Abstract
Koinobiont parasitoid females when attacking host species are faced with barriers at various levels, host behavioral defenses represent one of these barriers. We present data of the effects of host behavioral defenses on host preference of larval and adult parasitoid. We quantified the effects of defensive behaviors of the exotic host, Harmonia axyridis (Pallas), and the indigenous host, Coleomegilla maculata lengi Timberlake (Coleoptera: Coccinellidae), on the handling time and attack preference of the indigenous parasitoid, Dinocampus coccinellae Schrank (Hymenoptera: Braconidae). Successful egg development was also recorded as a consequence of host behavioral defense and unsuitability. Female parasitoids were offered in an interspecific choice test, adult or larvae of both H. axyridis and C. maculata, and in an intraspecific choice test, larval and adult stage of H. axyridis. Adult H. axyridis exhibited a greater number of defensive behaviors compared to adult C. maculata or larval H. axyridis resulting in significantly longer handling time by the parasitoid. Our results suggest that host acceptance cues used by the generalist parasitoid D. coccinellae are inadequate to evaluate adult H. axyridis. These results provide support to the hypothesis that H. axyridis represents an evolutionary trap for D. coccinellae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Koinobiont parasitoid females must overcome various levels of defenses in order to successfully parasitize their hosts. Host defensive behaviors influence the parasitoid parasitism success because they directly impact the parasitoid’s individual fitness by increasing host handling time, thus decreasing the instantaneous rate of fitness gain (de Farias and Hopper 1999; Gerling et al. 1990; Gross 1993; Völkl and Stadler 1996; Walker and Hoy 2003). In extreme cases, behavioral defenses can harm or even kill parasitoid females (Potting et al. 1999). Host behavioral defenses thus affect host acceptance, decreasing the rate of parasitism and eventually shaping parasitoid host-preference (Gross 1993).
Active host defensive behaviors are usually divided into two categories (Godfray 1994; Gross 1993). Firstly, behaviors that allow hosts to escape from a parasitoid female, such as aphids that evade parasitoids by walking away or dropping off the plant (Braendle and Weisser 2001; Chau and Mackauer 1997; Dill et al. 1990; Losey and Denno 1998), are categorized as evasive behaviors. Secondly, behaviors that drive away, disable or kill parasitoid females are categorized as aggressive behaviors. For example, caterpillars defend themselves aggressively by jerking their head, regurgitating, spitting and biting (Gentry and Dyer 2002; Lederhouse 1990; Potting et al. 1999; Singer and Stireman 2003).
The relationship between host defensive behaviors and female parasitoid host preference has mostly been studied by comparing female host choice between different host stages according to their behavioral defenses rather than comparing interspecific hosts (Allen 1990; Barrette et al. 2008; Chau and Mackauer 1997, 2000; Cornell et al. 1987; Gerling et al. 1990; Walker and Hoy 2003). Moreover, many studies have used a single host-choice experimental design which is more adapted to the evaluation of host suitability and thus more prone to erroneous conclusions regarding host preferences (Mansfield and Mills 2004).
We studied the effect of behavioral host defenses on parasitism using a model composed of an exotic host, Harmonia axyridis Pallas, an indigenous host, Coleomegilla maculata lengi Timberlake and a generalist indigenous parasitoid, Dinocampus coccinellae Schrank. The Asiatic multicolored lady beetle, H. axyridis, was introduced in the early 20th century to the southern United States as a biological control agent (Tedders and Schaefer 1994) and has since invaded most of North America (Chapin and Brou 1991; Colunga-Garcia and Gage 1998). The twelve-spotted ladybeetle, C. maculata, is an indigenous coccinellid species to North America (Gordon 1985) that co-occurs in some habitats with H. axyridis (Hoogendoorn and Heimpel 2004; Musser and Shelton 2003). The parasitoid D. coccinellae is a generalist Braconidae with a known host range of nearly 30 coccinellid species (Hodek and Honěk 1996). Dinocampus coccinellae produce only females by thelitokous parthenogenesis (Balduf 1926).
In Quebec, parasitism by D. coccinellae reaches levels of 32.1% on C. maculata but only 4.6% on H. axyridis (Firlej et al. 2005). This supported the general assumption that, in North America, H. axyridis shows a low susceptibility to pathogens, nematodes, parasitoids and predators (see review in Koch 2003). In addition, H. axyridis shows aggressive behaviors against heterospecific coccinellids in intraguild interactions (Michaud 2002; Sato et al. 2005; Snyder et al. 2004; Yasuda et al. 2001, 2004) but whether it displays such defensive behaviors against parasitoids has not been documented. Previous studies that looked at choice tests between different hosts by D. coccinellae females never considered defensive behaviors as a factor influencing parasitism success (Koyama and Majerus 2008; Obrycki 1989; Orr et al. 1992).
