Abstract
Field experiments and surveys were conducted to evaluate the efficacy of releasing Fopius arisanus (Sonan) and Psyttalia fletcheri (Silvestri) parasitoids for suppression of Bactrocera cucurbitae (Coquillett) infesting wild Coccinia grandis L. In 2003 and 2004, P. fletcheri releases combined with natural emergence from wild fly populations resulted in better fly suppression, compared to the control site. While P. fletcheri developed freely on melon fly, F. arisanus was less successful at producing its own progeny, yet causing mortality and a twofold decrease in pupae recovered from ivy gourds. Concurrent releases of both parasitoids exerted a compounded suppressive effect on the melon fly population 2–3 times higher than during the pre-release phase. A similar, less obvious, pattern occurred in 2004, due to reduction of the ivy gourd fruit canopy. In 2005, only P. fletcheri was released, with greatly reduced impact, due to ivy gourd destruction and by growers leaving crop culls in fields, producing large numbers of melon flies unaffected by parasitoid releases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melon fly, Bactrocera cucurbitae (Coquillett), is a key agricultural pest in Hawaii, USA. The fly infests cultivated crops such as cucumber (Cucumis sativa L.), watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai), cantaloupe (Cucurbita pepo L.), bittermelon (Momordica charantia L.), and zucchini (C. pepo). Two feral hosts, wild bittermelon (M. charantia), and ivy gourd (Coccinia grandis L.) are major melon fly wild hosts in crop environments (Clausen et al. 1965; Harris and Lee 1989). Crop infestation by melon fly in Hawaii causes millions of dollars in losses to growers from direct crop damage and losses in income due to quarantine restrictions imposed on export crops. Many natural enemies were introduced into Hawaii to control melon fly, but the most successful was Psyttalia fletcheri (Silvestri) (Braconidae) (Liquido et al. 1990; Uchida et al. 1990). The United States Department of Agriculture, Agricultural Research Service (USDA-ARS), the University of Hawaii College of Tropical Agriculture and Human Resources, and the Hawaii State Department of Agriculture are implementing an area-wide integrated pest management (AWIPM) program to suppress tephritid pests in Hawaii. The methods of control consist of population monitoring and male annihilation through male lure trapping, GF-120 protein bait spray treatments, field sanitation, sterile insect release technique (SIT), and parasitoid releases. Of the fruit fly control tactics used in the program, parasitoid release has received the least attention in terms of field evaluation. Considering that our laboratory has the capability to produce fruit fly parasitoids in large numbers, the on going AWIPM program has provided the opportunity to assess and quantify the potential impact of parasitoid augmentation when used alone as a strategy for suppression of fruit flies. Vargas et al. (2004) showed that releases of parasitoids in field cages suppressed melon flies infesting ivy gourd. We set up a study to evaluate the potential of releasing two parasitoids for suppressing melon fly in the field.
We initiated research with the objective of applying biological control to suppress melon fly populations infesting wild ivy gourd (C. grandis) in the field, based on the laboratory research reported by Bautista et al. (2004). Combining the use of the egg parasitoid Fopius arisanus (Sonan) (Braconidae) with P. fletcheri was shown to be a promising option under laboratory conditions that needed to be field tested. The north shore of the island of Oahu was chosen as the experimental site for augmentative parasitoid releases, because a large melon fly population in Laie and Kahuku infests a dense canopy of wild ivy gourd growing on trees and shrubs near orchards producing papaya. Reported here are studies on melon fly ecology in ivy gourd and the effects of augmentative releases of F. arisanus and P. fletcheri. Specifically, we studied (1) the population trends of melon fly in ivy gourd stands in Laie and Kahuku, and (2) the effect of open field releases of F. arisanus and P. fletcheri against melon fly infesting ivy gourd in Laie. Information was also generated on emergence and mortality of melon fly reared from ivy gourd fruit collections.
Materials and methods
In 2003, 2004, and 2005, weekly open field releases of F. arisanus and P. fletcheri (egg-larval and larval parasitoids, respectively) were made in Laie, while Kahuku served as the control site for comparison (Fig. 1). Both sites selected for this study consisted of a commercial papaya orchard (8–10 acres in size) bordered by a wild habitat of feral hosts of melon fly, primarily C. grandis. Approximately eight miles apart, the control site was located in Kahuku (N21°40′90″, W157°57′67″) while the parasitoid release site was in Laie (N21°38′26″, W157°55′69″). The two sites, although not necessarily identical, had comparable field sizes, stands of fruit-bearing papayas, and matching wild vegetation of C. grandis, all serving as breeding grounds for melon flies.
