Abstract
Invasive species are generally detected in new ecosystems long after their first arrival, making it difficult to elucidate pathways leading to successful invasion. In this study, the dispersal of a classical biological control agent, the mymarid egg parasitoid Gonatocerus ashmeadi, was monitored across ten islands in three major island groups in French Polynesia from the exact moment of its introduction into Tahiti to combat the invasive pest Homalodisca vitripennis. Within 10 months, the parasitoid spread quickly from Tahiti to widely separated islands (up to 1,400 km from Tahiti); presumably through the transportation of plant material containing parasitized H. vitripennis eggs. Gonatocerus ashmeadi thus functioned as a “biomarker”, providing an informal audit of the effectiveness of inter-island quarantine measures designed to curb the accidental spread of noxious organisms. Survey results suggest that invasive organisms, like deliberately released biological control agents, can be unintentionally and rapidly transmitted across vast distances by humans. Furthermore, even remote islands appear to experience relentless pressure from invasive propagules associated with human travel. Implications of survey work documenting the spread and impact of G. ashmeadi are discussed within the context of biological control programs, non-target impacts, and biosecurity initiatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the critical role natural dispersal plays in biological control programs, there is relatively little information on the dispersal characteristics of natural enemies (Corbett and Rosenheim 1996; Canto Sila et al. 2006). Specifically, how introduced species spread through a new geographic range and the factors affecting this dispersal are some of the most important applied problems in invasion ecology (Elton 1958; Sallam et al. 2001; Leibhold and Tobin 2007). The “natural laboratory” setting of island archipelagos, such as those of French Polynesia, facilitates the study of fundamental scientific issues regarding biological invasions and natural colonization processes (Gillespie and Roderick 2002). To this end, the intentional introduction of a parasitoid as a classical biological control agent into an insular natural laboratory provides a unique opportunity to monitor the spread of a biological control agent, as a surrogate, for examining the potential spread of an invasive species in a new ecosystem. Monitoring can commence from the exact moment of the introduction of the natural enemy with precise records of initial propagule pressure, introduction frequencies, and release localities being used to examine the invasion dynamics of the biological control agent.
The glassy-winged sharpshooter, Homalodisca vitripennis (Germar) [formerly Homalodisca coagulata (Say) (Takiya et al. 2006)] (Hemiptera: Cicadellidae), is an important exotic insect pest in the South Pacific that most likely originated from California (USA) after H. vitripennis invaded this state in the late 1980s (Sorensen and Gill 1996). Homalodisca vitripennis is native to the southeast USA and northeast Mexico (Sorensen and Gill 1996; Triapitsyn and Phillips 2000). This pest invaded French Polynesia in 1999 (Secretariat of the Pacific Community 2002), Hawaii in 2004 (Hoover 2004), Easter Island in 2005 (Sandra Ide personal communication 2005), and the Cook Islands in 2007 (Maja Poeshco personal communication 2007). The arrival of H. vitripennis on the island of Tahiti and the subsequent problems resulting from this invasion were discussed by Grandgirard et al. (2006). In 2004, a classical biological control program against the glassy-winged sharpshooter was initiated in French Polynesia using the highly specific egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), a solitary endoparasitoid that attacks eggs of Proconiini sharpshooters (Cicadellidae: Cicadellinae: Proconiini) (Triapitsyn et al. 1998), the tribe to which H. vitripennis belongs. Gonatocerus ashmeadi is <2 mm in length and has an average longevity of ~12 days (at 25°C) (Pilkington and Hoddle 2006). After a ~12 month risk assessment study (Grandgirard et al. 2007), G. ashmeadi was cleared for release by the French Polynesian Government, and 13,786 parasitoids were released at 27 sites on the island of Tahiti between May 2 and October 25 2005.
Within seven months post-release, pest populations on Tahiti were reduced by more than 95% and parasitism levels of H. vitripennis eggs averaged 80–100% (Grandgirard et al. 2008). Such high levels of parasitism in French Polynesia were likely favored by year round temperatures which favored optimal reproductive output (Pilkington and Hoddle 2006), an abundance of flowering plants in urban areas that could have provided resources that promoted increased longevity and fecundity of female parasitoids (Irvin and Hoddle 2007), and a complete absence of Proconiini sharpshooters in French Polynesia meant G. ashmeadi had no alternative host species for exploitation which ensured an extremely tight linkage between this natural enemy and its intended target, H. vitripennis (Grandgirard et al. 2007).