We suggest that host behavioral defenses, along with the host immune system, could be a major factor explaining the low susceptibility of the North Americain strain of H. axyridis to D. coccinellae. Dinocampus coccinellae females readily attack North American H. axyridis in laboratory (Hoogendoorn and Heimpel 2002) but Firlej et al. (2007) and Hoogendoorn and Heimpel (2002) also demonstrated that successful development of D. coccinellae eggs and immatures are very low within North American H. axyridis. Firlej et al. (2007) hypothesized that immune response of H. axyridis may play a role in parasitoid resistance by encapsulating D. coccinellae eggs. These postulations were confirmed by observations in electronic microscopy (Firlej et al. unpublished data).
Here, we focused on the effect of host behavioral defenses on host handling time and female D. coccinellae attack preference using paired-choice tests. Usually, parasitoid host preference is determined by recording oviposition in the offered host which directly relates to parasitoid fitness. Dinocampus coccinellae oviposition in H. axyridis remains difficult to evaluate due to the presence of an immunological resistance to D. coccinellae (Firlej et al. unpublished data). We therefore measured successful egg development as a fitness parameter taking into account that it reflected a combined effect of host behavioral defense and host unsuitability. Because D. coccinellae females can encounter larvae and adults of both host species together in fields (Musser and Shelton 2003), we tested the effect of host behavioral defenses on parasitism in situation of interspecific (D. coccinellae with a choice among C. maculata and H. axyridis as either larvae or adults) and intraspecific host choice (D. coccinellae with a choice among larvae and adults of H. axyridis).
Materials and methods
Rearing colonies
Coleomegilla maculata, H. axyridis (Coleoptera: Coccinellidae) and D. coccinellae (Hymenoptera: Braconidae) were collected from corn fields from southern Quebec, Canada (45°21′N; 73°09′W) and maintained in laboratory for approximately two years with regular introductions of wild individuals to maintain genetic variability. Coccinellids were reared on a liver-based artificial diet (Firlej et al. 2006), grounded pollen, Mediterranean flour moth eggs (Ephestia kuehniella Keller) and aphids (Acyrtosiphon pisum Harris). The coccinellids had ad libitum access to all food sources that were renewed every two days. Parasitoids were reared on C. maculata adults by placing 30 C. maculata adults with four D. coccinellae adult females every two days, for 24 h. Parasitized C. maculata were incubated for a three week period to allow larval parasitoid development. Emerged adult D. coccinellae were kept in Petri dishes (5 cm diameter) with water and honey. Insects were reared under 20 ± 2°C, 60 ± 10% RH and a LD 16:8 h photoperiod.
Test procedure
Both parasitoid and host behaviors were observed i) in interspecific paired-choice tests where a single two days old, experienced D. coccinellae female was placed in a Petri dish (10.5 cm diameter) with either three C. maculata and three H. axyridis adult females or three C. maculata and three H. axyridis fourth instar larvae, and ii) in intraspecific paired-choice tests where a single two days old, experienced D. coccinellae female was placed in a Petri dish (10.5 cm diameter) with three H. axyridis adult females and three H. axyridis fourth instar larvae. Adult, one week old C. maculata and female H. axyridis with uniform body size (mean pronotum width ±SE: 3.20 ± 0.25 mm for H. axyridis and 2.39 ± 0.09 mm for C. maculata) were used for experimentation. To avoid biased host preference, only female adult coccinellids were offered as D. coccinellae have been shown to prefer females over males (Davis et al. 2006). Fourth instar larvae with uniform body size (mean body weight ±SE: 18.06 ± 2.9 mg for H. axyridis and 11.63 ± 2.3 mg for C. maculata) were also used for experimentation. Each choice test represented a treatment with 15 replicates. New parasitoid female and hosts were used for each replicate. Preliminary experiments showed that experienced females laid 4.4 ± 2.8 eggs in the following choice tests regardless of the combination supplied. In order to experience the females, they were individually placed in Petri dishes (10.5 cm diameter) for 1 h with six hosts of the same type (species or stages) offered in the subsequent choice test. After given an experience with hosts, females were then isolated with water and honey for 1 h prior to the choice test. Prior to each test, all hosts were marked with a small drop of white acrylic paint, on elytra for adult hosts or on spines for larval hosts in order to facilitate their visual identification. Only hosts stung by wasps were kept in order to monitor parasitoid egg development. Preliminary observations showed that acrylic paint did not influence the host choice behavior of the female parasitoid and previous studies (Richerson and DeLoach 1972) showed that colors did not influence the female’s host preference.
