Abstract
An underlying assumption of classical biological control implies that intentionally introduced natural enemies will remain within the boundaries that delineate the program’s area of implementation. A weed biological control program targeting Melaleuca quinquenervia in Florida, USA has resulted in the release and establishment of Oxyops vitiosa and Boreioglycaspis melaleucae. An international survey of M. quinquenervia populations in 13 other states or countries where the insects have not been intentionally introduced was initiated to monitor the long range dispersal of O. vitiosa and B. melaleucae beyond the herbivores’ intended geographic range (Florida). Surveys in 2006 resulted in the discovery of B. melaleucae within the canopies of several M. quinquenervia trees near San Juan, Puerto Rico. In 2007, O. vitiosa was observed on the island of New Providence in the Bahamas but neither herbivore was detected on nearby Grand Bahama or Andros islands. In 2009, B. melaleucae was observed attacking M. quinquenervia trees in Los Angeles, California (USA). The herbivores have not been detected on other surveyed M. quinquenervia populations in Cuba, Jamaica, Texas (USA), Costa Rica, Brazil, Hawaii (USA) or South Africa. There is no evidence to suggest that herbivore colonization of New Providence, Puerto Rico, or California was influenced by linear distance between Florida and the recipient M. quinquenervia stand. While the dispersal pathway(s) remains unknown, biological control agents were detected from 200 to >3500 km from their original release location (Florida) and at locations that have strong links via tourism and trade as indicated by the number of airline flights connecting south Florida with colonized tree populations. Implications of this unintended spread are discussed in relation to permeability of biogeographical barriers and risk assessment of biological control agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of a classical weed biological control program is a multifaceted process that includes estimating inherent risks associated with the introduction of exotic species. Risk assessments involve, but are not limited to, quantifying a prospective biological control agent’s host range to estimate the potential for direct feeding and damage to vulnerable non-target species. This process is an exercise in maximizing predictive precision within the bounds of practicality (Forno and Heard 1997). Intuitively, increasing the number of test plant species presented to a herbivore is expected to increase the precision of host range predictions. In practice, however, most truly relevant information regarding a herbivore’s host range is derived from test species that are phylogenetically close relatives of the target weed (Wapshere 1974; Pemberton 2000; van Klinken and Heard 2000; Barratt et al. 2010). As described by Briese and Walker (2008), plant phylogenies can be used to refine test plant lists that may include many unnecessary species to a shorter and more informative list that accurately and efficiently characterizes a herbivore’s host range (Briese 2005; Sheppard et al. 2005).
A second factor used to refine test plant lists is the geographic area of the biological control program. All classical weed biological control programs have a strong spatial component, a geographic footprint that delineates the region of study and implementation. This geographic footprint plays a critical role in development of risk assessments and interpretation of inferences drawn from pre-release studies. The geographic region of interest is often defined by the distribution of the weed species within certain spatial constraints. These constraints may include natural geographic barriers like oceans, mountain ranges, deserts or other geological features that inhibit landscape level dispersal of released biological control agents (Briese and Walker 2002). Alternatively, physiological constraints (lower or upper lethal temperatures, diapause, etc.) that limit survival of a candidate biological control agent at certain latitudes, elevations, or climates further delineate a program’s geographic footprint (Boughton et al. 2009). It may be argued, therefore, that suitable host plants beyond these geographical and physiological barriers are not at risk from biological control introductions due to a lack of spatial overlap or a perceived inability of the agent to gain access to these areas even though they may be suitable for establishment (Briese and Walker 2002).
An underlying assumption of classical biological control implies that intentionally introduced natural enemies will remain within the identified boundaries that delineate the program’s footprint. Examples from the literature, however, demonstrate that this assumption is not uniformly valid. The South American cactus moth’s circuitous range expansion to North America, where it now threatens native cacti, illustrates this point. Prickly pear cacti in the genus Opuntia are native to the new world but have become invasive weeds elsewhere (Goeden and Andres 1999). Cactoblastis cactorum (Berg) is a specialist of cacti in the genus Opuntia and was imported from Argentina to Australia in 1925 for control of exotic Opuntia spp., ultimately resulting in complete suppression of nearly 24 million hectares of infested land (Dodd 1940; Goeden and Andres 1999). This success led to transfers of C. cactorum to other Opuntia infested regions, including the Caribbean island of Nevis in 1957 (Pemberton 1995; Stiling and Simberloff 2000). The moth eventually spread, either naturally or inadvertently through the ornamental trade, from Nevis to southern Florida, USA, where it developed new associations with native Opuntia species including the endangered O. corallicola (Small) Werdermann (Stiling 2002). Thus, risk to American Opuntia species from the introduction of C. cactorum into Australia was minimal due to multiple significant geographic barriers that limited movement between intended and “at risk” regions. The moth’s introduction to Nevis, in contrast, markedly increased risk to endemic American Opuntia populations due to regional proximity (or overlap) coupled with the herbivore’s inherent or human-mediated dispersal capabilities.
