plot. The PCA presented here reveals a strong shift in the relative proportion of the compounds from the elm blend due to insect feeding and oviposition.
Analysis of the volatile pattern of differently treated U. minor plants. Treatments: (squares) untreated U. minor plants (n = 6); (circles) Xanthogaleruca luteola feeding-treated U. minor plants (n = 13); (triangles) X. luteola oviposition- and feeding-treated U. minor plants (n = 17). a: Score plot and b: loading plot from principal component analysis (PCA) based on relative amounts of 20 volatile compounds (% of the total emission of compounds). A total of 58 % of the variance in the data is explained by the two significant principal components, as judged by cross-validation. The ellipse shown in the score plot defines the Hotelling’s T2 confidence region (95 %). *compound was shown to be attractive, ⊖compound was shown to be not attractive for the egg parasitoid Oomyzus gallercucae
Terpenoid emission enhances parasitisation in natural elm stands
In order to elucidate whether an enhanced emission of terpenoids really leads to an enhanced rate of parasitisation in natural elm stands, we tested the attractiveness of MeJA-induced elm towards O. gallerucae in the presence of the natural habitat odour of X. luteola-infested elm stands. It had already been established that elm leaves emit a blend of volatiles that consists almost exclusively of terpenoids when treated with JA. Major components are EBC and DMNT, and (E)-β-ocimene, α-humulene, germacrene D, γ-cadinene, and (E,E)-α-farnesene are present in minor amounts (Wegener et al. 2001). After 48 h exposure, egg clutches on all 15 MeJA treated trees had been parasitised, while the egg masses on four out of the 14 control trees were unparasitised (χ² = 4.97, p < 0.026). One control plant has been lost probably due to wild animals. 77 % of the 85 egg clutches retrieved from MeJA-induced and EF-treated elm plants were parasitised while only 55 % of the 54 egg masses retrieved from control plants, that were only treated by EF, were parasitized. The experimental plants did hardly experience additional beetle infestation (eggs or feeding) during exposure. To exclude the possibility that the number of egg masses laid on a plant had an influence on the parasitisation rate, we performed a regression analysis between the number of egg masses laid on a plant and the parasitisation rate, which revealed no relationship (R² = 10−5). Thus, the enhanced emission of terpenoids leads to an enhanced rate of parasitism in natural elm stands in the field.
Discussion
Our study demonstrated that O. gallerucae does perceive the background of uninfested trees, but is still able to orient to the eggs of its host. However, an odorous background of non-attractive host plant volatiles from feeding damaged elms or most abundant GLV (Z)-3-hexenyl acetate masks the attractive effect of the host-induced EBC to O. gallerucae. Volatile analysis revealed that the odour of X. luteola-infested oviposition and feeding treated elms was different from that of undamaged elms chiefly in the proportional reduction of Z3HexAc accompanied by a strong increase in sesquiterpenes such as EBC. We corroborated the ability of O. gallerucae to orientate towards X. luteola eggs on elms with increased sesquiterpene emission by the outcome of our field study. In natural elm stands of elms, where they were additionally exposed to the surrounding background odours from uninfested elms as well as from elms infested by different larval stages of the beetle, we demonstrated that the egg parasitoid parasitized more host egg masses due to an artificially induced blend of elm terpenoids. The results are consistent with our previous findings that O. gallerucae is attracted by individual terpenes such as the sesquiterpenes, EBC, (E,E)-α-farnesene, and α-humulene, and the homoterpene DMNT (Büchel et al. 2011).
Non-host background odour may sometimes mask the host-indicating odour of plants (Schroeder and Hilker 2008; Bruce and Pickett 2011). In our study the odour of elms subject to X. luteola feeding masked the attractive effect of the key host-induced sesquiterpene, EBC, towards O. gallerucae. Parasitoids of insect larvae such as Exorista japonica (Ichiki et al. 2008) and Cotesia marginiventris (Fontana et al. 2011) have been shown to be capable of recognizing background odours and to use them in combination with specific key odours in orientation. At present we lack sufficient knowledge of the insect olfactory system to understand why insect parasitoids can sometimes identify the relevant host plant odour cues against a background while other times components from the host plant blend are not recognised as host cues when perceived outside the context of that blend. However, sensory complexity may play a role and recent research suggests that less diverse vegetation structures can better facilitate host location of parasitoids than more diverse structures (Bezemer et al. 2010; Wäschke et al. 2013).