Two of us (Rene Bautista and Thomas Mangine) developed the cylindrical cage (Figs. 2, 3) and used it for rearing and releasing F. arisanus parasitoids and for releasing P. fletcheri parasitoids which needed to be evaluated. The P. fletcheri parasitoids used in this research were reared by the USDA-ARS fruit fly rearing unit. Parasitized pupae were placed in the bottom of the cylindrical cage in a screened tray. The parasitoids passed through the screen into the cylindrical cage where they were fed honey streaked on the outside of the cage and provided water through sponges in plastic tubes extending to the top of the cage (Fig. 3). To improve the latent effectiveness of the parasitoids released, they were fed honey and males and females were held together for 5–6 days before release to ensure sexual maturity and mating before release (Bautista et al. 2001). This procedure ensured that released parasitoids were ready to search for and parasitize fruit fly hosts in ivy gourd fruits immediately after release in the field.
Blankets of ivy gourd near papaya orchards were surveyed from November 2002 to December 2005. Baseline information on the relative abundance of melon fly was documented with lure traps and fruit collections before, during and after parasitoid releases. Likewise, the level of melon fly suppression was calculated from parasitoid eclosion and host pupal mortality among puparia recovered from fruit collections. Male lure traps, constructed from plastic bucket containers (Highland Plastics, Pasadena, CA, USA) and baited with a mixture of cue-lure and naled in a dental wick (Dibrom Concentrate, Valent USA Corp, Walnut Creek, CA, USA), were maintained in the ivy gourd sites to monitor melon fly relative abundance (Jackson et al. 2003; Vargas et al. 1990). Commencing 8 April 2003, and at weekly intervals thereafter until 18 July 2003, F. arisanus and P. fletcheri (both 5–6 days old) were liberated in Laie (release sites) within the wild vegetation near borders of papaya orchards. From March 24 to May 24, 2004 and March to September 2005 releases of P. fletcheri were made in Laie. Releases were facilitated using cylindrical screen cages (60 × 65 cm), that held 8–10,000 parasitoids each (Figs. 2, 3). The locations where parasitoids were liberated were rotated every week or more for better coverage and distribution of parasitoids in release sites. At weekly release rates of 50–100,000 parasitoids, about half a million or more P. fletcheri females were released over three months in 2003, two months in 2004, and five months in 2005. Wild ivy gourd provided a major source of melon flies that infest cultivated crops grown near our experimental sites.
The relative abundance of melon fly populations in our experimental sites was monitored with (a) male lure trap captures, expressed as catch per trap per day (CPTD), and (b) emergence of flies and parasitoids and host pupal mortality from infested ivy gourd and papaya fruits. During the 2004–2005 period, the release site was modified extensively by destruction by growers of the canopies of ivy gourd that produced large populations of wild melon flies. The fallow land in Laie was rented to several small-scale growers who planted, grew and harvested cucumber and zucchini squash crops. In Laie (release site) and Kahuku (control site), ivy gourd fruit samples were collected weekly to monitor fruit infestation and parasitism rates.
Data on the effect of parasitism was compared between the treatment area (Laie) and the control (Kahuku), based on trap catches, recovery, mortality, survival and impact of the parasitoids on melon fly in the fruit samples. For fruit collection data, numbers of melon flies, oriental fruit flies, and parasitoids (F. arisanus and P. fletcheri) recovered from fruits collected in Laie and Kahuku were pooled and summarized (fruit flies and parasitoids per kg of fruit). The parasitoid release data was analyzed with PROC GLM ANOVA (SAS 2005), following square-root transformation, to show significant differences (P < 0.05) between the treatment and control plot.