The role of plant phylogenies in host range testing has benefited from repeated scientific scrutiny (Pemberton 2000; van Klinken and Edwards 2002; Louda et al. 2003; Sheppard et al. 2005; Barratt et al. 2010). In contrast, less attention has been given to the influence of biogeographical barriers in host range predictions and the resulting inferences for widely distributed pest species (Louda and Stiling 2004; Petit et al. 2009). The assumption that weed biological control agents will remain within regional constraints has largely gone untested for most biological control programs. Herein, this assumption is evaluated for the introduced natural enemies of the internationally distributed tree Melaleuca quinquenervia (Cav.) S.T. Blake.
The myrtaceous tree M. quinquenervia occurs naturally along Australia’s eastern coast from Sydney in New South Wales to the northern tip of Queensland, in New Guinea, and in New Caledonia (Boland et al. 1987). Australian habitats that support M. quinquenervia populations typically include low-lying coastal wetlands behind heath-dominated headlands, riparian zones and brackish estuaries behind mangrove swamps (Rayamajhi et al. 2002).
Melaleuca quinquenervia has been widely disseminated over the course of the last century for ornamental, revegetation, and agroforestry purposes (Turner et al. 1998; Serbesoff-King 2003; Dray et al. 2006). This exotic tree was introduced into various locations in the United States and Caribbean but was planted and propagated extensively in southern Florida (Dray et al. 2006). After its introduction, M. quinquenervia spread at an estimated rate of 2850 ha year−1 (Center et al. 2000) and has proven to be a superior competitor to most, if not all, native vegetation occurring in forested and sawgrass dominated wetlands of the Florida Everglades (Turner et al. 1998). These M. quinquenervia wetland forests typically form dense stands characterized by continuous upper canopies with depauperate understories (Rayamajhi et al. 2009).
A classical weed biological control program targeting M. quinquenervia in Florida was initiated in the mid 1980s, with the expectation that introduced herbivores would limit invasion and complement conventional control tactics (Balciunas et al. 1994). The biological control program’s area of implementation was identified as the geographic range of M. quinquenervia in Florida, which encompassed much of the state’s peninsula. The adventive range of the exotic tree also includes various nearby Caribbean islands (Table 1) so the flora of these neighboring countries was also considered during development of test plant lists. Based on the flora of these regions, it was determined that biological control agents would require genus level specificity to be suitable for introduction into Florida. The curculionid weevil Oxyops vitiosa Pascoe (Coleoptera: Curculionidae) was the first candidate selected for quarantine-based host range assessments (Purcell and Balciunas 1994) and, once deemed sufficiently host specific, was released in south Florida in 1997 (Center et al. 2000; Pratt et al. 2003). Feeding by the weevil markedly reduces the tree’s reproductive potential and growth (Pratt et al. 2005; Tipping et al. 2008), but O. vitiosa pupates in the soil so persistent populations are rare in permanently flooded habitats where some M. quinquenervia stands persist. To enhance landscape-level suppression of M. quinquenervia, a second biological control agent, the psyllid Boreioglycaspis melaleucae Moore (Hemiptera: Psyllidae), was released in south Florida during the spring of 2002 (Center et al. 2006). By completing its life cycle entirely on the plant, B. melaleucae is less vulnerable to hydrological conditions and exploits a wider range of leaf ages than the weevil (Wineriter et al. 2003). Feeding by psyllids induces leaf senescence, eventually resulting in mortality of coppicing stumps and seedlings (Morath et al. 2006; Franks et al. 2006). Host specificity testing revealed that O. vitiosa and B. melaleucae are specialists of a species complex within the genus Melaleuca, which is restricted to Australasia. Following establishment, common garden experiments confirmed that feeding and development by O. vitiosa and B. melaleucae was restricted to Melaleuca species, as predicted in quarantine-based host range testing, and posed no direct threat to native or economically important species in the New World (Center et al. 2007; Pratt et al. 2009).
An areawide release effort from 2001 through 2008 resulted in 3.3 million M. quinquenervia biological control agents (combined total) redistributed to 407 locations and among 15 Florida counties (Balentine et al. 2009). Post release evaluations indicate that the geographic distribution of O. vitiosa encompasses 71% of the M. quinquenervia infestation in Florida. The distribution of B. melaleucae is slightly greater than its predecessor, despite being released five years later, with a range including 78% of the tree’s range in Florida. Although widely distributed, highest population densities of both herbivores occur in southern portions of the state (Pratt et al. 2003; Balentine et al. 2009).