Behaviors recorded
The number and duration of behaviors realized by parasitoid females (Table 1) were recorded with “The Observer” software (version 4.0; Noldus 1991). Occurrence of defensive behaviors expressed by hosts against parasitoids (Table 1) were noted. When first encountering a potential host, D. coccinellae female examine it with its antennae, the wasp then bends its abdomen with the ovipositor directed toward the host. In a rapid movement, the female inserts its ovipositor inside the host’s abdomen and either accepts the host and lays an egg or rejects it and removes its ovipositor without depositing an egg (Richerson and DeLoach 1972; Orr et al. 1992). Behavioral recordings were initiated when the parasitoid female was released in the Petri dish (10.5 cm diameter) and lasted for a period of 30 min. In all experiments, each parasitoid female interacted with at least one host type offered. Hosts stung were kept in rearing at 20 ± 2°C and 50–60% RH for five days (i.e. time needed to observe developing eggs inside host abdomen) and their abdomens were dissected thereafter in phosphate buffered saline (PBS) under binocular to check for egg presence. We therefore distinguished between (1) a host attacked by the female parasitoid, indicating host was accepted following visual and antennal examination, and (2) a successfully infected host shown by the successful egg deposition and egg development after a five-days incubation period.
To evaluate the proportion of ovipositor insertions that were not followed by egg deposition (host rejection), one inexperienced two days old D. coccinellae female was placed in the presence of one week old C. maculata or H. axyridis adult female and observed during 30 min. Each test was replicated ten times with new parasitoid females and hosts each time. The number of stings performed by the parasitoid female in each host was recorded and hosts were dissected immediately in PBS buffer to recover eggs laid inside each host with a stereomicroscope.
Statistical analysis
The sequence of host encounters and behaviors expressed can influence the female parasitoid’s subsequent behaviors. The Cox’s proportional hazard model (Cox 1972) has been used to identify behavioral mechanisms determining patch residence time of animals across several taxa, including for several parasitoid species (Wajnberg 2006; Wajnberg et al. 2006; Martel et al. 2008). We used the Cox’s proportional hazard model to estimate the influence of previous host encounter by D. coccinellae on the latency period prior to attacking a host. Here, we used “antennal examination” by D. coccinellae on one host type as covariate and measured its influence on the latency of occurrence of “ovipositor attack” on H. axyridis in interspecific comparison and on adult H. axyridis in intraspecific comparison. The equation model is:
Where h(t) is the hazard rate, h 0 (t) is the baseline hazard, t is the time elapsed since the last ovipositor attack on H. axyridis and β i the regression coefficients that give the relative contributions of p covariates z i . The effect of covariates is given by the term \( \exp \left\{ {\beta_{i} z_{i} } \right\} \), the hazard ratio. When the hazard ratio is greater than one, the covariate (here antennal examination) reduces the latency between two ovipositor attacks on H. axyridis. The significance of the model was assessed with a likelihood ratio test (Collett 1994).
Both mean number of host encounters and mean number of host behavioral defenses of each host type (species or stages) were compared within a choice test between each host types with a paired t-test (Sokal and Rohlf 1981). The proportion of each defensive behavior type were compared using a Wilcoxon signed rank-test (Sokal and Rohlf 1981). The total time spent on each host type (species or stages) was compared within each choice test by a paired t-test (Sokal and Rohlf 1981). We calculated the mean handling time by summing the time spent by the parasitoid manipulating one host type (antennal examination + ovipositor bending + ovipositor insertion) and dividing it by the sum of encounters with this host type. The parasitoid attack preference was calculated by dividing the number of encounters followed by an ovipositor insertion in a host type by the number of encounters with this host type. The number of eggs that successfully developed represented the number of developed eggs observed inside each host divided by the number of ovipositor insertions observed in those hosts. Because of the lack of independence between parasitoid interactions in paired-choice tests (Mansfield and Mills 2004), we paired data for the analysis. The mean handling time was compared within a treatment between host types (species or stages) with a paired t-test (Sokal and Rohlf 1981) whereas the attack preference and the number of eggs that successfully developed were compared with a Wilcoxon signed rank-test (Sokal and Rohlf 1981). Finally, the proportion of ovipositor rejection was compared between H. axyridis and C. maculata adults with a χ2 analysis (Sokal and Rohlf 1981). All statistical tests were performed with the JMPin software (SAS Institute Inc.) (Sall and Lehman 1996).