Olfactory backgrounds can also be necessary for the recognition of individual compounds as it was shown for the specialized egg parasitoid, Closterocerus ruforum. When searching for its host, the sawfly Diprion pini on Pinus sylvestris trees, this species is attracted to the sesquiterpene (E)-ß-farnesene, a compound emitted by egg-induced P. sylvestris. However, this attraction is also contingent upon the presence of the background odour of P. sylvestris (Mumm and Hilker 2005). An enhanced ratio of (E)-ß-farnesene with respect to other constitutive background pine volatiles has been suggested to be responsible for the orientation of C. ruforum to the eggs of its host (Beyaert et al. 2010).
Volatile terpenoids are frequently used as olfactory signals by insect egg parasitoids when searching for their herbivorous hosts (Colazza et al. 2004a, b; Beyaert et al. 2010; Bruce et al. 2010). In this study, we showed that elm trees infested with eggs and feeding stages of X. luteola release a volatile blend characterised by a strongly increased amount of terpenoids and some GLVs. Although Ulmus minor elms exhibit high variation in their volatile emission profiles (Wegener et al. 2001; Büchel et al. 2011; this study), we could show that the profiles from uninfested elms cluster together and can be separated from the profile of beetle-infested elm trees. In particular, the increased ratio of EBC in comparison to other compounds in beetle infested elm plants is responsible for this separation. In most plants species studied, herbivore infestation causes a strong increase in the overall amount of emitted volatiles accompanied by a shift in the ratios between certain volatile compounds which parasitoids can use for their orientation towards HIPVs (Turlings and Wackers 2004; Mumm and Dicke 2010).
GLVs represent another important group of herbivore-induced volatiles that could be used by egg parasitoids for orientation (Reddy et al. 2002; Penaflor et al. 2011). GLVs are typically released by plants after damage, and are often the first compounds released after herbivore attack (Matsui 2006; Schaub et al. 2010) and might cause the attraction of parasitoids in the field (James and Grasswitz 2005). However, in the tritrophic interaction between elm, elm leaf beetle eggs and egg parasitoids, we suggest that Z3HexAc (and possibly other GLVs) plays a role as a masking compound, rather than an attractive one. Although this substance is one of the most abundant host plant volatiles, it is unattractive by itself and, when presented in a mixture with EBC, Z3HexAc masked the host-indicating effect of this key terpenoid volatile. In fact, the significant reduction of Z3HexAc in relation to total volatiles in the odour of EF elms (Fig. 2a) in comparison to uninfested elms, could be responsible for the attractiveness of the EF odour (Meiners and Hilker 1997, 2000) either alone or in combination with EBC. Moreover, artificially damaged elms, which show an increased emission of Z3HexAc (Büchel and Meiners unpublished data), are repellent to O. gallerucae (Meiners and Hilker 1997). In another biological system, a marked reduction in emission of Z3HexAc after stemborer oviposition on African grass was shown to play an important role in the attraction to the braconid larval parasitoid, Cotesia sesamiae (Bruce et al. 2010).
Although the behaviour of herbivore parasitoids is often correlated with chemical changes in plant emission profiles as in our case (EF or F vs C), sometimes no significant changes in volatile emissions can be detected by standard GC–MS analysis (as in our case EF vs F) (McCormick et al. 2012 and references therein). This may be caused by a high variability in the emission of compound mixtures even in clonal plants (Büchel et al. 2011) caused by differing abiotic and biotic influences. For example, for caterpillars feeding on maize inbred lines growing in the field, neither total volatile emission nor any specific single compound within the blend could convincingly explain the differential parasitisation rates (Degen et al. 2012). Slight differences in emissions that are not detectibly significant may be important for the differential attraction of parasitoids (Gols et al. 2011). It is also possible that researchers, including ourselves, have completely missed the detection of further volatile compounds that play a key role in mediating attraction of parasitoids due to the limitations of the chemical methodology employed. Loivamäki et al. (2008) found that adding isoprene to odour of herbaceous plants had a repellent effect on the parasitoid Diadegma semiclausum, but not on Cotesia rubecula, which does not perceive isoprene.