Materials and methods
Surveys for spreading herbivores
An international survey was implemented to monitor the long range dispersal of O. vitiosa and B. melaleucae beyond the herbivores’ intended geographic range of Florida. First, a combination of herbaria searches, literature reviews, and various unpublished reports were used to develop a database of adventive M. quinquenervia populations (Table 1). Herbaria responding with geographic data included: ARCH, BISH, BRIT, EAP, F, FLAS, FSU, FTG, G, GH, HNMN, JBSD, LSU, MO, MOL, MU, NY, PIHG, SWF, USF, US (see Thiers 2011 for interpretation of herbaria codes). Surveys of adventive populations consisted of two phases: (1) confirming the existence of M. quinquenervia at reported locations and (2) searching the trees for the presence of the herbivores or signs of their feeding (see Balentine et al. 2009). Global positioning system (GPS) data were gathered for each confirmed M. quinquenervia population as potential areas of colonization. Their distances from the Florida coast line were measured using ArcMap (ver. 9.3, ESRI, Redlands, CA, USA). For each confirmed location, M. quinquenervia trees were randomly selected and herbivore presence or absence was determined by searching canopy vegetation during a 30-min evaluation period. Surveys were conducted once per year, during the dry season when herbivore densities and signs of damage are greatest (Balentine et al. 2009). The frequency of annual surveys varied among sites based on local cooperator availability (Table 1). It should be noted that a few sites were surveyed only once or twice during the monitoring process (i.e. Cuba, Costa Rica, Jamaica, etc.) and therefore caution should be taken when interpreting these results. Infrequently monitored sites, however, were surveyed later in the sampling effort (2009–2011), affording more time for colonization, population buildup, and thus increased likelihood for detection. Biological control agents discovered during surveys were collected, identified by the authors based on morphological features, and voucher specimens were deposited with the California State Collection of Arthropods or Florida State Collection of Arthropods (see Pratt et al. 2006; Pratt et al. 2008; Pratt and Arakelian 2011).
Dispersal pathways radiating from south Florida to surrounding regions include natural as well as human-mediated mechanisms. Confirming pathway use requires intercepting the dispersing herbivore in transit and was beyond the scope of this survey (Work et al. 2005). However, patterns of dispersal and detection from among the possible recipient locations can provide insight to the likelihood of pathway use (Petit et al. 2009). Long range dispersal from Florida to unintended areas may include active flight or other more passive forms of dispersion including “rafting” on debris, propulsion from wind currents, or “hitchhiking” on vessels of transportation (Browne and Peck 1996; Drake and Farron 1998; Kiritani and Yamamura 2003). Long range dispersal is strongly influenced by distance between the propagule source and recipient areas suitable for colonization, as well as propagule pressure (density) and life stage (Simberloff 2009). Based on principles of island biogeography, we hypothesize that the probability of colonization is negatively correlated with distance and infer that detection of herbivores at “near” versus “far” recipient M. quinquenervia stands is evidence of natural dispersal. For the purposes of this study, “near” M. quinquenervia populations were those sites <1,000 km from the Florida coastline (the Bahamas, Cuba, Jamaica) while “far” populations included those beyond 1,000 km.
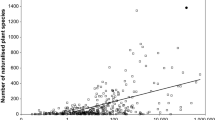
Airline transportation systems also serve as important invasion pathways for the long range dispersal of insects and are indicators of trade connectivity between two regions (Kiritani and Yamamura 2003; Work et al. 2005). Successful colonization of new habitats is often attributed to the frequency of invasion attempts or opportunities (Crawley 1989; Wilson et al. 2009). By assuming that flight frequency was a metric for colonization opportunities, we hypothesize that the probability of M. quinquenervia herbivore colonization is positively correlated with the number of flights arriving in foreign locations from southern Florida. To investigate the frequency of airline connections, transportation statistics for direct (non-stop) flights between international airports in southern Florida and destinations that harbored adventive populations of M. quinquenervia were tabulated. This was done by searching the air carrier database of the USA Department of Transportation (http://www.bts.gov/). These statistics include monthly data reported by certified USA and foreign air carriers on the combined number of passengers, freight, and mail transported flights departing the USA. Data were sorted by the flight destination, origin, year, and number of departures performed. Origin was limited to the four international airports that are sympatric with the M. quinquenervia biological control agents’ ranges in southern Florida (Fort Lauderdale Hollywood International Airport (FLL), Miami International Airport (MIA), Palm Beach International Airport (PBI), Southwest Florida International Airport (RSW)). All Florida airports are <1 km from M. quinquenervia stands that support high densities of the introduced natural enemies. Destination was represented by the nearest international airport to the monitored M. quinquenervia population. The mean distance between monitored M. quinquenervia populations and the nearest international airports was 55.2 (SE: 17.5, range: 1–232) km. The resulting data represented the number of all flights (passenger, cargo, and mail combined) originating from southern Florida and arriving in an area of interest per year. The mean number of flights per year was calculated by averaging data from years 2005 through 2007. The non-parametric Kruskal–Wallis test was used to investigate the influence of distance and flight frequency on the probability of herbivore recruitment (PROC NPAR1WAY, SAS ver. 9.1, SAS 1999).
Results
Thirteen M. quinquenervia populations were confirmed and monitored from 2005 to 2011 (Table 1). Surveys conducted in January 2006 resulted in the discovery of B. melaleucae on leaves of M. quinquenervia trees growing near San Juan, Puerto Rico. An island-wide assessment by Pratt et al. (2006) documented that psyllid densities and associated damage were greatest near the San Juan (Luis Muñoz Marín International) Airport but decreased with increasing distance from the greater San Juan area. In 2007, O. vitiosa was observed on the island of New Providence in the Bahamas but neither herbivore was detected on nearby Grand Bahama or Andros islands (Pratt et al. 2008). Upon first discovery, O. vitiosa was observed within 4 km of the Nassau International Airport. In 2009, B. melaleucae was also observed attacking M. quinquenervia trees within a neighborhood community approximately 5 km west of the Los Angeles, California (USA) International Airport (Arakelian 2009). To date, O. vitiosa and B. melaleucae have not been detected in Cuba, Jamaica, Texas (USA), Costa Rica, Brazil, Hawaii (USA) or South Africa.
