Abstract
The effectiveness of the egg parasitoid Trichogrammatoidea armigera Nagaraja (Hymenoptera: Trichogrammatidae) in controlling Heliocheilus albipunctella de Joannis (Lepidoptera: Noctuidae), a major insect pest of pearl millet in the Sahel was assessed during two consecutive years in Niger on-station and on-farm conditions. We found that released T. armigera were able to find and parasitize host eggs within pearl millet fields both on-station and in farmers’ fields. On-station releases of T. armigera led to an average 4.86-fold increase in T. armigera parasitism compared to control fields, where no parasitoids were released. Likewise, on-farm releases of T. armigera led to up to 5.31-fold more egg parasitism by T. armigera in release fields than in control. Our results suggest the effectiveness of T. armigera and lays the groundwork for using T. armigera in augmentative biological control of H. albipunctella in the Sahel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cropping system in the Sahel typically includes pearl millet, Pennisetum glaucum (L.) R. Br. (Poaceae) associated with cowpea, Vigna unguiculata (L.) Walp. (Fabaceae), and sometimes sorghum, Sorghum bicolor (L.) Moench (Poaceae). Niger is the largest producer of pearl millet with 7.2 million ha (FAO 2019). Over the years, pearl millet grain yields have remained stagnant, usually below 500 kg ha−1. Production is affected by many abiotic and biotic factors, including insect pests. The most important insect pest for pearl millet in the Sahel is the millet head miner (MHM), Heliocheilus albipunctella de Joannis (Lepidoptera: Noctuidae). This insect is responsible for chronic yield losses of up to 85% (Krall et al. 1995; Gahukar and Ba 2019). Control measures (cultural control, host-plant resistance, and the application of pesticides) are ineffective, impractical, or not economically feasible (Gahukar and Ba 2019).
Biological control with the larval parasitoid Habrobracon (= Bracon) hebetor Say (Hymenoptera: Braconidae) has emerged as the most attractive solution for controlling MHM (Gahukar et al. 1986; Bhatnagar 1987). Recently, augmentative releases of H. hebetor have been successful against MHM in the Sahel (Payne et al. 2011; Ba et al. 2013, 2014; Baoua et al. 2014, 2018) and a biological control program with H. hebetor was designed for Sahelian countries (Guerci et al. 2018). However, the parasitoid H. hebetor only targets the third and later instar larvae of MHM when the insect has already started feeding on millet grains (Gahukar and Ba 2019). Early control of MHM may be better achieved with releases of egg parasitoids, especially trichogrammatid species (Hymenoptera: Trichogrammatidae). trichogrammatids have been widely used for biological control of Lepidopteran pests for many decades in different cropping systems (Li 1994; Smith 1996; Jalali et al. 2016; van Lenteren et al. 2018; Parra and Coelho 2019). They have interesting features that make them popular in biological control programs worldwide. They have a short cycle, high reproductive potential, and are usually inexpensive and easy to produce in large numbers on eggs of factitious hosts (Li 1994; Greenberg et al. 1996; Parra 2010; Wang et al. 2014; Jalali et al. 2016). Recently, the parasitoid Trichogrammatoidea armigera Nagaraja (Hymenoptera: Trichogrammatidae) was observed to parasitize 12.4% and 17% of MHM eggs in the field in Senegal and Niger, respectively (Karimoune et al. 2018; Sow et al. 2018). Although parasitoids of the genus Trichogrammatoidea (Hymenoptera: Trichogrammatidae) are less studied than the related genus Trichogramma (Hymenoptera: Trichogrammatidae), they share similar features (Smith 1996) and are both successfully used in augmentative biological control (Newton 1988; Newton and Odendaal 1990; Mohamed et al. 2016; Khatun et al. 2017; Cagnotti et al. 2018). A prerequisite for the use of Trichogramma in augmentative biological control requires that large numbers of parasitoids can be efficiently reared (Parra 2010). In the case of MHM, culture of the parasitoid T. armigera has been successfully established on eggs of the rice moth, Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) in Niger (Karimoune et al. 2018). Additionally, laboratory performance of trichogrammatid can be indicative of field performance as indicated by Coelho et al. (2016). In the laboratory, T. armigera was found to parasitize nearly 80% of MHM eggs (Karimoune et al. 2018). These results suggest strong potential for the use of T. armigera in augmentative releases for MHM control. The present study was a follow-up of early laboratory studies (Karimoune et al. 2018) and is aimed at evaluating the field performance of T. armigera after releases against MHM.
Materials and methods
Study environment
The experiments were conducted during two consecutive cropping seasons (May–October in 2017 and 2018) at a research station and in farmers’ pearl millet fields in Niger. The on-station experiment was conducted at the research campus for the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) at Sadore in Niger (latitude 13° 15′ N, longitude 2° 18′ E). The on-farm experiments were performed in selected farmers’ fields in villages surrounding the ICRISAT campus in the region of Tillabery that lies between latitudes 13° 13′ 41.03″ and 13° 25′ 09.00″ N, and longitudes 02° 08′ 56.09″ and 02° 18′ 16.05″ E. This agroecosystem has a unimodal rainfall pattern with the rainy season extending from mid-May to mid-October. Total annual rainfall amounts recorded in 2017 and 2018 were 592 mm and 606 mm, respectively. The temperature during the pearl millet growing season was in the range of 20–40 °C, with an average of 28 °C. During this period, the area had continuous pearl millet crops covering almost 80% of the cultivated area, usually in association with cowpea. Farmers grow 2–3 local pearl millet varieties on 3–10 ha. No irrigation, chemical fertilizers, or pesticides were applied to the pearl millet crops.