As part of the biological control program against H. vitripennis in French Polynesia, we hypothesized that the spread of G. ashmeadi on and among islands as well as across vastly separated archipelagos would follow a stratified dispersal process (Hengeveld 1989; Petit et al. 2008b). Stratified dispersal is a combination of: (1) short-distance localized dispersal by the organism (e.g., flying or walking), and (2) rapid long-distance dispersal assisted by abiotic factors (e.g., wind) or biotic factors (e.g., the unintentional human transportation of H. vitripennis egg masses infected by G. ashmeadi). The insular island system of French Polynesia makes it very easy to separate short-distance dispersal across an island from long-distance movement between islands and archipelagoes.
Controlled releases and subsequent monitoring of natural enemies within the context of a biological control program provides a rare opportunity for rigorous quantitative ecological studies investigating processes underlying deliberately orchestrated invasions. Such evaluations are important because unintentional introduction of natural enemies to new areas via unregulated movement (e.g., plants bearing infective propagules such as parasitized eggs), may pose significant risk to non-target species if non-targets were not previously considered in risk assessment studies because they inhabited areas outside of the initial release foci of the biological control program. Therefore, biological control programs in “natural laboratories”, such as the islands of French Polynesia, and subsequent research on the spread of natural enemies can potentially improve the efficacy of biosecurity procedures to contain the spread of invasive species and to enhance the safety of biological control programs. Understanding the long distance dispersal capability of natural enemies and the possible role of humans in unintentionally moving natural enemies are important aspects affecting the outcomes of biological control programs in terms of both impact on the intended target and safety to non-target species throughout the range of the target pest.
Consequently, the long-distance spread of natural enemies, such as G. ashmeadi, can serve as “biomarkers” to help identify the invasion pathways of their hosts, in this instance, the pest H. vitripennis. Here we present data on the long-distance dispersal of G. ashmeadi throughout French Polynesia and evaluate the influence of unintentional human transportation on the spread of this deliberately released upper trophic level organism.
Material and methods
The deliberate release of Gonatocerus ashmeadi in Tahiti
Detailed release methods for G. ashmeadi in Tahiti are described in Grandgirard et al. (2008). Briefly, G. ashmeadi was first released at two monitoring sites on the north of the island of Tahiti: (1) at sea level in Papenoo (17°30′25.01′′S; 149°27′30.47′′W), and (2) at 800 m in Pirae (17°34′21.58′′S; 149°31′26.79′′W) (Fig. 1). The Papenoo site was characterized by: (1) an extremely high host density, with an average of 156 H. vitripennis nymphs collected on Hibiscus rosa-sinensis (Malvaceae) hedges in one minute of sampling effort (Petit et al. 2008a); (2) a disturbed habitat composed almost exclusively of exotic ornamental vegetation; (3) a mean annual temperature averaging 27°C (Grandgirard et al. 2008), and (4) a predominant easterly wind (Laurent et al. 2004). The high elevation monitoring site at Pirae (800 m elevation, 10 km southwest from Papenoo release site, 6 km south from the coast) was characterized by: (1) a very low host density of H. vitripennis, with an average of 1.3 nymphs collected on native Metrosideros colina in one minute of sweep net sampling effort (Petit et al. 2008a); (2) a habitat consisting almost exclusively of mixed native vegetation, and (3) a mean annual temperature averaging 20.5°C (Grandgirard et al. 2008).
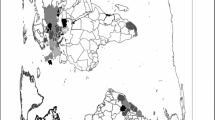
Gonatocerus ashmeadi dispersal after release on Tahiti. Black stars are parasitoid release sites in Papenoo (beginning on May 2 2005) and Pirae (beginning on June 9 2005). Dark grey patch is the G. ashmeadi colonization range on July 26 2005 (a), on August 23 2005 (b) and on September 20 2005 (c), and on November 8 2005 (d) as determined by sweep net sampling and laboratory rearing of field collected H. vitripennis egg masses
From May 2 2005 to June 30 2005, 6,574 parasitoids were released in Papenoo. From June 7 2005 to October 25 2005, 1,652 individuals were released in Pirae. Subsequent releases took place at a number of other sites: (1) on August 25 2005, a total of 755 parasitoids were released across 20 sites on the peninsula on the southern part of Tahiti (~17°44′35.44′′W; 149°19′03.72′′W; 10 m elevation), and (2) on September 5 2005, 1,495 and 1,317 parasitoids were released at two sites in Papeete, the international sea port (17°32′12.65′′S; 149°34′08.28′′W; 12 m elevation), and the international airport (17°33′23.29′′S; 149°36′40.84′′W; 7 m elevation), respectively. These sites had conditions that were very similar to those detailed for Papenoo. G. ashmeadi was only released on one island in French Polynesia, this being Tahiti, as part of the classical biological control program against H. vitripennis.