Winged dispersal represents one pathway by which O. vitiosa and B. melaleucae may have spread beyond their intended geographic range. Distances from Florida’s coastline to possible recipient M. quinquenervia populations are listed in Table 1. At least six populations of the exotic tree are less than 1,000 km from peninsular Florida, with approximately 200 km of open water separating Grand Bahama from Florida. The recruitment of Florida’s biological control agents, however, was not influenced by linear distance between Florida and the recipient M. quinquenervia stand (χ1 2 = 0.01, P = 0.93), suggesting that flight by biological control agents across the open ocean was not likely.
Accidental anthropogenic transportation is another common mechanism of long range dispersal of insects worldwide. The mean number of flights departing from the four international south Florida airports was nearly 350,000 annually when averaged across 2005–2007. Of the total number of flights, 15% landed at airports within 200 km from known adventive M. quinquenervia stands or trees. The most common destination of direct flights that linked south Florida with other exotic populations of the tree included the Bahamas, with nearly 20,000 flights annually (Table 1). Other common destinations included Puerto Rico, Jamaica, Colombia, and Los Angeles (USA). Various adventive populations of the tree are not or only weakly linked by direct air transit with south Florida, including the Hawaiian islands, South Africa and various Caribbean islands of the Lesser Antilles (Table 1). Evidence suggests that the destination of dispersing M. quinquenervia biological control agents was influenced by the mean number of annual flights linking south Florida with other adventive tree populations (χ1 2 = 4.88, P = 0.03).
Discussion
The M. quinquenervia biological control agents that were intentionally introduced into Florida have dispersed far beyond their intended adventive range. While unexpected, the movement of O. vitiosa and B. melaleucae to Caribbean islands highlights the biotic connectivity between the Antilles and peninsular Florida. Intuitively, one may assume that factors related to island biogeography (island size and proximity) may be important drivers influencing biotic exchange and serve as a framework to explain the movement of the dispersing herbivores. One may hypothesize that among the Caribbean islands, for instance, dispersing biological control agents are more likely to spread to the island of Cuba due to its close proximity to the mainland (330 km) and much larger surface area in relation to the other islands within the Greater and Lesser Antilles. A similar hypothesis may be generated for the Bahamian Archipelago with the largest (Andros) or the nearest (Grand Bahama) islands more likely to recruit dispersing herbivores as compared to the smaller and relatively more distant island of New Providence. In contrast to these predictions, B. melaleucae bypassed nearer islands harboring M. quinqueneriva and was detected over 1665 km south of Florida on the island of Puerto Rico in 2005. Similarly, O. vitiosa was observed on the island of New Providence in 2006, which is the smallest, more distant, and least infested of the Bahamian islands surveyed. Most recently (2009) B. melaleucae was recovered from M. quinqueneriva trees in Los Angeles California (USA) and, assuming the psyllid originated from Florida populations, this dispersal event spanned the North American Continent with no observed satellite colonies distributed between donor and recipient locations. Alternatively, the B. melaleucae population in Los Angeles may have originated in the native range of Australia rather than Florida. Franks et al. (2011) examined mtDNA sequence data for Australian and Floridian populations of B. melaleuca and determined that the adventive range possessed two (A and B) of the eight haplotypes sampled in eastern Australia (A–H). Therefore, evaluation of the mtDNA for the Los Angeles psyllid population can provide insight to the herbivore’s origin if the haplotypes differ from the two types that occur in both Australia and Florida (A and B). Regardless of origin, these data underscore the unpredictability of long range dispersal events based on spatial and geographical constraints. Emigration of the M. quinquenervia biological control agents was not restricted by large water bodies, mountain ranges, or extended expanses of host-free lands.
Although spatial proximity or island size may not account for the observed pattern of the herbivore’s recruitment, frequency of trade and tourism may. Human activities play an important role in accidental insect invasions, with the most common introduction pathways including international transportation of airplane luggage and cargo (Kiritani and Yamamura 2003). Dobbs and Brodel (2004) reported, for instance, that over 10% of airplanes on the Miami tarmac contained insects in their cockpits, cabins or cargo areas. Nearly 32,000 airline flights depart Florida for the Bahamas annually and approximately 20,000 of these arrived on the three islands studied herein. Within the Bahamas, the mean number of annual flights to New Providence was markedly greater (13,982) than those destined for Grand Bahama (5,452) or Andros (559). A similar pattern of long range dispersal also exists among the other adventive M. quinquenervia stands monitored herein (Table 1), with herbivore recruitment associated with locations that experience a high level of connectivity with southern Florida, as indicated by the large numbers of direct flights originating from the region. Considering the frequent transport of tourists and cargo between southern Florida and the newly colonized locations, the premise that the biological control agents were inadvertently carried or “hitchhiked” to their new ranges is a plausible or even likely explanation.