Insect cultures
The insects mass-reared for this study included the millet head miner, H. albipunctella, the rice moth, C. cephalonica, and the parasitoid wasp, T. armigera. The moths of H. albipunctella were collected from five light traps that were set up within the 500 ha area of the ICRISAT Sadore campus. The light trap utilized a 250-W mercury vapor white incandescent bulb wired to a grid. The bulb was positioned above a wire mesh cage (1.38 m width × 1.93 m height), which rested on a metal support set 2.43 m above the ground level. The light trap was operated during the rainy season from June to October in 2017 and 2018. Collected moths were transferred to egg-laying wire mesh cages (30 cm × 30 cm) in the laboratory. A piece of freshly harvested millet panicle was placed in the cage as a surface for MHM moths to lay eggs. The panicle was examined daily, and eggs were collected for use as sentinel eggs for on-station trials.
A colony of C. cephalonica was established in the laboratory at ICRISAT Sadore from wild insects collected from farmers’ granaries in Niger in 2015. The insects were routinely reared on a mixture of pearl millet grain and flour in plastic buckets at ambient temperature (Ba et al. 2014). Adults typically emerged after one month. The eggs of C. cephalonica were used for rearing the parasitoid T. armigera and as sentinel eggs for on-station trials. The eggs were irradiated to halt their development, as suggested by Parra (2010), to avoid cannibalization of both parasitized and unparasitized eggs by hatched larvae from unparasitized eggs.
The T. armigera parasitoid population was initially started from field-collected eggs of MHM from different regions of Niger as described by Karimoune et al. (2018). Eggs were kept in Petri dishes in the laboratory at the ambient conditions described above until the emergence of adults. Emerging T. armigera were collected daily, sexed, and placed in tubes containing freshly laid eggs of the rice moth, C. cephalonica. The host eggs were glued on white rectangular cards (4.5 cm × 1.75 cm), and a drop of honey was placed at the corner of the card to provide food for adult parasitoids. The culture was routinely maintained by exposing a first set of 30 eggs (one day old) of C. cephalonica to a mated T. armigera female. This was followed by subsequent sets of 30 eggs (one day old) every day until the death of the female. The females were given a new male for mating every three days for 24 h. The average life span of T. armigera females was approximately 12 days. The development from eggs to adults was completed in seven days. Each female produced approximately 100 individuals in a balanced sex ratio.
Preparation of the T. armigera card for on-station and on-farm field releases
All T. armigera parasitoids used in the releases were from laboratory colonies reared on a factitious host, C. cephalonica. Parasitoid cards (12.5 cm × 9 cm dimension) were prepared seven days prior to field releases. Each card had 14,000 irradiated eggs of C. cephalonica parasitized by 250 T. armigera mated females for six days. T. armigera adults began emerging from cards the day they were placed in the field, and, after three days, all the parasitoids had emerged. Each card produced approximately 8800 parasitoids. The parasitoid cards were placed individually in a thick envelope to protect them from sunlight, direct rainfall, and predation. The envelope was perforated to create small holes that allow emerging T. armigera adults to disperse. Given that no recommendation currently exists for the number of T. armigera to be released, we released a number of 150,000 parasitoids ha−1 (at a sex ratio of 1:1), considering available data on T. armigera life table (Karimoune et al. 2018) and the most frequently recommended doses for other Trichogramma species in other settings (Bigler 1986; Wang et al. 2014; Tang et al. 2017).
Experimental design for on-station trials
This experiment was performed once in the rainy season of 2017, and twice in the rainy season of 2018. In 2017, the millet was planted in mid-June and panicles began emerging in mid-August. The first trial in 2018 was conducted in an early-planted farm (mid-May) where millet panicles emerged in mid-July, whereas the second trial was conducted in a late-planted farm (mid-July) where millet panicles emerged in mid-September. For each of the three trials an improved popular millet variety named HKP (80 days maturing cycle, sensitive to MHM) was planted at the spacing arrangement of 1 m × 1 m. For each trial, the millet in six fields of 1600 m2 each and all consecutive fields were separated by 200 m of grassland. The six fields were divided into two groups of three: (1) release fields that were each supplied with T. armigera parasitoids and (2) control fields that did not receive parasitoids.