Results
Interspecific host choice
Adults
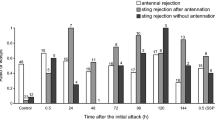
The parasitoid encountered H. axyridis adults significantly more often than C. maculata adults (t 14 = −3.20, P = 0.0064) (Fig. 1). Adults of H. axyridis expressed more behavioral defenses in the presence of the parasitoid than did C. maculata (t 14 = 2.14, P = 0.0500) (Table 2) and H. axyridis adults expressed more leg movements than did C. maculata against the parasitoid (z = 2.35, P = 0.0186) (Fig. 2). The mean handling time was longer (t 14 = 4.02, P = 0.0013) and the number of eggs that successfully developed was lower (z = 10.50, P = 0.0310) for H. axyridis than C. maculata adults (Table 2). When assessed five days after oviposition, no eggs were found in H. axyridis adults. The attack preference did not differ between host species (z = 28.00, P = 0.117) (Table 2). Dinocampus coccinellae spent significantly more time interacting with H. axyridis (75.4%) than with C. maculata (24.6%) (t 14 = 4.28, P = 0.0008).
Larvae
The mean number of encounters with larvae of the two host species did not differ significantly (t 14 = 0.24, P = 0.8146) (Fig. 1). When a female D. coccinellae was given the choice between larvae of H. axyridis and C. maculata, the mean number of host defenses, the mean handling time, the attack preference and the number of eggs that successfully developed did not differ (Table 2). Also, H. axyridis and C. maculata larvae did not exhibited different behavioral defenses against the parasitoid (Fig. 2). D. coccinellae spent 45.4% of their time with H. axyridis and 54.5% with C. maculata (t 14 = −0.67, P = 0.5119).
Intraspecific host choice
We observed a higher mean number of encounters with adult compared to larvae of H. axyridis (t 14 = −3.96, P = 0.0014) (Fig. 1). Harmonia axyridis adults displayed higher mean number of behavioral defenses in the presence of the parasitoid compared to the larvae (t 14 = −2.73, P = 0.0016) (Table 2). Furthermore, the proportion of the different behavioral defenses used by H. axyridis against the parasitoid differed between adults and larvae (z = −2.21, P = 0.0269) (Fig. 2): adults used more escape to defend themselves against D. coccinellae than did larvae. The mean handling time (t 14 = 4.64, P = 0.0005) and attack preference (z = 35.50, P = 0.025) were higher for H. axyridis adults. However, the number of eggs that successfully developed was lower (z = 22.50, P = 0.004) in H. axyridis adults than in larvae (Table 2). Dinocampus coccinellae spent significantly more time with adult H. axyridis (79.7%) than with larvae (20.3%) (t 14 = 6.74, P < 0.0001).
Host rejection
No significant differences were observed in the proportion of host rejection following ovipositor insertion between adult H. axyridis and C. maculata (χ 21 = 0.15, P = 0.6983). In C. maculata and H. axyridis adults, respectively 28 and 24% of the ovipositor insertion were followed by egg deposition.
Cox proportional hazards model
In the interspecific choice tests, the mean latency between two ovipositor attacks on H. axyridis ranked from 246.4 ± 122.2 s when parasitoid females were confronted with adults of both coccinellid species to 200.2 ± 85.4 s when confronted to larvae of both species. When confronted to adults and larvae of H. axyridis, latency between two ovipositor attacks on adults decreased to 179.5 ± 83.3 s. Table 3 describes the influence of antennal examination on the aforementioned latency in interspecific and intraspecific choice tests. Antennal examination of adult or larval C. maculata had no significant influence on the latency period prior to an ovipositor attack on adult H. axyridis by the female parasitoid. Antennal examination of adult H. axyridis had no influence on the latency period prior to an ovipositor attack on adult H. axyridis when the parasitoid was confronted to adults of both host species. That being said, antennal examinations significantly decreased by 1.176 the latency period when in contact with larvae of both host species. Antennal examination of adult H. axyridis resulted in a decrease of 1.310 of the latency period before an ovipositor attack of adult H. axyridis by the female parasitoid when confronted to both larvae and adults of H. axyridis.
Discussion
As expected, the exotic host H. axyridis expressed more behavioral defenses against females of the indigenous parasitoid D. coccinellae compared to the indigenous host C. maculata. These defenses significantly increased the parasitoid handling time from 135% to 217% depending of the host considered.