These long range dispersal data may also provide insight to the likelihood that the remaining natural enemy-free M. quinquenervia populations will recruit these herbivores without intentional human assistance. The exotic tree populations in Colombia and to a lesser degree Jamaica, Dominican Republic, and Costa Rica experience relatively high levels of transportation connectivity with southern Florida and are therefore predicted to have a greater probability of colonization by the biological control agents than other populations (Table 1). The island of Grand Bahama, however, may have the highest probability of colonization due to the second highest number of flights originating from southern Florida but also due to the new colonization of O. vitiosa within the archipelago, which further increases its connectivity with adventive weevil populations in the Caribbean. Therefore, these new adventive populations of the M. quinquenervia herbivores serve as foci that may facilitate expansion to areas that were previously less likely to be invaded due to lack of transport connectivity and intra-island connectivity within the Caribbean may play an important role of the continued spread of these biological control agents. The islands of Hawaii, for instance, were previously considered less vulnerable to unintentional spread by the M. quinquenervia biological control agents due to limited pathways of invasion yet adventive satellite populations in Los Angeles (USA) markedly increase linkages between the mainland and herbivore-free tree populations in Hawaii.
The presence of these newly arrived herbivores is interpreted differently among the recipient land managers. In the Bahamas and Puerto Rico, for instance, this unintended spread is considered by some to be a fortuitous benefit to their ongoing and underfunded effort to control the spread of M. quinquenervia (Pratt et al. 2006; Pratt et al. 2008). In California, where the tree is not considered invasive, land managers perceive the psyllid’s arrival as an added complication to the aesthetic maintenance of the communities’ street trees (Pratt and Arakelian 2011).
Unintended spread of introduced biological control agents is not limited to the M. quinquenervia system. Geographic range expansions of biological control agents can be categorized into at least two general groups: those that spread beyond political boundaries and those that overrun geographical barriers that were assumed to curtail their spread. Recently, the houndstongue root weevil Mogulones cruciger Herbst, which was released in Canada in 1997, has dispersed south across the US border where it may feed on native Boraginaceae (Andreas et al. 2008). Similarly, the seed head fly Urophora quadrifasciata (Meigen) was introduced into Canada in the early 1970s but spread across the USA border and can now be found in much of North America, including the states of Arizona and Arkansas (Story 1985; Duguma et al. 2009). In contrast, the eriophyid mite Eriophyes chondrillae (Canestrini) and the rust fungus Puccinia chondrillina Bubak and Sydow were released for control of Chondrilla juncea L. in the western USA but spread north to British Colombia, Canada (Julien and Griffiths 1998). The tephritid fly Procecidochares utilis Stone was introduced into India in 1963 for control of Ageratina adenophora (Sprengel) but has spread to neighboring Nepal and China (Wang 1989).
There are also numerous examples of biological control agents that have dispersed beyond geographic barriers that historically were considered impermeable. In addition to the South American cactus moth cited earlier, the scale parasitoid Aphytis lepidosaphes Compere was intentionally introduced into California in 1948–1949 but has since been recovered at various locations where it was not intentionally released including: Florida, Hawaii, Puerto Rico, El Salvador, Argentina, Turkey, Israel and Australia (DeBach 1974). Various biological control agents of Lantana camara L., including the agromyzid flies Calycomyza lantanae (Frick) and Ophiomyia lantanae (Froggatt), have spread from areas of intentional release (i.e. Australia) to Malaysia and Micronesia (Julien and Griffiths 1998; Muniappan and Reddy 2003). The gracillariid Dialectica scalariella (Zeller) was introduced to Australia for the control of Echium candicans L. but has since dispersed to New Zealand (Julien and Griffiths 1998).The South American bruchid beetle Acanthoscelides macrophthalmus (Schaeffer) was introduced to South Africa for the control of Leucaena leucocephala (Lam.) but is now found in Australia as well as Cyprus (Vassiliou and Papadoulis 2008).
Over time, regulatory and advisory organizations that oversee weed biological control agent introductions have demonstrated greater awareness for the potential of herbivore dispersal beyond its intended range. The Technical Advisory Group (TAG) for biological control agents of weeds in the USA, and its predecessors, conducted informal and reciprocal reviews of proposed introductions with Canadian officials beginning in 1962 (APHIS-PPQ 1998). The reviewing body began requesting formal comments from both Canadian and Mexican officials in 1971 based on the knowledge “that an introduced organism recognizes no political boundaries and its introduction need(s) to be considered on a continental basis” (APHIS-PPQ 1998). Considering the data presented herein, we propose that this process can be more inclusive through formal consultation and comment from Caribbean or other nearby countries to address the potential of unintended spread. In addition, petitions for the introduction of weed biological control agents can be improved by delineating the attainable geographic range of the introduced herbivore based on the target weed’s and alternative host plant’s distributions rather than environmental barriers that restrict dispersal. A recent example of this process involves a Longitarsus sp. that was proposed for biological control of Heliotropium amplexicaule Vahl but was not permitted for release in Australia. Its rejection was attributable to the questionable permeability of geographic barriers expected to limit the herbivore’s dispersal and its use of allopatric native non-target species even though they did not overlap with the weed’s current geographic range (Briese and Walker 2008).