Within each field, millet panicles were randomly selected. A stake flag was used to mark the central point of the field (= 0 m) and in both directions along the NW–SE, SW–NE, E-W and S–N axis at 10 m and 20 m from the central point of the field. At each station, seven panicles were marked. A modified protocol of that described by Chapman et al. (2009) and Geetha and Balakrishnan (2010) was used for infesting millet heads with sentinel eggs before parasitoid releases. In 2017, we were able to infest only 2.68 panicles per station because of limited MHM eggs. For this reason, in 2018, the marked panicles were infested with either MHM or rice moth eggs to account for the shortage of MHM eggs. The viability of this method was supported by early laboratory findings suggesting equal parasitism of rice moth and MHM eggs by T. armigera (Karimoune et al. 2018). We managed to infest 5.15 panicles per station in the 2018 early-planted trial and 6.43 panicles per station in the 2018 late-planted trial. All eggs used in these experiments were irradiated to halt larval development, thus preventing eggs from hatching. Irradiation of MHM and rice moth eggs does not affect their acceptability by T. armigera (Karimoune et al. 2018). Infestation was performed at the 30% exertion development stage of the panicle to mimic the preferred oviposition stage of the millet head miner (Karimoune et al. 2018; Owusu et al. 2004). To mimic the preferred oviposition site (Gahukar and Ba 2019), eggs were adhered with non toxic glue to the rachis of the panicle within 3–5 cm of the tip/head. Each selected panicle was infested with 40 eggs of either MHM or rice moth. In 2017, 100% of the treated panicles were infested with MHM eggs, whereas only 7% and 25% of the treated panicles were infested in the 2018 early-planted and late-planted trials, respectively.
Infestation of millet panicles with host eggs occurred on the same day as the placement of parasitoid cards. The parasitoids were released once at the central point of the field using cards with parasitized C. cephalonica eggs the day T. armigera adults began emerging. The envelopes bearing the parasitoid cards were fastened to a millet stalk at 0.5 m above ground in the central point of the field. All the parasitoids typically emerged in three days (Karimoune et al. 2018). No parasitoids were released in any of the control fields. Eggs of MHM and C. cephalonica that were placed on millet panicles in both release and control fields were recollected six days after exposure and taken back to the laboratory for incubation and observation of parasitism, emergence of parasitoids, and dead eggs.
Experimental design for on-farm trials
This experiment was conducted in the rainy season in 2017 and 2018 in selected farmers’ fields in the district of Say. For each season, the experiment was performed within three different villages (Deyyebon, Gilliel, and Worseno in 2017; and Mansaré-Boura, Lelehi-Koynounga, and Boumba in 2018). In each village, we selected one early-planted millet farm of at least 5 ha. Early-planted farms are typically more likely to be infested by the millet head miner (Gahukar and Ba 2019). Within that farm, we delineated two plots of 400 m2 at two ends of the field, such that the two plots were separated by at least 200 m. The two plots were assigned the following treatments: (1) T. armigera release fields that were supplied with T. armigera cards and (2) control fields that did not receive parasitoids. Each village represents a block with two treatments and three villages represent three replicates. For parasitoid releases, we placed the parasitoid cards after the millet panicle emergence stage and detection of the presence of MHM eggs in the field. We performed one release of the parasitoids at the dose of 150,000 per hectare.
Before the release of T. armigera, data on MHM infestation (number of heads-bearing eggs) was recorded on 100 randomly selected millet heads for both T. armigera release and control fields. The same observations were performed six days after parasitoid releases, but this time, 100 panicles bearing MHM eggs were taken to the laboratory for assessment of egg parasitism. The eggs were reared in the laboratory at ambient temperature until the emergence of parasitoids. Emerging parasitoids were identified and counted.
Data analysis
The parasitism percentage was calculated by the ratio of the total number of parasitized eggs divided by the total number of collected eggs. For on-station parasitism, MHM and rice moth parasitism was aggregated and computed by treatments as early findings suggest equal parasitism of T. armigera for both host species (Karimoune et al. 2018). For each season, data on parasitism and parasitized eggs with viable offspring were subjected to one-way ANOVA to compare release fields with control fields for both on-station and on-farm data. In addition, data of all years were combined together and subjected to two-way ANOVA to compare treatments and years and their interactions. Data were all subjected to arcsine transformation prior to analysis of variance with the SAS software version 9.1 (SAS 2003).
For on-station trials, we also assessed T. armigera parasitism at 0, 10, and 20 m distance from release points. When ANOVAs were significant, means were compared by the Student–Newman–Keuls tests at the 5% level. All parameters are presented as mean ± SE.
Results
Parasitism of MHM eggs following on-station releases of T. armigera
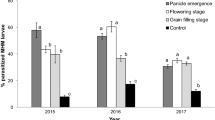
On average, 74.2 ± 1.81% of sentinel eggs were not recovered from infested millet panicles. However, significantly more egg parasitism by T. armigera was recorded in release fields (55–90%) than in control fields (12–16%), irrespective of season and year (2017: F1,4 = 566.14, P < 0.001; 2018-early planting: F1,4 = 40.38, P = 0.003; 2018 late planting: F1,4 = 14.23, P = 0.02; Fig. 1). The overall parasitism for all three years was significantly higher in release fields than control fields (71.51 ± 6.94% vs. 14.42 ± 1.34%, respectively; F1,12 = 102.88, P < 0.001). However, a significant difference was not found for the interaction between years and treatments (F2,12 = 2.94; P = 0.09). Once released T. armigera dispersed from release point and parasitism was significantly higher at the release point than 10 and 20 m away in both 2018 experiments (Table 1). Likewise, for the combined two experiments, parasitism was significantly higher at the release point than 10 and 20 m away (F2,20 = 14.85, P < 0.001).