Measuring the dispersal of Gonatocerus ashmeadi
Dispersal of G. ashmeadi was monitored over the entire island of Tahiti after its initial introduction. Monitoring was conducted also on an additional nine islands in French Polynesia infested by H. vitripennis, even though the parasitoid had not been deliberately released on these islands as part of the biological control program. On each island, 25–50 geographical sites were sampled. Survey sites were <1 km apart (in major towns) to a maximum of 10 km (around the coast) separation. Distances between sampling sites were influenced by the size of major towns, island topography, altitude, and the availability of roads and hiking trails into remote wilderness areas. At a minimum, ten sites were surveyed in and around the main city of each island, 20 sites around coastal areas, and ten inland, either in valleys or at altitude on steep interior mountains. The sampling plan was designed to provide information that would enable the construction of a representative map of G. ashmeadi population distributions for surveyed islands.
In urban areas, parasitoids were sampled for using an insect sweep net on Hibiscus rosa-sinensis (Malvaceae) hedges for one minute. In natural areas (i.e., undeveloped wilderness areas and undisturbed mountain tops), there were often no H. rosa-sinensis, so preferred native plants for H. vitripennis, either Metrosideros collina (Myrtaceae), Weinmannia sp. (Cuonicaceae), or Vaccinium sp. (Ericaceae) were sampled in the absence of H. rosa-sinensis. In addition to sweep netting, the presence or absence of G. ashmeadi was further verified by returning harvested H. vitripennis egg masses to the laboratory for rearing of parasitoids or nymphs. At least ten fresh uneclosed H. vitripennis egg masses were held in labeled individual Petri dishes in the laboratory at 25°C for parasitoid or H. vitripennis emergence. The presence or absence of G. ashmeadi for each sampling method (i.e., sweep netting and rearing) for each sampling point was recorded for each surveyed island and egg mass rearing was used to provide estimates on percentage parasitism (i.e., {the number of parasitized eggs identified by a parasitoid emergence hole/[total number of parasitized eggs and unparasitized eggs from which H. vitripennis nymphs emerged]} × 100) of H. vitripennis eggs by G. ashmeadi.
Surveys for G. ashmeadi were conducted monthly in Tahiti, from June 15 to December 12 2005; every other month on Moorea 17 km to the west of Tahiti (both Tahiti and Moorea are part of the Windward Islands group) from June 9 to January 1 2005. Every third month surveys were made in the Leeward Islands (~200–300 km west of Tahiti; on Huahine from July 1 2005 to March 29 2006; Bora Bora from June 23 2005 to April 3 2006; Raiatea from June 24 to December 20 2005; Tahaa on June 30 and December 21 2005 only; Maupiti on December 18 2005 only). Surveys were conducted every four months in the Marquesas archipelago (~1,400 km northeast of Tahiti; on Nuku Hiva on November 21 2005 and April 22 2006), and the Australs archipelago (~600 km south Tahiti; on Rurutu on January 23 and May 9 2006, on Tubuai on January 19 and May 4 2006). See Grandgirard et al. (2008) and Petit et al. (2008a) for island maps showing monitoring sites.
Results
Dispersal of Gonatocerus ashmeadi on Tahiti
By July 26 2005 (78 days after release) G. ashmeadi had colonized sites 1,000 m West and East from the Papenoo release site on Tahiti (Fig. 1a). No additional dispersal beyond this leading edge of G. ashmeadi on Tahiti was observed. By August 23 2005 (113 days after release), G. ashmeadi had colonized sites 4,600 m West and 5,000 m East from the release site (Fig. 1b), and two additional parasitoid colonies were detected at: (1) Pirae, 10 km west of the release site, and (2) Maraa, 45 km south–west of the release site (Fig. 1b). The G. ashmeadi colony in Pirae was highly localized and did not exceed 500 m in width along the shoreline with 100% parasitism of eggs (n = 13 egg masses examined). In Maraa, the G. ashmeadi colony did not exceed 300 m in width along the shore line and parasitism was 18% (n = 22 egg masses). No other widely dispersed G. ashmeadi populations were detected over the rest of Tahiti. On September 1 2005, a new parasitoid colony was detected in Paea, 35 km south–west of the Papenoo release site (Fig. 1b). The width of this new colony did not exceed 300 m along the shore line and parasitism was 66% (n = 6 egg masses).