The assumption that introduced biological control agents will remain within the identified boundaries that delineate the program’s geographic footprint is not supported by the M. quinquenervia system as well as other biological control projects. Considering the ever-increasing levels of globalization, are there are any geographical barriers that can meaningfully restrict the spread of introduced biological control agents (Vermeij 2005)? We propose that the dramatic increase of international trade and tourism has resulted in the development of complex pathways that render historic barriers irrelevant to curtailing the spread of biological control agents. The reality of long-range dispersal and unintended spread in biological control underscores the need to conduct risk assessments that focus less on “at risk” species within strict geographical barriers and more on broadly defining the agent’s host range (Briese and Walker 2008). This will be accomplished as greater attention is placed on exploring patterns of a herbivore’s host plant use in comparison to the degree of phylogenetic relatedness to the target weed over larger geographic ranges. Inferences drawn from host use patterns in relation to plant phylogenies provide greater insights to the risk of direct non-target damage across geographic and political barriers.
References
Andreas JE, Schwarzländer M, De Clerck-Floate R (2008) The occurrence and potential relevance of post-release, nontarget attack by Mogulones cruciger, a biocontrol agent for Cynoglossum officinale in Canada. Biol Control 46:304–311
Animal and Plant Health Inspection Service-Plant Protection and Quarantine (APHIS-PPQ) (1998) Reviewer’s manual for the technical advisory group for biological control of weeds. PPQ 03/98–01 animal and plant health inspection service (APHIS). U.S. Department of Agriculture, Riverdale
Arakelian G (2009) Melaleuca psyllid. Co. Los Angeles, Department of Ag. Com. Weights Measures. http://acwm.co.la.ca.us/pdf/Melalpsyllid.pdf. Accessed 10 Mar 2011
Balciunas JK, Burrows DW, Purcell MF (1994) Field and laboratory host ranges of the Australian weevil, Oxyops vitiosa, a potential biological control agent of the paperbark tree, Melaleuca quinquenervia. Biol Control 4:351–360
Balentine KM, Pratt PD, Dray FA Jr, Rayamajhi MB, Center TD (2009) Geographic distribution and regional impacts of Oxyops vitiosa (Coleoptera: Curculionidae) and Boreioglycaspis melaleucae (Hemiptera: Psyllidae), biological control agents of the invasive tree Melaleuca quinquenervia. Environ Entomol 38:1145–1154
Barratt BIP, Howarth FG, Withers TM, Kean JM, Ridley GS (2010) Progress in risk assessment for classical biological control. Biol Control 52:245–254
Boland DJ, Brooker MIH, Chippendale GM, Hall N, Hyland BPM, Johnston RD, Kleinig DA, Turner JD (1987) Forest trees of Australia. Nelson Wadsworth, Melbourne
Boughton AJ, Bennett CA, Goolsby JA, Pemberton RW (2009) Laboratory host range testing of Neomusotima conspurcatalis (Lepidoptera: Crambidae), a potential biological control agent of the invasive weed, Old World climbing fern, Lygodium microphyllum (Lygodiaceae). Biocontrol Sci Tech 19:369–390
Briese DT (2005) Translating host-specificity test results into the real world: the need to harmonize the yin and yang of current testing procedures. Biol Control 35:208–214
Briese DT, Walker A (2002) A new perspective on the selection of test plants for evaluating the host-specificity of weed biological control agents: the case of Deuterocampta quadrijuga, a potential insect control agent of Heliotropium amplexicaule. Biol Control 25:273–287
Briese DT, Walker A (2008) Choosing the right plants to test: the host-specificity of Longitarsus sp. (Coleoptera: Chrysomelidae) a potential biological control agent of Heliotropium amplexicaule. Biol Control 44:271–285
Browne J, Peck SB (1996) The long-horned beetles of south Florida (Cerambycidae: Coleoptera): biogeography and relations with the Bahama Islands and Cuba. Can J Zool 74:2154–2169
Center TD, Van TK, Rayachhetry M, Buckingham GR, Dray FA Jr, Wineriter S, Purcell MF, Pratt PD (2000) Field colonization of the melaleuca snout beetle (Oxyops vitiosa) in south Florida. Biol Control 19:112–123
Center TD, Pratt PD, Tipping PW, Rayamajhi MB, Van TK, Wineriter S, Dray FA Jr, Purcell MF (2006) Field colonization, population growth, and dispersal of Boreioglycaspis melaleucae Moore, a biological control agent of the invasive tree Melaleuca quinquenervia. Biol Control 39:363–374
Center TD, Pratt PD, Tipping PW, Rayamajhi MB, Van TK, Wineriter S, Dray FA Jr (2007) Initial impacts and field validation of host range for Boreioglycaspis melaleucae Moore (Hemiptera: Psyllidae), a biological control agent of the invasive tree Melaleuca quinquenervia (Cav.) Blake. Environ Entomol 36:569–576