Parasitism of MHM eggs following on-farm releases of T. armigera
On average, 25.99 ± 6.98% of millet panicles were naturally infested by MHM in farmers’ fields in 2017. The infestation dropped to only 16.05 ± 3.10% in 2018. However, there was no significant difference for head miner infestation between control and release fields in 2017 (F1,4 = 0.001, P = 0.98) and 2018 (F1,4 = 0.44; P = 0.54). The infested panicles had, on average, 11.57 ± 1.64 eggs of the head miner in 2017 and 12.40 ± 2.34 eggs in 2018. Likewise, there was no significant difference between number of eggs recorded in the panicles of control and release fields in 2017 (F1,4 = 1.24, P = 0.32) and 2018 (F1,4 = 0.96, P = 0.38).
The releases of T. armigera caused up to 60% egg parasitism by T. armigera in release fields compared to up 15% in control fields (2017: F1,4 = 26.39, P = 0.007; 2018: F1,4 = 8.57, P = 0.04; Fig. 2). The overall egg parasitism by T. armigera for two years was significantly higher in release fields than control fields (45.40 ± 7.72% vs. 10.53 ± 3.86%, respectively; F1,8 = 32.48, P < 0.001). However, a significant difference was not found for the interaction between years and treatments (F2,8 = 2.40, P = 0.16).
Emergence of T. armigera adults from eggs parasitized on-station and on-farm
Typically, the parasitized eggs in release and control fields yielded similar numbers of T. armigera progeny in both on-station and on-farm fields regardless of the season, except for the 2018 early-planting on-station experiment (Table 2). For all three years, up to 88% (on-station) and 98% (on-farm) parasitized eggs yielded viable T. armigera adults with no significant difference between release and control fields (On-station: F1,12 = 0.15, P = 0.70; On-farm: F1,8 = 0.43, P = 0.53). Likewise, a significant difference was not found for the interaction between years and treatments for on-station (F2,12 = 2.23, P = 0.15) and on-farm parasitoid emergence (F1,8 = 1.36, P = 0.27).
Discussion
In the current study, we found the natural parasitism of MHM eggs due to T. armigera in the field to vary between 5 and 16% in 2017 and 2018 on sentinel eggs or naturally laid eggs. This level of parasitism is similar to recent results on the natural parasitism of MHM eggs by T. armigera in the same area (Karimoune et al. 2018). This low level of natural parasitism justified the need for augmentative releases (van Driesche and Bellows 1996; Collier and van Steenwyk 2004). Indeed, from the different experiments, releases of T. armigera led to significantly higher parasitism of MHM eggs compared to control fields where parasitoids were not released. Similar findings were reported for other trichogrammatid egg parasitoids in open field conditions in corn cropping systems infested with the Asian corn borer, Ostrinia furnacalis Guenée (Lepidoptera: Crambidae), and the earworm, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) in China (Wang et al. 2014). Likewise, in Latin America, trichogrammatid parasitoid releases significantly increase egg parasitism of Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae), Diatraea spp. (Lepidoptera: Pyralidae), and Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in crops such as cotton, corn, and sugarcane (Bueno and van Lenteren 2002; Díaz et al. 2012).
Although releases of T. armigera increase MHM egg parasitism by 4–5 folds, the overall egg parasitism ranged between 30–90%. This was not unexpected as observed in other settings with other Trichogramma species (Bigler 1986; Bourchier and Smith 1998; Parra 2010). However, releases of T. armigera will subsequently reduce MHM larvae population in the field. These larvae will be exposed to natural predation and parasitism (Gahukar and Ba 2019) and ultimately controlled by augmentative releases of the larval parasitoid H. hebetor (Ba et al. 2013, 2014; Baoua et al. 2014, 2018). However, this needs further testing as the interaction of different parasitoid species can lead to contrasting results (Briggs and Latto 2001; Finke and Denno 2005; Grieshop et al. 2006; Yamamoto et al. 2007; Bigler et al. 2010).
As indicated by van Lenteren and Bigler (2010), the quality of parasitoids reared in large numbers is critical for use in biological control programs. This suggests that mass rearing should not affect parasitoid performance once released in the field. Furthermore, it can be interesting when released parasitoids produce viable progeny especially when the target pest lays eggs over several weeks. This can evade the need for multiple releases of parasitoids in the same season. Interestingly, our data suggests successful development of T. armigera after releases with typical levels of viable progeny that were similar for control and release fields. Given that MHM eggs are laid during a period of six weeks (Gahukar and Ba 2019), the initially released parasitoids will parasitize the first batch of eggs encountered and the emerging progeny could parasitize subsequent eggs being laid by MHM.