By September 15 2005, G. ashmeadi was found at every monitoring site located at 10 km intervals around the coastline of Tahiti (Fig. 1c). Homalodisca vitripennis egg masses were collected at each sampling site on this date, and parasitism rates were approaching 100% all around the island of Tahiti (>10 egg masses collected at all 25 monitoring sites). An exception was an area from Maraa to the peninsula on the west side of Tahiti, where parasitism was measured at just 14–66%. Inland, the dispersal of G. ashmeadi was observed at 65 m of elevation in Papenoo but not at 210 m of elevation (1,200 m from the initial release site). Similarly, parasitoid dispersal and establishment was absent after repeated releases at 800 m elevation at Pirae.
By October 1, November 2 and December 12 2005, G. ashmeadi was found at every monitoring site along the coast of Tahiti, and egg parasitism was consistently exceeding 90%. Inland, the dispersal of G. ashmeadi was observed at 800 m of elevation in Pirae on October 11 2005, and at 1,400 m of elevation in Pirae on November 8 2005, suggesting that the parasitoid was present from sea level to high altitude inland sites by this date (Fig. 1d).
Long-distance dispersal of Gonatocerus ashmeadi from Tahiti to other islands in French Polynesia
Moorea is the closest island to Tahiti and is separated by 17 km of open ocean. No releases of G. ashmeadi were made on Moorea as part of the biological control program to control H. vitripennis. Parasitoids were not detected on Moorea on June 9 2005 and August 16 2005, but G. ashmeadi was found at two sites on September 5 2005 (four months after its first release in Tahiti). The first site on Moorea with G. ashmeadi was at the seaport of Vaiare where parasitism was 100% (n = 25 egg masses). The second site was Pihaena 18 km from the sea port and parasitism was 17% (n = 6 egg masses). On September 26 2005, G. ashmeadi was collected by sweep netting at every monitoring site around the coastline of Moorea. Homalodisca vitripennis eggs were collected on this date and parasitism was approaching 100% at every coastal site sampled (n > 10 egg masses collected from 14 monitoring sites) on Moorea.
On Huahine in the Leeward Islands (175 km northwest of Tahiti), G. ashmeadi was not detected on July 1 2005 but was found on September 19 2005, 4.5 months after the first release in Tahiti. Detection of G. ashmeadi on Huahine was restricted to Fare, the main town, and did not exceed 300 m in width from the harbor area with a 25% parasitism rate (n = 29 egg masses) (Fig. 2a). Sampling revealed that G. ashmeadi was absent from the rest of the island, including the airport. On December 28 2005, G. ashmeadi was collected on most of the monitoring sites around Huahine but was absent on the southern peninsula (Fig. 2b). On March 31 2006, six months after the first record of G. ashmeadi on Huahine, the parasitoid was widespread across most of the monitoring sites including the southern peninsula (Fig. 2c).
On Raiatea (210 km northwest of Tahiti), G. ashmeadi was not observed on June 24 2005 or September 19 2005, but it was detected on the east side of the island by Agricultural Service officials on October 15 2005, five months after the first release on Tahiti. By December 20 2005, G. ashmeadi was widespread across the entire island of Raiatea.
On Bora Bora (260 km northwest of Tahiti), G. ashmeadi was absent on June 23, and September 17 2005, but it was detected over the entire island on December 24 2005, 7.5 months after first release on Tahiti. Similarly, G. ashmeadi was absent on Tahaa (230 km northwest of Tahiti, and 4 km north of Raiatea) on June 30 2005, but it was found at every monitoring site on December 27 2005. G. ashmeadi was detected on Maupiti (315 km northwest of Tahiti) on December 18 2005.
In the Australs archipelago, G. ashmeadi was detected on the island of Rurutu (~600 km south of Tahiti) on January 19 2006, 8.5 months after first release in Tahiti and it was present at most monitoring sites across the island. The parasitism rate was high at Moerai, the main town on Rurutu (71% parasitism of eggs, n = 140 egg masses examined). At Avera, a village on the south end of Rurutu, parasitism was 43% (n = 51 egg masses). On Tubuai (another island in the Australs archipelago, 640 km south of Tahiti), G. ashmeadi was absent on January 23 2005, but it was detected at Matura, the main town, on February 20 2005 by Agricultural Service officials with a parasitism rate of 9% (n = 20 egg masses). On May 4 2006, the parasitoid was widespread in all areas of Tubuai infested by H. vitripennis.
In the Marquesas archipelago, on the island of Nuku Hiva (~1,400 km northeast of Tahiti), G. ahsmeadi was absent on November 21 2005, but a massive decrease of H. vitripennis abundance was observed at Taiohae, the main town, in February 2006 by Agricultural services officials, suggesting an invasion of the parasitoid. On April 22 2006, G. ashmeadi was detected at Taiohae and Haakaui (7 km west from Taiohae), with a parasitism rate of 95% (n = 59 egg masses) and 87% (n = 49 egg masses), respectively.