Results
The CPTDs from November 2002 to March 2003 (prerelease period) showed high levels of melon fly populations in both experimental sites (Fig. 4). Within two weeks after P. fletcheri was liberated in 2003, the number of flies trapped in the release site dropped substantially (from ca. 200 to <70 CPTD). This trend leveled off to between 88 and 117 CPTD during the next four months, while parasitoid releases were on-going. During the same period melon fly trap catches in the control site consistently increased in numbers, reaching a peak in early June 2003, when a CPTD of 286 flies was recorded (Fig. 4). Melon fly trap catches increased by fivefold in June–August 2005, when the cucurbit crops were abandoned. The number of P. fletcheri (Fig. 5) recovered from cohorts of melon fly puparia infesting ivy gourd fruits (1.5–3 kg sampled weekly) increased to as high as <600 per kg fruit during and after releases of parasitoids (April–July 2003). Although wild P. fletcheri was present in the natural habitat, it only accounted for less than 7% parasitism of melon fly, compared to ca. 30% when releases of P. fletcheri were undertaken.
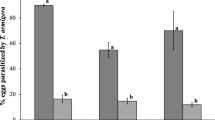
The incidence of melon fly puparia that aborted development increased substantially as releases of parasitoids progressed (Fig. 5). While percent dead puparia averaged ca. 20% before P. fletcheri releases were undertaken, it increased to an average high of 41% during releases, and up to two weeks after releases had ceased. A combination of two mortality factors affected the results: parasitoids that successfully enclosed from host puparia, and dead host puparia, that reduced the number of potential flies that could have otherwise developed into reproducing adults (Table 1). Based on the compounded effects exerted by F. arisanus and P. fletcheri on melon fly populations, we estimated the level of suppression [% impact of parasitoid release = (No. enclosed parasitoids + dead puparia) × 100/total number pupae] to be 2–3 times higher (Fig. 6a) than during the pre-parasitoid release phase of the study. A similar trend is also shown on Fig. 6b. The strategy of liberating P. fletcheri in the natural habitat where highly preferred wild hosts, such as ivy gourd, are present, thus ensuring a reservoir of reproducing melon flies, could prove effective in suppressing movement or migration of egg-laying flies to and from cultivated crops. Where melon fly populations are high, parasitoid augmentation (with P. fletcheri) would be more effective if integrated with other compatible fruit fly control measures first applied to bring down the target pest population to manageable levels.
Discussion
The efficacy of P. fletcheri against melon fly can be underestimated by basing field evaluation solely on the number of parasitoids that emerged from the host puparia. Other mortality factors, such as the observed field incidence of unenclosed host puparia, confirming previous laboratory observations that P. fletcheri caused as much as 40% death in melon fly pupae (Bautista et al. 2004), should be taken into consideration to account for total impact of parasitoids on the melon fly. Based on the results reported by Nishida (1953), melon fly releases should focus on wild rather than cultivated habitats, because gravid females migrate to cultivated crops to oviposit in fruits, and go back out to roost or seek refuge in the wild. Where melon fly populations are extremely high, suppression by release of parasitoids is possible to a certain degree. In 2003, 785 melon flies and 75 oriental fruit flies were recovered from ivy gourd fruit in Laie, compared with seven melon flies and nine oriental fruit flies recovered from ivy gourd fruit in Kahuku. In 2004, 417 melon flies and 99 oriental fruit flies were recovered from ivy gourd fruit in Laie, compared with 152 melon flies and four oriental fruit flies recovered in Kahuku. However, combining parasitoid releases with other compatible strategies to reduce melon fly populations is a far more effective strategy. We believe that a substantial reduction of viable puparia could have resulted from latent mortality inflicted by F. arisanus and P. fletcheri on the developing host, concurring with the up to 40% mortality demonstrated in laboratory (Bautista et al. 2004). In Laie, the melon flies reproduced in large numbers in the ivy gourd canopy and some remained in the canopy, while others migrated to adjacent fields. Both parasitoids collectively suppressed melon fly in ivy gourd in 2003 (Fig. 6a) and 2004 (Fig. 6b). When the ivy gourd canopy was partially destroyed in May 2004, this habitat modification forced more of the flies to switch from ivy gourd to cultivated (cucumber, squash, and papaya) crops, and farmers consequently applied protein bait sprays to control melon fly. The parasitoid releases were less effective in controlling melon fly infesting cucurbit crops, due to bait sprays and fruit harvest before development of the wasps was completed. Although we increased the number of parasitoids released in 2005, the melon fly population became uncontrollable when the growers discontinued crop hygiene practices and bait sprays, due to their high cost. Parasitoid releases were very effective in suppressing melon fly infesting ivy gourd, but less effective on cucumber or zucchini, where fly larvae can burrow deep enough inside fruits to avoid being parasitized.