Our study showed positive behavior characteristics for T. armigera as a biological control agent in pearl millet crops. Results suggest the feasibility of augmentative releases of parasitoids in pearl millet cropping systems as previously reported for the larval parasitoid H. hebetor in the same system (Ba et al. 2014; Baoua et al. 2014, 2018; Amadou et al. 2017). We found that released T. armigera were able to find and parasitize host eggs within the pearl millet field both for sentinel eggs on-station and naturally infested eggs on farmers’ fields. According to Jones et al. (2014), sentinel eggs could dramatically underestimate actual rates of parasitism. In our study, the contrary was observed: the sentinel eggs had significantly more parasitism than wild eggs. Sentinel eggs were also found to be good for estimating parasitism following Trichogramma parasitoid releases in many settings (Bourchier and Smith 1998; Wright et al. 2001; Mansfield and Mills 2002; Gardner et al. 2012; Tang et al. 2017). The difference in parasitism by wild and sentinel eggs could be due to the duration of exposure. Sentinel eggs were irradiated and therefore had more exposure to T. armigera than wild eggs. Wild eggs could have been there for only a few hours and then collected before being parasitized by T. armigera. Nevertheless, the parasitism of sentinel eggs by T. armigera could have been overestimated because of the lower number of exposed eggs to released parasitoid ratio. As suggested by on-station data, nearly 75% of the sentinel eggs were not recovered after exposure. This could be due to predation; the millet panicles are visited by several species of mirid bugs and earwigs that prey on MHM eggs (Baoua et al. 1997; Sow et al. 2018). Predation of sentinel eggs has also been reported in other settings (Figueiredo et al. 2002; Chapman 2007; Cornelius et al. 2016; Herlihy et al. 2016). The sentinel eggs could have also been washed away by rain. In fact, 14–40 mm of rainfall was recorded in the 1–4 days following sentinel egg exposure in 2017 and 2018. The data on sentinel eggs is also a good indication of how rain and predators contribute to the reduction of MHM population.
T. armigera parasitism on wild MHM eggs in farmers’ fields was higher in 2017 compared to 2018, whereas MHM infestation was lower. This suggests a typical density-dependent behavior as reported previously for T. armigera (Karimoune et al. 2018), related species such as Trichogrammatoidea sp. nr. lutea (Girault) (Kalyebi et al. 2005), and other Trichogramma parasitoids (Gross et al. 1984; Wang and Ferro 1998). In our experiment, we released the same number of T. armigera regardless of MHM infestation. Since we did not have a recommendation for this parasitoid, we used available data on T. armigera life table and most frequently recommended doses for other Trichogramma species to start with a dose of 150,000 T. armigera per hectare. These findings indicate that the numbers to be released per millet acreage need to be adjusted according to the level of MHM infestation to avoid the release of excess numbers that could lead to superparasitism as reported for Trichogramma (Sousa and Spence 2000; Dorn and Beckage 2007; DaSilva et al. 2016), and thus reduce parasitoid efficiency (Knipling 1977; Hoddle et al. 1997; Crowder 2007).
Parasitism at the release point was higher than 10 m and 20 m from the release point. This is consistent with previous studies reporting a decrease in parasitism of Trichogramma with increasing distance from the point of release (Greatti and Zandigiacomo 1995; Chapman et al. 2009). In our study, the 10 m and 20 m distances recorded between 46 and 66% parasitism, which is still high. This was higher than the 8 m dispersal observed for T. pretiosum released to control Lepidopteran pests in soybean (Bueno et al. 2012) and the 11 m dispersal for T. brassicae released for O. nubilalis control in corn (Mills et al. 2006). Other studies reported dispersal distances of 45 m and 365 m at four days and 14 days post-release, respectively, for T. ostriniae in corn (Wright et al. 2001; Gardner et al. 2012) and potato (Chapman et al. 2009). We cannot speculate about longer dispersal distance for T. armigera as our investigation stopped at 20 m from the release point and only lasted six days after post-release. As indicated by several studies, Trichogramma dispersal and performance is mainly habitat-specific, which is affected by weather, crop plant architecture, pest cycle, and predation (Thorpe 1985; Andow and Prokrym 1990; Smith 1996; Romeis et al. 1997, 1998; McCravy and Berisford 1998; Cloyd and Sadof 2000; Gingras et al. 2008). For these reasons, the conditions for field release of T. armigera needs further investigation. Most important, the timing for T. armigera release needs to be identified. MHM moth flight is usually limited to six weeks, and females lay eggs on emerging panicles of millet (Gahukar and Ba 2019). Therefore, there is a narrow window for parasitoid release. Our future investigation will also include the number of parasitoids to be released per area of millet and the number of releases. We will also investigate the effect of T. armigera on MHM damage and grain yield and the effect of combined releases of T. armigera and the larval parasitoid H. hebetor on controlling MHM and the overall effect on millet grain yield. Finally, further investigation will be needed for assessing the economic viability of the technology.
This study showed that augmentative releases of the egg parasitoid T. armigera significantly increases the parasitism of MHM eggs in the field. This finding lays the groundwork for use of T. armigera for complementing the existing biocontrol program with the larval parasitoid, H. hebetor in an integrated approach for controlling the MHM in the Sahel.