Crawley MJ (1989) Chance and timing in biological invasions. In: Drake JA, Mooney HA, Castri Fd, Groves RH, Kruger FJ, Rejmanek M, Williamson M (eds) Biological invasions: a global perspective. Wiley, New York, pp 407–423
DeBach P (1974) Biological control by natural enemies. Cambridge University Press, London
Dobbs TT, Brodel CF (2004) Cargo aircraft as a pathway for the entry of nonindigenous pests into south Florida. Fla Entomol 87:65–78
Dodd AP (1940) The biological campaign against prickly pear. Commonwealth Prickly Pear Board, Brisbane
Drake VA, Farron RA (1998) The influence of atmospheric structure and motions on insect migration. Ann Rev Entomol 33:183–210
Dray FA Jr, Bennett BC, Center TD (2006) Invasion history of Melaleuca quinquenervia (Cav.) S.T. Blake in Florida. Castanea 71:210–225
Duguma D, Kring TJ, Wiedenmann RN (2009) Seasonal dynamics of Urophora quadrifasciata on spotted knapweed in the Arkansas Ozarks. Can Entomol 141:70–79
Forno W, Heard T (1997) Compiling a plant list for testing the host range of agents. In: Julien M, White G (eds) Biological control of weeds: theory and practical application. Australian Centre for International Agricultural Research, Canberra, pp 71–75
Franks SJ, Kral AM, Pratt PD (2006) Herbivory by introduced insects reduces growth and survival of Melaleuca quinquenervia seedlings. Environ Entomol 35:366–372
Franks SJ, Pratt PD, Tsutsui ND (2011) The genetic consequences of a demographic bottleneck in an introduced biological control insect. Conserv Genet 12:201–211
Goeden RD, Andres LA (1999) Biological control of weeds in terrestrial and aquatic environments. In: Bellows TS, Fisher TW (eds) Handbook of biological control. Academic Press, San Diego, pp 871–890
Julien MH, Griffiths W (1998) Biological control of weeds: a catalog of agents and their target weeds, 4th edn. CABI Publishing CAB International, Wallingford
Kiritani K, Yamamura K (2003) Exotic insects and their pathways for invasion. In: Ruiz GM, Carlton JT (eds) Invasive species: vectors and management strategies. Island Press, Washington, pp 44–67
Louda SM, Stiling P (2004) The double-edged sword of biological control in conservation and restoration. Conserv Biol 18:50–53
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Nontarget effects–the Achilles’ Heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Ann Rev Entomol 48:365–396
Morath SU, Pratt PD, Silvers CS, Center TD (2006) Herbivory by Boreioglycaspis melaleucae (Hemiptera: Psyllidae) accelerates foliar degradation and abscission in the invasive tree Melaleuca quinquenervia. Environ Entomol 35:1372–1378
Muniappan R, Reddy GVP (2003) Fortuitous introduction of two natural enemies of Lantana camara to Chuuk. Proc Hawaiian Entomol Soc 36:123–124
Pemberton RW (1995) Cactoblastis cactorum in the United States: An immigrant biological control agent or an introduction of the nursery industry? Am Entomol 41:230–232
Pemberton RW (2000) Predictable risk to native plants in weed biolgoical control. Oecologia 125:489–494
Petit JN, Hoddle MS, Grandgirard J, Roderick GK, Davies N (2009) Successful spread of a biocontrol agent reveals a biosecurity failure: elucidating long distance invasion pathways for Gonatocerus ashmeadi in French Polynesia. BioControl 54:485–495
Pratt PD, Arakelian G (2011) First report of the biological control agent Boreioglycaspis melaleucae (Hemitptera: Psyllidae) in California, USA. Fla Entomol 94:724–725
Pratt PD, Slone DH, Rayamajhi MB, Van TK, Center TD (2003) Geographic distribution and dispersal rate of Oxyops vitiosa (Coleoptera: Curculionidae), a biological control agent of the invasive tree Melaleuca quinquenervia in south Florida. Environ Entomol 32:397–406
Pratt PD, Rayamajhi MB, Van TK, Center TD, Tipping PW (2005) Herbivory alters resource allocation and compensation in the invasive tree Melaleuca quinquenervia. Ecol Entomol 30:316–326
Pratt PD, Rayamajhi MB, Bernier LS, Center TD (2006) Geographic range expansion of Boreioglycaspis melaleucae in Puerto Rico. Fla Entomol 89:529–531
Pratt PD, Rayamajhi MB, Center TD (2008) Geographic range expansion of Oxyops vitiosa to the Bahamaian Archipelago. Fla Entomol 91:695–697
Pratt PD, Rayamajhi MB, Center TD, Tipping PW, Wheeler GS (2009) The ecological host range of an intentionally introduced herbivore: a comparison of predicted versus actual host use. Biol Control 49:146–153
Purcell MF, Balciunas JK (1994) Life history and distribution of the Australian weevil Oxyops vitiosa, a potential biological control agent for Melaleuca quinquenervia. Ann Entomol Soc Am 87:867–873