When studying the effect of odours on parasitoids it is important to measure not just attraction, but also the parasitisation rate in the field, since attraction might not necessarily lead to parasitisation (reviewed in Meiners and Peri 2013). Roland et al. (1995) demonstrated that the application of borneol in an apple orchard increased the density of the tachinid fly Cyzenis albicans (Fall.), a parasitoid of the winter moth, Operophtera brumata L. (Lepidoptera: Geometridae), but not its parasitisation rate. In a semi-field study Qiu et al. (2012) demonstrated higher parasitism rates of P. brassicae larvae on shoot-JA induced cabbage plants and the parasitoid Cotesia glomerata visited JA-induced plants more often than controls. In this study, we demonstrated that increased terpenoid emission from MeJA-induced elm trees in the field caused a higher parasitisation rate of X. luteola eggs by O. gallerucae from the second generation. It needs to be tested in future studies whether the first (=overwintering) generation of parasitoids responds differently, because of e.g. different background odours, levels of natural infestation, and plant age. To get a more complete picture on the importance of background odours in the field these stand factors should moreover be varied in future studies on different parasitoid populations with varying density.
The use of phytohormone elicitors such as jasmonate has often been employed for attracting predators and parasitoids of pests since this treatment typically triggers plants to produce endogenous volatiles in quantities that are comparable to those induced by herbivore feeding (Rohwer and Erwin 2008; Simpson et al. 2011). The attraction of natural enemies to jasmonate-induced plant volatiles under field conditions has been demonstrated for tomato, tobacco, and maize plants (Kessler and Baldwin 2001; Thaler et al. 2001; Ozawa et al. 2008). Von Mérey et al. (2012) found that application of MeJA increased the emission of sesquiterpenes in maize seedlings in the field, yet with marginal effects on parasitism rates. In elms, the oviposition-induced attraction of parasitoids can be mimicked by applying JA (or MeJA), which is known to induce an attractive blend of volatiles that consists almost exclusively of terpenoids like EBC and DMNT (Wegener et al. 2001).
Studies investigating parasitoid orientation to volatiles too often ignore natural interactions involving wild plants, herbivores and parasitoids (Gols and Harvey 2009; Wäschke et al. 2013). A focus on crop plants might oversee that volatile attractants or masking compounds for parasitoids can be completely absent in certain commercial plant varieties (e.g., Tamiru et al. 2011). In addition, constitutive or herbivore-induced volatiles do not always attract parasitoids in the same way in the field as observed in the lab. Our study indicates how host location in a wild plant-herbivore-parasitoid interaction might proceed under natural conditions. First, we could show that O. gallerucae is able to locate elms in the field when they release key induced volatiles. Second, the response of this egg parasitoid to the key induced volatiles was demonstrated to be affected by the odorous background. Additional progress in understanding how parasitoids effectively exploit cues for host plant discrimination requires further attention to the ecological context of such interactions, especially regarding background odour. This is necessary to optimize integrated pest management or novel biological control measures using host plant volatiles to attract insect parasitoids of agricultural and forest pests.
References
Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1734:91–111
Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Bernasconi Ockroy ML, Turlings TCJ, Edwards PJ, Fritzsche-Hoballah ME, Ambrosetti L, Bassetti P, Dorn S (2001) Response of natural populations of predators and parasitoids to artificially induced volatile emissions in maize plants (Zea mays L.). Agric For Entomol 3:201–209
Beyaert I, Wäschke N, Scholz A, Varama M, Reinecke A, Hilker M (2010) Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim Behav 79:1077–1086
Bezemer T, Harvey J, Kamp A, Wagenaar R, Gols R, Kostenko O, Fortuna T, Engelkes T, Vet L, van der Putten W, Soler R (2010) Behaviour of male and female parasitoids in the field: influence of patch size, host density, and habitat complexity. Ecol Entomol 35:341–351
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochem 72:1605–1611
Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR (2010) Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol Lett 6:314–317
Bruinsma M, Dicke M (2008) Herbivore-induced indirect defense: from induction mechanisms to community ecology. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, Berlin, Germany, pp 31–60