References
Amadou L, Baoua I, Ba M, Haussmann B, Altiné M (2017) Gestion de la chenille mineuse de l'épi du mil par des lâchers du parasitoïde Habrobracon hebetor Say au Niger. Cah Agric 26:55003
Andow DA, Prokrym DR (1990) Plant structural complexity and host finding by a parasitoid. Oecologia 82:162–165
Ba MN, Baoua IB, N’Diaye M, Dabiré-Binso C, Sanon A, Tamò M (2013) Biological control of the millet head miner Heliocheilus albipunctella in the Sahelian region by augmentative releases of the parasitoid wasp Habrobracon hebetor: effectiveness and farmers’ perceptions. Phytoparasitica 41:569–576
Ba MN, Baoua IB, Kaboré A, Amadou L, Oumarou N, Dabire-Binso C, Sanon A (2014) Augmentative on-farm delivery methods for the parasitoid Habrobracon hebetor Say (Hymenoptera: Braconidae) to control the millet head miner Heliocheilus albipunctella (de Joannis) (Lepidoptera: Noctuidae) in Burkina Faso and Niger. BioControl 59:689–696
Baoua IB, Gingras J, Tourneur JC (1997) Feeding habits of Forficula senegalensis (Derm.: Forficulidae) on millet in the sudanese-sahelian zone of Niger: crop content analysis. BioControl 42:537–542
Baoua I, Amadou L, Oumaro N, Payne W, Roberts J, Stefanova K, Nansen C (2014) Estimating effect of augmentative biological control on grain yields from individual pearl millet heads. J Appl Entomol 138:281–288
Baoua IB, Ba MN, Amadou L, Kaboré A, Dabiré-Binso CL (2018) Field dispersal of the parasitoid wasp Habrobracon hebetor (Hymenoptera: Braconidae) following augmentative release against the millet head miner Heliocheilus albipunctella (Lepidoptera: Noctuidae) in the Sahel. Biocontrol Sci Technol 28:404–415
Bhatnagar VS (1987) Conservation and encouragement of natural enemies of insect pests in dryland subsistence farming: problems, progress and prospects in the Sahelian zone. Int J Trop Insect Sci 8:791–795
Bigler F (1986) Mass production of Trichogramma maidis Pint et Voeg and its field application against Ostrinia nubilalis Hbn. in Switzerland. J Appl Entomol 101:23–29
Bigler F, Babendreier D, van Lenteren JC (2010) Risk assessment and non-target effects of egg parasitoids in biological control. In: Cônsoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, New York, pp 413–442
Bourchier RS, Smith SM (1998) Interactions between large-scale inundative releases of Trichogramma minutum (Hymenoptera: Trichogrammatidae) and naturally occurring Spruce budworm (Lepidoptera: Tortricidae) parasitoids. Environ Entomol 27:1273–1279
Briggs CJ, Latto J (2001) Interactions between the egg and larval parasitoids of a gall forming midge and their impact on the host. Ecol Entomol 26:109–116
Bueno VHP, van Lenteren JC (2002) The popularity of augmentative biological control in Latin America: history and state of affairs. In: First international symposium on biological control of arthropods, proceedings of the International Organization of Bbiological Control, Honolulu, Hawai, USA, 14–18 Jan, pp 180–184
Bueno RCOF, Parra JRP, Bueno AF (2012) Trichogramma pretiosum parasitism and dispersal capacity: A basis for developing biological control programs for soybean caterpillars. Bull Entomol Res 102:1–8
Cagnotti CL, Riquelme VM, Botto EN, Lopez SN (2018) Dispersion and persistence of Trichogrammatoidea bactrae (Nagaraja) over Tuta absoluta (Meyrick), in tomato greenhouses. Neotrop Entomol 47:553–559
Chapman AV (2007) Optimizing Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) releases to control European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae) in bell pepper. MSc thesis, Virginia Polytechnic Institute and State University, Blacksburg
Chapman AV, Kuhar TP, Schultz PB, Brewster CC (2009) Dispersal of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in potato fields. Environ Entomol 38:677–685
Cloyd RA, Sadof CS (2000) Effects of plant architecture on the attack rate of Leptomastix dactylopii (Hymenoptera: Encyrtidae) a parasitoid of the citrus mealybug (Homoptera: Pseudococcidae). Environ Entomol 29:535–541
Coelho A Jr, Rugman-Jones PF, Reigada C, Stouthamer R, Parra JR (2016) Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). PLoS ONE 11(1):e0146153
Collier T, van Steenwyk RA (2004) critical evaluation of augmentative biological control. Biol Control 31:245–256
Cornelius ML, Dieckhoff C, Vinyard BT, Hoelmer KA (2016) Parasitism and predation on sentinel egg masses of the brown marmorated stink bug (Hemiptera: Pentatomidae) in three vegetable crops: importance of dissections for evaluating the impact of native parasitoids on an exotic pest. Environ Entomol 45:1536–1542
Crowder DW (2007) Impact of release rates on the effectiveness of augmentative biological control agents. J Insect Sci 7:15
DaSilva CSB, Morelli R, Parra JRP (2016) Effects of self-superparasitism and temperature on biological traits of two neotropical Trichogramma (Hymenoptera: Trichogrammatidae) species. J Econ Entomol 109:1555–1563
Díaz MF, Ramírez A, Poveda K (2012) Efficiency of different egg parasitoids and increased floral diversity for the biological control of noctuid pests. Biol Control 60:182–191
Dorn S, Beckage NE (2007) Superparasitism in gregarious hymenopteran parasitoids: ecological, behavioural and physiological perspectives. Physiol Entomol 32:199–211