Populations of Bactrocera dorsalis (Hendel) were also monitored using male lure (methyl eugenol) traps and emergence data from field-collected papayas. The highest levels of B. dorsalis (almost 800 per trap per day) occurred in Laie and Kahuku in July 2004, while melon fly populations in Laie oscillated, but generally remained below 500 per trap per day. No emergence of P. fletcheri from papaya fruits was recorded. From 2,927 papaya-bred puparia from Laie, 777 F. arisanus, 1,189 B. dorsalis and 31 B. cucurbitae emerged. Most F. arisanus came from B. dorsalis infesting papaya as its primary host.
Finally, numerous studies have demonstrated the feasibility of parasitoid augmentation to control fruit flies. In Hawaii, releases of Diachasmimorpha tryoni (Cameron) (at 20,000 per km2 area) more than tripled C. capitata parasitism rates (Wong et al. 1991). Melon fly emergences from fruits were reduced up to 21-fold and numbers of parasitoids were increased 11-fold when P. fletcheri was released in field cages (Vargas et al. 2004). In open field releases of P. fletcheri into ivy gourd patches throughout the Kailua-Kona area (Hawaii island), parasitism rates were increased by 4.7-fold in release plots compared to those in control plots, but resulted in no significant (P > 0.05) reduction in emergence of flies from fruits (Vargas et al. 2004). In Florida, release of 20,000–60,000 D. longicaudata (Ashmead) per week (in 5 and 13 km2 areas) reduced populations of Caribbean fruit fly, Anastrepha suspensa (Loew), by 95% (Sivinski et al. 1996). Other workers in Guatemala have reported successful control of C. capitata in 10 ha coffee farms by augmentative release of D. longicaudata combined with SIT (Cancino-Diaz et al. 1996). In Mexico, aerial releases of D. longicaudata resulted in increased parasitism rates in mango orchards and a 2.7-fold reduction of Anastrepha spp. populations in backyard orchards (Montoya et al. 2000). The shipments of F. arisanus parasitoids to collaborators resulted in successful acclimatization of the ovo-pupal parasitoid F. arisanus in Réunion island for the biological control of the Peach fruit fly, Bactrocera zonata (Quilici et al. 2008; Rousse et al. 2005). The parasitoid is well established now in Réunion breeding on B. zonata, as they are in Hawaii on B. dorsalis.
The releases of F. arisanus and P. fletcheri showed great promise for control of melon fly infesting wild ivy gourd in an undisturbed environment, but their impact at suppressing melon infesting cull fruit piled in the field and the back of farmers sheds is limited, supporting the importance of crop sanitation in helping fruit fly suppression (Piñero et al. 2009).
References
Bautista RC, Harris EJ, Vargas RI (2001) The fruit fly parasitoid Fopius arisanus: reproductive attributes of pre-released females and the use of added sugar as a potential food supplement in the field. Entomol Exp Appl 101:247–255
Bautista RC, Harris EJ, Vargas RI, Jang EB (2004) Parasitization of melon fly (Diptera: Tephritidae) by Fopius arisanus and Psyttalia fletcheri (Hymenoptera: Braconidae) and the effects of fruit fly substrates on host preferences by parasitoids. Biol Control 30:156–164
Cancino-Diaz J, Ruiz JLS, Aguilar E (1996) Evaluacion de liberaciones inundativas de parasitoids Diachasmimorpha longicaudata sobre povlaciones de Ceratitis capitata len fincas en Guatemala C.A. In: Proceedings of the second meeting of the working group on fruit flies of the Western Hemisphere, 3–8 November 1996, Vina del Mar, Chile, p 68
Clausen CP, Clancy, DW, Chock QC (1965) Biological control of the oriental fruit fly (Dacus dorsalis Hendel) and other fruit flies in Hawaii. U S Dep Agr Tech Bull 1322
Harris EJ, Lee CYL (1989) Influence of bitter melon, Momordica charantia L. (Cucurbitaceae), on distribution of melon fly, Dacus cucurbitae Coquillet (Diptera: Tephritidae), on the island of Molokai, Hawaii. Proc Hawaii Entomol Soc 29:49–56