When taken together, all island survey data showed that G. ashmeadi colonized every surveyed island group in French Polynesia infested by H. vitripennis within 10 months of its first release (i.e., May 2 2005) on Tahiti, even though G. ashmeadi was only released on one island (i.e., Tahiti) in French Polynesia (Fig. 3).
Human-mediated dispersal of Gonatocerus ashmeadi in French Polynesia. The black star is the initial release of G. ashmeadi on Tahiti on May 2 2005. Dates indicate first detection of G. ashmeadi on islands in various archipelagoes and parasitoid spread to other countries (i.e., Easter Island) from French Polynesia
Discussion
Local monitoring and regional surveillance of G. ashmeadi after its introduction to French Polynesia demonstrated a highly stratified dispersal behavior with natural (i.e., flying) continuous local diffusion (Petit et al. 2008a) punctuated by rapid long-distance discontinuous dispersal (shown here). From its initial release on the island of Tahiti in May 2005, G. ashmeadi spread rapidly to very distant island archipelagoes [~1,400 km from Tahiti (e.g., Marquesas Islands)] within 10 months. This long distance dispersal most likely occurred through the unregulated transport of plant material by humans that was unintentionally bearing H. vitripennis egg masses parasitized by G. ashmeadi.
On the island of Tahiti, several parasitoid populations were found at distant and isolated locations from the leading edge of the colonizing wave, suggesting that human transport of plants bearing infected egg masses might have contributed to the dispersal of G. ashmeadi. However, the possibility of natural discontinuous dispersal on Tahiti, perhaps aided by strong winds cannot be totally discounted (Petit et al. 2008a). Similarly, it is possible that the nearby island of Moorea (separated from Tahiti by 17 km of ocean) could have been colonized naturally by a small number of founding parasitoids that underwent long-distance dispersal via wind assisted events from Tahiti. The wind dispersal hypothesis as the predominant means of spreading G. ashmeadi through French Polynesia, is much less likely for the rapid dispersal of G. ashmeadi to much more distant islands [Huahine, Raiatea (~200 km from Tahiti)] and even extremely remote archipelagoes [Marquesas and Australs (~1,400 and 600 km from Tahiti, respectively)]. Gonatocerus ashmeadi was even detected in Easter Island in April 2006, at more that 4,000 km from Tahiti (Marcos Bleeche personal communication). These data strongly suggest that human-mediated spread of G. ashmeadi was primarily responsible for parasitoid arrival in these extremely remote islands.
The French Polynesian government’s decision to carry out a biological control program on Tahiti following a rigorous biosafety and scientifically based risk assessment approach enabled a relatively detailed evaluation of the invasion and colonization processes of G. ashmeadi to distant islands. Sampling data suggests that transport of plants bearing infected egg masses was probably a more significant factor in moving G. ashmeadi among islands than adult ‘stowaways’ on aircraft that visit many of these islands on a daily basis from Tahiti. In Huahine (Leeward Islands), G. ashmeadi was observed first on plants in the immediate vicinity of the sea port. In this instance, the parasitoid colonized areas around the sea ports first and then later areas close to airports. In Bora Bora and Maupiti (Leeward Islands), airports are located on small offshore coral islands (commonly referred to as “motus”) a short distance from the main island. Gonatocerus ashmeadi was not observed on these “motus” in Bora Bora or Maupiti but it was detected on the main islands. These observations suggest that G. ashmeadi did not travel on planes as adults independently of plant material (in which case, one would expect the first colonies to have been detected on vegetation around airports because G. ashmeadi would have attacked H. vitripennis egg masses here once escaping from the plane).
In Tahiti, the parasitoid spread only 200 m in the first month of its release, whereas it took approximately the same amount of time (between August 16 and September 26 2005) for G. ashmeadi to spread over the entire island of Moorea (132 km²). It seems likely that by September 2005, propagule pressure on Moorea was very high and coupled with multiple human-mediated introductions from Tahiti causing simultaneous inoculations all over Moorea resulted in rapid colonization of this island. Inter-island exchanges between Tahiti and Moorea are frequent, with 15 ferry and 25 plane rotations a day and there are no plant protection controls between these two islands. In comparison, Huahine is much more isolated from Tahiti and this probably explains why G. ashmeadi took up to six months to colonize the entire island of Huahine from the first detection, despite its smaller size (75 km²) when compared with Moorea (134 km2) because fewer inoculations were being made with infected plant material bearing parasitized H. vitripennis egg masses from Tahiti or Moorea as a result of lower visitation frequencies.