FAO (2019) FAOSTAT Database Query, Food and Agriculture Organization of the United Nations, Rome. https://faostat3.fao.org/browse/Q/QC/E. Accessed 5 Dec 2019
Figueiredo MLC, Della Lucia TMC, Cruz I (2002) Effect of Telenomus remus Nixon (Hymenoptera: Scelionidae) density on control of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) egg masses upon release in a maize field. Rev Bras Milho Sorgo 1:12–19
Finke DL, Denno RF (2005) Predator diversity and the functioning of ecosystems: The role of intraguild predation in dampening trophic cascades. Ecol Lett 8:1299–1306
Gahukar RT, Guevremont H, Bhatnagar VS, Doumbia YO, Ndoye M, Pierrard G (1986) A review of the pest status of the millet spike worm, Raghuva albipunctella de Joannis (Noctuidae: Lepidoptera) and its management in the Sahel. Int J Trop Insect Sci 7:457–463
Gahukar RT, Ba MN (2019) An updated review of research on Heliocheilus albipunctella (Lepidoptera: Noctuidae), in Sahelian West Africa. J Integr Pest Manag 10(1):1–9
Gardner J, Wright MG, Kuhar TP, Pitcher SA, Hoffmann MP (2012) Dispersal of Trichogramma ostriniae in field corn. Biocontrol Sci Technol 22:1221–1233
Geetha N, Balakrishnan R (2010) Dispersal pattern of Trichogramma chilonis Ishii in sugarcane field. J Biol Control 24:1–7
Gingras D, Dutilleul P, Boivin G (2008) Effect of plant structure on searching strategy and searching efficiency of Trichogramma turkestanica. J Insect Sci 8:28
Greatti M, Zandigiacomo P (1995) Postrelease dispersal of Trichogramma brassicae Bezdenko in corn fields. J Appl Entomol 119:671–675
Greenberg SM, Norlund DA, King EG (1996) Mass production of Trichogramma spp.: experiences in the former Soviet Union, China, the United States and western Europe. Biocontrol News Inf 17:51–60
Grieshop MJ, Flinn PW, Nechols JR (2006) Biological control of Indianmeal moth (Lepidoptera: Pyralidae) on finished stored products using egg and larval parasitoids. J Econ Entomol 99:1080–1084
Gross HR Jr, Lewis WJ, Beevers M, Nordlund DA (1984) Trichogramma pretiosum (Hymenoptera: Trichogrammatidae): Effects of augmented densities and distributions of Heliothis zea (Lepidoptera: Noctuidae) host eggs and kairomones on field performance. Environ Entomol 13:981–985
Guerci MJ, Norton GW, Ba MN, Baoua I, Alwang J, Amadou L, Moumouni O, Karimoune L, Muniappan R (2018) Economic feasibility of an augmentative biological control industry in Niger. Crop Prot 110:34–40
Herlihy MV, Talamas EJ, Weber DC (2016) Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three Maryland habitats. PLoS ONE 11(3):e0150275
Hoddle MS, van Driesche R, Sanderson J (1997) Biological control of Bemisia argentifolii (Homoptera: Aleyrodidae) on poinsettia with inundative releases of Encarsia formosa (Hymenoptera: Aphelinidae): are higher release rates necessarily better? Biol Control 10:166–179
Jalali SK, Mohanraj P, Lakshmi BL (2016) Trichogrammatids. In: Omkar (ed) Ecofriendly pest management for food security. Academic Press, Cambridge, pp 139–181
Jones AL, Jennings DE, Cerruti RRH, Shrewsbury PM (2014) Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug, Halyomorpha halys. Biol Control 78:61–66
Kalyebi A, Overholt WA, Schulthess F, Mueke JM, Hassan SA, Sithanantham S (2005) Functional response of six indigenous trichogrammatid egg parasitoids (Hymenoptera: Trichogrammatidae) in Kenya: influence of temperature and relative humidity. Biol Control 32:164–171
Karimoune L, Ba NM, Baoua IB, Muniappan R (2018) The parasitoid Trichogrammatoidea armigera Nagaraja (Hymenoptera: Trichogrammatidae) is a potential candidate for biological control of the millet head miner Heliocheilus albipunctella (de Joannis) (Lepidoptera: Noctuidae) in the Sahel. Biol Control 127:9–16
Khatun MF, Alam SN, Dutta NK, Sarker D (2017) Inundative release of egg and larval parasitoids as a component of IPM of leaf eating caterpillars of cabbage. Mysore J Agric Sci 51:136–139
Knipling EF (1977) The theoretical basis for augmentation of natural enemies. In: Ridgway RL, Vinson SB (eds) Biological control by augmentation of natural enemies. Plenum Press, New York, pp 79–123
Krall S, Youm O, Kogo SA (1995) Panicle insect pest damage and yield loss in pearl millet. In: Nwanze KF, Youm O (eds) Proceeding of an international consultative workshop on panicle insect pest of sorghum and millet. ICRISAT Sahelian Centre, Niamey, pp 135–145
Li LY (1994) Worldwide use of Trichogramma for biological control on different crops: a survey. In: Wajnberg E, Hassan SA (eds) Biological control with egg parasitoids. CAB International, Wallingford, pp 37–54
Mansfield S, Mills NJ (2002) Host egg characteristics, physiological host range, and parasitism following inundative releases of Trichogramma platneri (Hymenoptera: Trichogrammatidae) in walnut orchards. Environ Entomol 31:723–731
McCravy KW, Berisford CW (1998) Parasitism by Trichogramma spp. (Hymenoptera: Trichogrammatidae) in relation to Nantucket pine tip moth (Lepidoptera: Tortricidae) egg density and location. Environ Entomol 27:355–359
Mills NJ, Babendreier D, Loomans AJM (2006) Methods for monitoring the dispersal of natural enemies from point source releases associated with augmentative biological control. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment. CAB International, Wallingford, pp 114–131