Büchel K, Malskies S, Mayer M, Fenning T, Gershenzon J, Hilker M, Meiners T (2011) How plants give early herbivore alert: volatile terpenoids attract parasitoids to egg-infested elms. Basic Appl Ecol 5:403–412
Colazza S, Fucarino A, Peri E, Salerno G, Conti E, Bin F (2004a) Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J Exp Biol 207:47–53
Colazza S, McElfresh JS, Millar JG (2004b) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30:945–964
Conti E, Zadra C, Salerno G, Leombruni B, Volpe D, Frati F, Marucchini C, Bin F (2008) Changes in the volatile profile of Brassica oleracea due to feeding and oviposition by Murgantia histrionica (Heteroptera: Pentatomidae). Eur J Entomol 105:839–847
De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Degen T, Bakalovic N, Bergvinson D, Turlings TCJ (2012) Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore-induced volatile emissions. PLoS One 7(10):e47589
Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38:148–165
Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990) Plant strategies of manipulating predator-prey interactions through allelochemicals - prospects for application in pest control. J Chem Ecol 16:3091–3118
Eriksson L, Johansson E, Kettaneh-Wold N, Wold S (2001) Multi- and megavariate data analysis; principles and applications. Umetrics Academy, Sweden
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One 7(8):e43607
Fontana A, Held M, Fantaye CA, Turlings TCJ, Degenhardt J, Gershenzon J (2011) Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J Chem Ecol 37:582–591
Gols R, Harvey JA (2009) Plant-mediated effects in the Brassicaceae on the performance and behaviour of parasitoids. Phytochem Reviews 8:187–206
Gols R, Bullock J, Dicke M, Bukovinszky T, Harvey J (2011) Smelling the wood from the trees: non-linear parasitoid responses to volatile attractants produced by wild and cultivated cabbage. J Chem Ecol 37:795–807
Hajek A (2004) Natural enemies: an introduction to biological control. Cambridge University Press, Cambridge, UK
Hilker M, McNeil J (2008) Chemical and behavioural ecology in insect parasitoids: how to behave optimally in a complex odourous environment. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioural ecology of insect parasitoids: from theoretical approaches to field applications. Blackwell, Oxford, UK, pp 92–112
Hilker M, Meiners T (2011) Plants and insect eggs: how do they affect each other? Phytochem 72:1612–1623
Hunter MD (2002) A breath of fresh air: beyond laboratory studies of plant volatile-natural enemy interactions. Agric For Entomol 4:81–86
Ichiki R, Kainoh Y, Kugimiya S, Takabayashi J, Nakamura S (2008) Attraction to herbivore-induced plant volatiles by the host-foraging parasitoid fly Exorista japonica. J Chem Ecol 34:614–621
James DG, Grasswitz TR (2005) Synthetic herbivore-induced plant volatiles increase field captures of parasitic wasps. BioControl 50:871–880
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Kielbaso JG, Kennedy MK (1983) Urban forestry and entomology: a current appraisal. In: Frankie GW, Koehler CS (eds) Urban entomology: interdisciplinary perspectives. Praeger Science, New York, USA, pp 423–440
Kwong RM, Field RP (1994) Elm leaf beetle history and distribution in southern Victoria. Plant Protect Quart 9:43–47
Loivamaki M, Mumm R, Dicke M, Schnitzler J-P (2008) Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc Natl Acad Sci USA 105:17430–17435
Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
McCormick CA, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Meiners T, Hilker M (1997) Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). Oecologia 112:87–93
Meiners T, Hilker M (2000) Induction of plant synomones by oviposition of a phytophagous insect. J Chem Ecol 26:221–232
Meiners T, Peri E (2013) Chemical ecology of insect parasitoids: essential elements for developing effective biological control programmes. In: Wajnberg E, Colazza S (eds) Recent advances in chemical ecology of insect parasitoids. Wiley-Blackwell, Oxford, UK, pp 191–224
Meiners T, Westerhaus C, Hilker M (2000) Specificity of chemical cues used by a specialist egg parasitoid during host location. Entomol Exp Appl 95:151–159
Meiners T, Hacker N, Anderson P, Hilker M (2005) Response of the elm leaf beetle to host plants induced by oviposition and feeding: The infestation rate matters. Entomol Exp Appl 115:171–177
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 88:628–667
Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30:337–343
Mumm R, Schrank K, Wegener R, Schulz S, Hilker M (2003) Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J Chem Ecol 29:1235–1252
Ozawa R, Shiojiri K, Sabelis M, Takabayashi J (2008) Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomol Exp Appl 129:189–199