Rayamajhi MB, Purcell MF, Van TK, Center TD, Pratt PD, Buckingham GR (2002) Australian paperbark tree (Melaleuca). In: Driesche RGV, Blossey B, Hoddle MS, Lyon S, Reardon R (eds) Biological control of invasive plants in the Eastern United States. Forest Health Technology Enterprise Team, Morgantown, pp 130–171
Rayamajhi MB, Pratt PD, Center TD, Tipping PW, Van TK (2009) Decline in exotic tree density facilitates increased plant diversity: the experience from Melaleuca quinquenervia invaded wetlands. Wet Ecol Manage 17:455–467
SAS (1999) The SAS System for Windows, Version 8. SAS Institute Inc., Cary
Serbesoff-King K (2003) Melaleuca in Florida: a literature review on the taxonomy, distribution, biology, ecology, economic importance and control measures. J Aquatic Plant Manage 41:98–112
Sheppard AW, van Klinken RD, Heard TA (2005) Scientific advances in the analysis of direct risks of weed biological control agents to nontarget plants. Biol Control 35:215–226
Simberloff D (2009) The role of propagule pressure in biological invasions. Ann Rev Ecol Evol Syst 40:81–102
Stiling P (2002) Potential non-target effects of a biological control agent, prickly pear moth, Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae), in North America, and possible management actions. Biol Invasions 4:273–281
Stiling P, Simberloff D (2000) The frequency and strength of nontarget effects of invertebrate biological control agents of plant pests and weeds. In: Follet PA, Duan JJ (eds) Nontarget effects of biological control. Kluwer Academic, Boston, pp 31–43
Story JM (1985) First report on the dispersal into Montana of Urophora quadrifasciata (Diptera: Tephritidae), a fly released in Canada for biological control of spotted knapweed and diffuse knapweed. Can Entomol 117:1061–1062
Thiers B (2011) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ Accessed 10 Mar 2011
Tipping PW, Martin MR, Pratt PD, Center TD, Rayamajhi MB (2008) Suppression of growth and reproduction of an exotic invasive tree by two introduced insects. Biol Control 44:235–241
Turner CE, Center TD, Burrows DW, Buckingham GR (1998) Ecology and management of Melaleuca quinquenervia, an invader of wetlands in Florida. USA Wetl Ecol Manage 5:165–178
van Klinken RD, Edwards OR (2002) Is host-specificity of weed biological control agents likely to evolve rapidly following establishment? Ecol Lett 5:590–596
van Klinken RD, Heard TA (2000) Estimating fundamental host range: a host-specificity study of a potential biocontrol agent for Prosopis species (Leguminosae). Biocontrol Sci Tech 10:331–342
Vassiliou VA, Papadoulis G (2008) First record of Acanthoscelides macrophthalmus (Schaeffer) (Coleoptera: Bruchidae) in Cyprus. Entomol Hellenica 17:52–55
Vermeij GJ (2005) Invasion as expectation: a historical fact of life. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer Associates, Inc., Sunderland, pp 315–340
Wang R (1989) Biological control of weeds in China: a status report. In: Delfosse ES (ed) Proceedings of the VII international symposium on biological control of weeds. Istituto Sperimentale per la Patologia Vegetale, Rome, pp 689–693
Wapshere AJ (1974) A strategy for evaluating the safety of organisms for biological weed control. Ann Appl Bio 77:201–211
Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM (2009) Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol 24:136–144
Wineriter SA, Buckingham GR, Frank JH (2003) Host range of Boreioglycaspis melaleucae Moore (Hemiptera: Psyllidae), a potential biocontrol agent of Melaleuca quinquenervia (Cav.) S.T. Blake (Myrtaceae), under quarantine. Biol Control 27:273–292
Work TT, McCullough DG, Cavey JF, Komsa R (2005) Arrival rate of nonindigenous insect species into the United States through foreign trade. Biol Invasions 7:323–332
Acknowledgments
We thank Roy van Driesche and Mark Hoddle for their invitation to present these data as part of the Biological Control for Nature Conference. We are indebted to various collaborators who located and monitored M. quinquenervia populations worldwide, including Dana Prince in Texas, Gevork Arakelian in California, Lourdes Bernier in Puerto Rico, Amy Ferriter and Melanie Williams in the Bahamas, Dan Clark in Hawaii and Costa Rica, Amy Ferriter and Ramona Oviedo in Cuba, Ernita van Wyk in South Africa, Kurt McLaren in Jamaica, and Greg Wheeler in Brazil. We wish to also thank Keith Bradley for conducting the herbaria search, which was completed as part of the TAME Melaleuca Areawide Program (http://tame.ifas.ufl.edu). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Mark Hoddle
Rights and permissions
About this article
Cite this article
Pratt, P.D., Center, T.D. Biocontrol without borders: the unintended spread of introduced weed biological control agents. BioControl 57, 319–329 (2012). https://doi.org/10.1007/s10526-011-9412-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9412-4