Mohamed HO, El-Heneidy AH, Ali GAE, Awad AA (2016) Non-chemical control of the pink and spiny boll worms in cotton fields at Assuit Governorate, Upper Egypt, II-utilization of the egg parasitoid, Trichogrammatoidea bactrae Nagaraja. Egypt J Biol Pest Control 26:807–813
Newton PJ (1988) Movement and impact of Trichogrammatoidea cryptophlebiae Nagaraja (Hymenoptera: Trichogrammatidae) in citrus orchards after inundative releases against the false codling moth, Cryptophlebia leucotreta (Meyrick) (Lepidoptera: Tortricidae). Bull Entomol Res 78:85–99
Newton PJ, Odendaal WJ (1990) Commercial inundative releases of Trichogrammatoidea cryptophlebiae [Hym.: Trichogrammatidae] against Cryptophlebia leucotreta [Lep.: Tortricidae] in citrus. BioControl 35:545–556
Owusu OE, Youm O, Hall DR, Green SV (2004) Observations on factors affecting attraction and oviposition preferences of the millet head miner, Heliocheilus albipunctella to pearl millet panicles. Int Sorghum Millets Newsl 45:72–74
Parra JRP (2010) Mass rearing of egg parasitoids for biological control programs. In: Cônsoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, New York, pp 267–292
Parra JRP, Coelho A Jr (2019) Applied biological control in Brazil: from laboratory assays to field application. J Insect Sci 19(2):1–6
Payne W, Tapsoba H, Baoua IB, Ba MN, N’Diaye M, Dabire- Binso C (2011) On-farm biological control of the pearl millet head miner: realization of 35 years of unsteady progress in Mali, Burkina Faso and Niger. Int J Agric Sustain 9:186–193
Romeis J, Shanower TG, Zebitz CPW (1997) Volatile plant infochemicals mediate plant preference of Trichogramma chilonis. J Chem Ecol 23:2455–2465
Romeis J, Shanower TG, Zebitz CPW (1998) Physical and chemical plant characters inhibiting the searching behaviour of Trichogramma chilonis. Entomol Exp Appl 87:275–284
SAS (2003) SAS version 9.1 for Windows. SAS Institute, Cary
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Sousa JM, Spence JR (2000) Effects of mating status and parasitoid density on superparasitism and offspring fitness in Tiphodytes gerriphagus (Hymenoptera: Scelionidae). Ann Entomol Soc Am 93:548–553
Sow A, Brévault T, Delvare G, Haran J, Benoit L, Coeur d'Acier A, Galan M, Thiaw C, SotiV SM (2018) DNA sequencing to help identify crop pests and their natural enemies in agro-ecosystems: the case of the millet head miner Heliocheilus albipunctella (Lepidoptera: Noctuidae) in sub-Saharan Africa. Biol Control 121:199–207
Tang R, Babendreier D, Zhang F, Kang M, Song K, Hou ML (2017) Assessment of Trichogramma japonicum and T. chilonis as potential biological control agents of yellow stem borer in rice. Insects 8:19
Thorpe KW (1985) Effects of height and habitat type on egg parasitism by Trichogramma minutum and T. pretiosum (Hymenoptera: Trichogrammatidae). Agric Ecosyst Environ 12:117–126
van Driesche RG, Bellows TS (1996) Augmentation of parasitoids, predators, and beneficial herbivores. In: van Driesche RG, Bellows TS (eds) Biological control. Springer, Boston, pp 178–200
van Lenteren JC, Bigler F (2010) Quality control of mass reared egg parasitoids. In: Cônsoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, New York, pp 315–340
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biol Control 63:39–59
Wang B, Ferro DN (1998) Functional Responses of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) to Ostrinia nubilalis (Lepidoptera: Pyralidae) under laboratory and field conditions. Environ Entomol 27:752–758
Wang ZY, He KL, Zhang F, Lu X, Babendreier D (2014) Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol Control 68:136–144
Wright MG, Hoffmann MP, Chenus AS, Gardner J (2001) Dispersal behavior of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) in sweet corn fields: Implications for augmentative releases against Ostrinia nubilalis (Lepidoptera: Crambidae). Biol Control 22:29–37
Yamamoto D, Henderson R, Corley LS, Iwabuchi K (2007) Intrinsic, inter-specific competition between egg, egg–larval, and larval parasitoids of plusiine loopers. Ecol Entomol 32:221–228
Acknowledgements
Funding for this research was provided by the United States Agency for International Development under Cooperative Agreement No. AID- OAA-A-13-00047 with the Kansas State University Feed the Future Collaborative Research on Sorghum and Millet Innovation Lab (SMIL). The project was implemented by ICRISAT and partner institutions (University Dan Dicko Dankoulodo of Maradi, Virginia Polytechnic Institute and State University) as part of the CGIAR Research Program on Grain Legumes and Dryland Cereals (GLDC-CRP). The authors are also grateful to farmers who graciously allow collecting data in their millet fields.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Dirk Babendreier
Rights and permissions
About this article
Cite this article
Karimoune, L., Ba, M.N., Baoua, I.B. et al. Field performance of the parasitoid wasp, Trichogrammatoidea armigera (Hymenoptera: Trichogrammatidae) following releases against the millet head miner, Heliocheilus albipunctella (Lepidoptera: Noctuidae) in the Sahel. BioControl 65, 389–399 (2020). https://doi.org/10.1007/s10526-020-10015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-10015-0