Inter-island exchanges of foliage and flowers are a common and integral part of traditional Polynesian culture and this behavior may have meditated the rapid long distance spread of G. ashmeadi in French Polynesia (and beyond). For example, flower necklaces and elaborate foliage costumes are part of Polynesian daily life as well as use in special ceremonies. The walls of hotels, shops, and houses are commonly covered with fresh leaf decorations which are replaced regularly. Cordyline and ferns leaves, where H. vitripennis eggs are commonly found, are amongst the most regularly used plants for decorations and ceremonies and are moved readily across and between islands for festive purposes. Furthermore, gardening (vegetables and ornamentals) is an extremely important component of Polynesian culture. New plant types are regularly collected and exchanged from one community to another, often across island groups. As examples, G. ashmeadi was detected at a commercial plant nursery 113 days after release, 10 km west of the sea level release site on Tahiti. Later, on September 10 2005, parasitoids were found on plants at French Polynesia’s premier agricultural festival in Punaauia (near Tahiti’s international airport) where hundreds of farmers, gardeners and horticultural enthusiasts from all over French Polynesia sell and buy plants during this week long event. These examples illustrate how plant exchanges between distant island groups could easily occur thereby inadvertently assisting in the rapid spread of G. ashmeadi (and unwanted pest species) throughout French Polynesia.
The establishment of G. ashmeadi throughout French Polynesia was strongly related to host density and the subsequent availability of host eggs for parasitism. The establishment of G. ashmeadi was non-existent at very low H. vitripennis densities (<2 nymphs minute−1 of sweep net sampling in Pirae (Petit et al. 2008a). However, the parasitoid was able to establish through unassisted dispersal on islands with an average host density of seven H. vitripennis nymphs minute−1 of sweep net sampling (i.e., Tahaa in Leeward Islands (Petit et al. 2008a) and Tubuai in Australs which had an average of ten nymphs minute−1 of sweep net sampling (Petit et al. 2008a). From these field observations, it would appear that a critical host threshold is necessary to increase the ease with which G. ashmeadi can establish following accidental introduction into new areas. Therefore, under prevailing environmental conditions in French Polynesia, a minimum threshold host density of approximately five to seven H. vitripennis nymphs sweep net sampled in one minute may be needed for G. ashmeadi establishment and spread from small accidentally introduced founder populations.
A noxious pest whose invasion speed exponentially accelerates with time through long-distance spread is much harder to control than one exhibiting a constant rate of expansion through natural dispersal (Fagan et al. 2002). The present study has shown that the natural enemy of H. vitripennis, G. ashmeadi, was capable of rapid spread via long distance dispersal and quick establishment at all sites colonized by the pest when host densities exceeded five nymphs minute−1 of sweep net sampling. Furthermore, the rate of spread of G. ashmeadi has appeared to be vastly faster than that of H. vitripennis at 17 vs. 5 km year−1, respectively, (Petit et al. 2008b). Parasitoid spread and establishment through long distance unintentional human movement was faster than that observed for H. vitripennis, with G. ashmeadi taking 10 months to colonize all of French Polynesia versus six years for H. vitripennis. The longer time period exhibited by H. vitripennis that was needed for colonization may have been due to Allee effects experienced by small founding populations during the initial phase of an invasion (Petit et al. 2008b). In direct comparison, high H. vitripennis densities mitigated establishment barriers for G. ashmeadi following accidental introductions which facilitated rapid spread of this biological control agent.
This study has provided a detailed example of the spread of an “invasive species” in a discontinuous island system, and has demonstrated the importance of unintentional human-mediated dispersal assisting rapid and widespread distribution of an upper trophic level organism. Officially, plant movement between Tahiti and the Leeward Islands, Marquesas and Australs archipelagoes must be authorized by the French Polynesian Plant Protection Territorial Service. Since September 2004, heightened quarantine measures were implemented to reduce H. vitripennis dispersal among islands (Grandgirard et al. 2006). Notably, potted plants were officially required to be defoliated to remove H. vitripennis egg masses, treated with insecticides to kill nymphs and adults, and then fumigated with methyl bromide. Treated plants were then inspected for H. vitripennis and rejected if any life stages were found. Cut flowers and foliage were inspected for the presence of H. vitripennis eggs before being moved between islands. If no eggs were found on cut ornamental flowers and foliage, they were treated with a prophylactic application of an aerosolized insecticide. If eggs were found, shipments were rejected or fumigated with methyl bromide. Indications of numerous human-mediated dispersal events of G. ashmeadi as parasitized H. vitripennis eggs suggests that these measures were insufficient or not enforced rigorously enough to prevent spread of G. ashmeadi outside of island groups where establishment was intended.