Jackson CG, Vargas RI, Suda DY (2003) Populations of Bactrocera cucurbitae (Diptera: Tephritidae) and its parasitoid, Psyttalia fletcheri (Hymenoptera: Braconidae), in Coccinia grandis (Cucurbitaceae), or ivy gourd, on the island of Hawaii. Proc Hawaii Entomol Soc 36:39–46
Liquido NJ, Cunningham RT, Nakagawa S, Uchida G (1990) Survey of Dacus cucurbitae Coquillet (Diptera: Tephritidae) infestations in the cultivated and weedy forms of Momordica charantia L. (Cucurbitae). Proc Hawaii Entomol Soc 30:31–36
Montoya P, Liedo P, Benrey B, Cancino J, Barrera JF, Sivinski J, Aluja M (2000) Biological control of Anastrepha (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol Control 18:216–224
Nishida T (1953) Ecological study of the melon fly, Dacus cucurbitae Coquillet, in the Hawaiian Islands. Ph.D. Dissertation, University of California, Berkeley, CA
Piñero JC, Mau RFL, Vargas RI (2009) Managing oriental fruit fly (Diptera: Tephritidae), with spinosad-based protein bait sprays and sanitation in papaya orchards in Hawaii. J Econ Entomol 102:1123–1132
Quilici S, Rousse P, Deguine JP, Simiand C, Franck, A, Gourdon F, Mangine T, Harris EJ (2008) Successful acclimatization of the ovo-pupal parasitoid Fopius arisanus in Réunion island for the biological control of the Peach fruit fly, Bactrocera zonata. Communication presented at the 1st Meeting of TEAM (“Tephritid Workers of Europe Africa and the Middle East”), 7–8 April 2008, Palma de Mallorca, Spain
Rousse P, Harris EJ, Quilici S (2005) Fopius arisanus, an egg-pupal parasitoid of Tephritidae: overview. Biocontrol News Info 26:59N–69N. (http://www.cabi.org/default.aspx?site=170&page=1016&pid=34). CAB International, Wallingford, UK
SAS (2005) JMP statistics and graphics guide, release 6. SAS Institute, Cary, NC, USA
Sivinski JM, Calkins CO, Baranowki R, Harris D, Brambila J, Diaz J, Burns RE, Holler T, Dodson D (1996) Suppression of Caribbean fruit fly (Anastrepha suspensa (Loew) Diptera: Tephritidae) population through augmented releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol Control 6:177–185
Uchida GK, Vargas RI, Beardsley JW, Liquido NJ (1990) Host suitability of wild cucurbits for melon fly, Dacus cucurbitae Coquillet, in Hawaii, with notes on their distribution and taxonomic status. Proc Hawaii Entomol Soc 30:37–52
Vargas RI, Stark JD, Nishida T (1990) Population dynamics, habitat preference, and seasonal distribution patterns of oriental fruit fly and melon fly in an agricultural area. Environ Entomol 19:1820–1828
Vargas RI, Long J, Miller NW, Delate K, Jackson CG, Uchida GK, Bautista RC, Harris EJ (2004) Releases of Psyttalia fletcheri (Hymenoptera: Braconidae) and sterile flies to suppress melon fly (Diptera: Tephritidae) in Hawaii. J Econ Entomol 97:1531–1539
Wong TTY, Ramadan MM, McInnis DO, Mochizuki N, Nishimoto JI, Herr JC (1991) Augmentative releases of Diachasmimorpha tryoni (Hymenoptera: Braconidae) to suppress a Mediterranean fruit fly (Diptera: Tehpritidae) population in Kula, Maui, Hawaii. Biol Control 1:2–7
Acknowledgements
We thank Drs. Grant McQuate and Stella Chang for their review of an earlier version of this manuscript. We thank Thomas Mangine and Steven Graham for their technical assistance in completion of this research. We thank Leonard Peters (Laie Properties) for approval to use the Laie test site. We thank Kenneth Kamiya (Brigham Young University Farms) who has supported for many years field testing of parasitoid strains. We also acknowledge the support of Clyde Fukuyama for permission to use his Kahuku papaya farm as control plot.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dirk Babendreier
Rights and permissions
About this article
Cite this article
Harris, E.J., Bautista, R.C., Vargas, R.I. et al. Suppression of melon fly (Diptera: Tephritidae) populations with releases of Fopius arisanus and Psyttalia fletcheri (Hymenoptera: Braconidae) in North Shore Oahu, HI, USA. BioControl 55, 593–599 (2010). https://doi.org/10.1007/s10526-010-9282-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-010-9282-1