Penaflor M, Erb M, Miranda LA, Werneburg AG, Bento JMS (2011) Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol 37:1304–1313
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Qiu BL, van Dam NM, Harvey JA, Vet LEM (2012) Root and shoot jasmonic acid induction differently affects the foraging behavior of Cotesia glomerata under semi-field conditions. BioControl 57:387–395
Randlkofer B, Obermaier E, Hilker M, Meiners T (2010) Vegetation complexity - The influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl Ecol 11:383–395
Reddy GVP, Holopainen JK, Guerrero A (2002) Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J Chem Ecol 28:131–143
Richens RH (1983) ELM. Cambridge University Press, Cambridge, UK
Rohwer CL, Erwin JE (2008) Horticultural applications of jasmonates: A review. J Hort Science Biotechnol 83:283–304
Roland J, Denford KE, Jiminez L (1995) Borneol as an attractant for Cyzenis albicans, a tachinid parasitoid of the winter moth, Operophtera brumata L (Lepidoptera, Geometridae). Can Entomol 127:413–421
Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A (2010) Real-time monitoring of herbivore induced volatile emissions in the field. Physiol Plant 138:123–133
Schroeder R, Hilker M (2008) The relevance of background odor in resource location by insects: a behavioral approach. Bioscience 58:308–316
Simpson M, Gurr GM, Simmons AT, Wratten SD, James DG, Leeson G, Nicol HI (2011) Insect attraction to synthetic herbivore-induced plant volatile-treated field crops. Agric For Entomol 13:45–57
Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant mite interactions - variation caused by biotic and abiotic factors. J Chem Ecol 20:1329–1354
Tamiru A, Bruce TJA, Woodcock CM, Caulfield JC, Midega CAO, Ogol CKPO, Mayon P, Birkett MA, Pickett JA, Khan ZR (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14:1075–1083
Thaler JS, Stout MJ, Karban R, Duffey SS (2001) Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol Entomol 26:312–324
Turlings TCJ, Wackers FL (2004) Recruitment of predators and parasitoids by herbivore-injured plants. In: Cardé RT, Millar GJ (eds) Advances in insect chemical ecology. Cambridge University Press, Cambridge, UK, pp 21–75
van Den Dool H, Dec. Kratz P (1963) A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr 11:463–471
van Driesche RGV (2012) The role of biological control in wildlands. BioControl 57:131–137
van Driesche RGV, Bellows TS (1996) Biology of arthropod parasitoids and predators. In: van Driesche RGV, Bellow TS (eds) Biological Control. Chapman & Hall and International Thompson Publishing, New York, USA, pp 309–335
Vet LEM, Dicke M (1992) Ecology of Infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 1:141–172
Von Mérey GE, Veyrat N, De Lange E, Degen T, Mahuku G, Valdez RL, Turlings TCJ, D’Alessandro M (2012) Minor effects of two elicitors of insect and pathogen resistance on volatile emissions and parasitism of Spodoptera frugiperda in Mexican maize fields. Biol Control 60:7–15
Wäschke N, Meiners T, Rostas M (2013) Foraging strategies of parasitoids in complex chemical environments. In: Wajnberg E, Colazza S (eds) Recent advances in chemical ecology of insect parasitoids. Wiley-Blackwell, Oxford, UK, pp 37–63
Wegener R, Schulz S, Meiners T, Hadwich K, Hilker M (2001) Analysis of volatiles induced by oviposition of elm leaf beetle Xanthogaleruca luteola on Ulmus minor. J Chem Ecol 27:499–515
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemometrics and Intelligent Laboratory Systems 2:37–52
Acknowledgments
We are grateful for the support of the Deutsche Forschungsgemeinschaft (Me 1810/4-1.2 and Fe 778/1-1.2) and of the Max Planck Society. We are very grateful to Monika Hilker for hosting the project at the Freie Universität Berlin and for critical comments on an earlier version of the manuscript. We are grateful for help of Swantje Malskies and Jakob Eckert with the biotests and Michael Reichelt (Max Planck Institute (MPI), Jena) and Frank Müller (Freie Universität (FU), Berlin) with volatile collections and GC–MS analyses. Efthymia Kazantzidou (MPI) and Ute Braun (FU) provided help with plant and insect maintenance, Sybille Lorenz (MPI) took care for the elm tissue culture. Andy Sheppard and members of the CSIRO European Laboratory supported the field assay. Two anonymous reviewers provided very helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stefano Colazza.
Rights and permissions
About this article
Cite this article
Büchel, K., Austel, N., Mayer, M. et al. Smelling the tree and the forest: elm background odours affect egg parasitoid orientation to herbivore induced terpenoids. BioControl 59, 29–43 (2014). https://doi.org/10.1007/s10526-013-9544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9544-9