While not its primary intention, this research effectively used G. ashmeadi as a “biomarker” to track the invasion pathways of H. vitripennis and to assess the efficacy of plant quarantine control measures in the South Pacific. Survey results across four remote island groups in French Polynesia showed that plant quarantine measures were insufficient to curtail the spread of a large, well known, and easily identified invasive plant pest, H. vitripennis. The rapid and vast geographical spread of G. ashmeadi was a major success for the biological control program in the sense that it dramatically and rapidly reduced H. vitripennis at very low cost throughout the pest’s range. It can also be argued that this rapid and uncontrolled spread of G. ashmeadi was a failure of the biological control program in French Polynesia. The initial establishment of G. ashmeadi in French Polynesia was originally intended for Tahiti only. However, this natural enemy spread beyond the intended release range of the program and arrived on island groups that had not been studied fully for non-target impacts. This unintended dispersal raised the concern that native cicadellids could unknowingly be at risk for potential attack by G. ashmeadi. However, this concern has not been realized, and non-target impacts by G. ashmeadi have not been observed in French Polynesia.
It is becoming increasingly important for biological control programs to carry out broad safety studies prior to natural enemy releases. Ideally, non-target impact studies should occur over a suitable geographic range that could climatically accommodate the natural enemy. Identifying the geographic range likely to be exposed to the biological control agent requires an understanding of its colonization capability, dispersal capacity in relation to human behavior, the presence of suitable host species (i.e., target and non-target species), and the effectiveness of biosecurity measures for curtailing unwanted spread. The importance of these points can be illustrated by the self-introduction of Cactoblastis cactorum Berg (Lepidoptera: Pyralidae), a very successful biological control agent of weedy Opuntia spp., after it self-introduction into the southeastern USA from the Caribbean. This invasive moth now threatens rare and native Opuntia spp. in North America (Hight et al. 2002).
For G. ashmeadi, the appropriate area of concern for potential non-target impact assessment studies could have included the central and eastern Pacific. However, this vast range would have greatly increased the cost and scope of the H. vitripennis biological control program to the point of infeasibility and curtailment of the biological control program would have allowed the pest to continue its spread from highly infested areas into new areas of the South Pacific.
There is little doubt that healthy H. vitripennis eggs were leaving Tahiti and arriving on other island groups in French Polynesia, and countries (e.g., Easter Island and the Cook Islands) despite strengthened biosecurity controls to prevent unwanted spread of H. vitripennis from French Polynesia to other areas within the South Pacific. Results of survey work conducted here suggest that aggressive plant pests arriving in Tahiti have a high probability of being able to colonize even the most remote islands of French Polynesia and neighboring countries through the poorly regulated movement of plants. Survey results presented here underscore the immense challenge facing Plant Protection Services in French Polynesia and throughout the South Pacific region. Increased regulation and resources for government action, while necessary, are unlikely to be effective alone. Also needed is a change in the public’s appreciation of the problems posed by invasive species and their modes of spread. Alongside enhanced enforcement of quarantine regulations, improved infrastructure for inspections and personnel training, greater education and outreach efforts, and penalties for perpetrators must all be used to modify public behavior and attitudes towards the illegal transport of biological materials that can vector unwanted invasive propagules.
References
Canto Silva CR, Kolberg R, Romanowski HP, Redaelli LR (2006) Dispersal of the egg parasitoid Gryon gallardoi (Brethes) (Hymenoptera: Scelionidae) in tobacco crops. Br J Biol 66:9–17
Corbett A, Rosenheim JA (1996) Quantifying movement of a minute parasitoid, Anagrus epos (Hymenoptera: Mymaridae), using fluorescent dust marking and recapture. Biol Control 6:35–44
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Fagan W, Lewis MA, Neubert MG, Van den Driessche P (2002) Invasion theory and biological control. Ecol Lett 5:148–157
Gillespie RG, Roderick GK (2002) Arthropods on islands: evolution and conservation. Ann Rev Entomol 47:95–632
Grandgirard J, Hoddle MS, Roderick GK, Petit JN, Percy D, Putoa R, Garnier C, Davies N (2006) Invasion of French Polynesia by the glassy-winged sharpshooter, Homalodisca coagulata (Hemiptera : Cicadellidae): a new threat to the South Pacific. Pac Sci 60:29–438
Grandgirard J, Hoddle MS, Petit JN, Percy D, Roderick GK, Davies N (2007) Pre-introductory risk assessment studies for Gonatocerus ashmeadi (Girault) (Hymenoptera: Mymaridae) for use as a classical biological control agent against Homalodisca coagulata (Say) (Hemiptera: Cicadellidae) in the Society islands (French Polynesia). Biocontrol Sci Technol 17:809–822
Grandgirard J, Hoddle MS, Petit JN, Roderick GK, Davies N (2008) Engineering an invasion: classical biological control of the glassy-winged sharpshooter, Homalodisca vitripennis, by the egg parasitoid Gonatocerus ashmeadi in Tahiti and Moorea, French Polynesia. Biol Invasions 10:135–148
Hengeveld R (1989) Dynamics of biological invasions. Chapman and Hall, London
Hight SD, Carpenter JE, Bloem KA, Bloem S, Pemberton RW, Stiling P (2002) Expanding geographic range of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla Entomol 85:527–530
Hoover W (2004) New invader may threaten crops. Published in: the Honolulu Advertiser, May 14, 2004, Honolulu
Irvin NA, Hoddle MS (2007) Evaluation of floral resources for enhancement of fitness of Gonatocerus ashmeadi, an egg parasitoid of the glassy-winged sharpshooter, Homalodisca vitripennis. Biol Control 40:80–88
Laurent V, Maamaatuaiahutapu K, Maiau J, Varney P (2004) Atlas climatologique de la Polynésie française. Météo France—Direction Interrégionale de la Polynésie française
Leibhold AM, Tobin PC (2007) Population ecology of insect invasions and their management. Ann Rev Entomol 53:387–408
Petit JN, Hoddle MS, Grandgirard J, Roderick GK, Davies N (2008a) Short-distance dispersal behavior and establishment of the parasitoid Gonatocerus ashmeadi (Hymenoptera: Mymaridae) in Tahiti: implications for its use as a biological control agent against Homalodisca vitripennis (Hemiptera: Cicadellidae). Biol Control 45:344–352
Petit JN, Hoddle MS, Grandgirard J, Roderick GK, Davies N (2008b) Invasion dynamics of the glassy-winged sharpshooter Homalodisca vitripennis in French Polynesia. Biol Invasions 10:955–967
Pilkington LJ, Hoddle MS (2006) Reproductive and developmental biology of Gonatocerus ashmeadi (Hymenoptera: Mymaridae), an egg parasitoid of Homalodisca coagulata (Hemiptera: Cicadellidae). Biol Control 37:266–275
Sallam MN, Overholt WA, Kairu E (2001) Dispersal of the exotic parasitoid Cotesia flavipes in a new ecosystem. Entomol Exp Appl 98:211–217
Secretariat of the Pacific Community (2002) Incursion of glassy-winged sharpshooter Homalodisca coagulata in French Polynesia. Pest Alert, Plant Protection Service. http://www.spc.int/PPS/PestAlerts/PestAlertNo24.pdf. Accessed Nov 4 2008
Sorensen JT, Gill RJ (1996) A range extension of Homalodisca coagulata (Say) (Hemiptera: Clypeorrhyncha: Cicadellidae) to southern California. Pan Pac Entomol 72:160–161
Takiya DM, McKamey SH, Cavichioi RR (2006) Validity of Homalodisca and of H. vitripennis as the name for glassy-winged sharpshooter (Hemiptera: Cicadellidae: Cicadellinae). Ann Entomol Soc Am 99:648–655
Triapitsyn SV, Phillips PA (2000) First record of Gonatocerus triguttatus (Hymenoptera: Mymaridae) from eggs of Homalodisca coagulata (Homoptera: Cicadellidae) with notes on the distribution of the host. Fla Entomol 83:200–203
Triapitsyn SV, Mizell RF, Bossart JL, Carlton CE (1998) Egg parasitoids of Homalodisca coagulata (Homoptera: Cicadellidae). Fla Entomol 81:241–243
Acknowledgments
We thank the director of the Service du Développement Rural for providing technical and strategic support on this program. We thank Dr. Charles Garnier, Leopold Stein and Rudolph Putoa for their helpful assistance in organizing the program, and the technicians in charge of the insect rearing: Suzanne Liloi, William Ellacott, René Tupana and Joseph Mamatui. Financial support for research on H. vitripennis has been provided by the French Polynesian Government (Convention no 4.0328). Additional support has come from the University of California at Riverside and Berkeley and the University of California, Division of Agriculture and Natural Resources, the California Department of Food and Agriculture, and the Secretariat of the Pacific Community. This paper is contribution #164 of the UC Berkeley Richard B. Gump South Pacific Research Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dirk Babendreier.
Rights and permissions
About this article
Cite this article
Petit, J.N., Hoddle, M.S., Grandgirard, J. et al. Successful spread of a biocontrol agent reveals a biosecurity failure: elucidating long distance invasion pathways for Gonatocerus ashmeadi in French Polynesia. BioControl 54, 485–495 (2009). https://doi.org/10.1007/s10526-008-9204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-008-9204-7