Host defenses occurrence and effect
Adult H. axyridis appeared to exhibit more defensive behaviors than adult C. maculata or larval H. axyridis and frequently disrupted the ovipositional sequence of D. coccinellae, significantly increasing parasitoid handling time. This supported previous findings demonstrating that H. axyridis is the most aggressive and better defended species among coccinellid guild (Michaud 2002; Sato et al. 2005; Snyder et al. 2004; Yasuda et al. 2001, 2004) and that young stages are less defensive than adults against parasitoids (Chau and Mackauer 2000; Gerling et al. 1990; Mackauer et al. 1996; Walker and Hoy 2003). We demonstrated that H. axyridis could express behavioral defenses not only against predators of the same guild, but also against parasitoids.
The parasitoid took longer to handle adult H. axyridis compared to the other interspecific adults or intraspecific larvae. Defensive behaviors were shown to protect hosts by increasing parasitoid handling time (Gerling et al. 1990; Gross 1993; Potting et al. 1999; Walker and Hoy 2003). Usually, better-defended hosts receive fewer eggs than hosts with weaker defenses (Gross 1993). However, D. coccinellae are able to reject hosts on the basis of internal examination following ovipositor insertion (Davis et al. 2006; Sloggett et al. 2004). Based on the number of eggs recovered immediately after oviposition, the proportion of host rejections did not differ between adult exotic and indigenous hosts, 76% for C. maculata and 72% for H. axyridis, respectively. Furthermore, following host acceptance and egg deposition, the egg can still be destroyed by the host’s immune system (Strand and Pech 1995). The fact that no parasitoid egg was recovered from adult H. axyridis five days after oviposition suggests that these eggs have been destroyed by the host’s immune system. While the capacity of the cellular immune response of adult H. axyridis to encapsulate D. coccinellae eggs has been shown (Firlej, personal observation) the immune response of C. maculata against D. coccinellae eggs has never been studied.
Host preference
Similar studies have been conducted in Britain where D. coccinellae females were shown to have the same attack rate on H. axyridis and on the seven-spotted lady beetle, Coccinella septempuncata, although H. axyridis greater mobility resulted in a higher encounter rate with the parasitoid females (Koyama and Majerus 2008). Similarly, we demonstrated that D. coccinellae females encountered more often adults of H. axyridis over other hosts (C. maculata adults or H. axyridis larvae). Contrary to the findings reported in Koyama and Majerus (2008), we showed that attack rate was higher on adult H. axyridis (Table 2). This suggests that D. coccinellae females prefer adults rather than larvae H. axyridis. Criteria of host preference were well studied in the multi-host species system associated to D. coccinellae. Females D. coccinellae prefer large hosts, female over male and adults over larvae for parasitization (Davis et al. 2006; Geoghegan et al. 1998; Obrycki 1989; Orr et al. 1992; Richerson and DeLoach 1972). In theory, this preference is adaptive as parasitism is more successful and development time is shorter in adult hosts (Firlej et al. 2007; Geoghegan et al. 1998; Obrycki et al. 1985). However, adult H. axyridis used in our study did not support D. coccinellae progeny development as previously demonstrated (Firlej et al. 2007). This would indicate that female D. coccinellae inaccurately assessed the internal quality of adult H. axyridis. The criteria used by D. coccinellae to assess host quality, such as its size or stage, do not appear to be associated with parasitism success when interacting with adults of the exotic host H. axyridis.
Our results indicate that attacking the larval stage of H. axyridis is a better choice. Contrary to what has been observed with other coccinellid host species (Geoghegan et al. 1998; Obrycki et al. 1985), larvae of H. axyridis were more suitable for the development D. coccinellae than adults (Firlej et al. 2007). This suggests that adult H. axyridis found parasitized by D. coccinellae in field samples (Firlej et al. 2005; Hoogendoorn and Heimpel 2002) probably originated from D. coccinellae attacks on H. axyridis larvae.
These findings could also be interpreted as an indication that the parasitoid was better adapted to overcome the behavioral defenses of the indigenous host C. maculata rather than those of the exotic host H. axyridis. Supposing that the exotic host species was to encounter indigenous natural enemies in its new environment, its behavioral defenses could favor its escape from biological control by natural enemies without any coevolutionary history (Keane and Crawley 2002; Prenter et al. 2004). However, these results are based on a single replicate of host interactions (one exotic species vs one indigenous species), and further tests using other sets of exotic/indigenous species would be needed to confirm this interpretation.
The fact that the D. coccinellae population used in our experiment was reared on C. maculata could have brought a bias to our results. Parasitoid laboratory rearing on H. axyridis is impossible considering the low success of parasitism (Firlej et al. 2007). The D. coccinellae females used here were reared on C. maculata and have never experienced contact with H. axyridis. However, to reduce this bias, the female parasitoids were giving an experience with H. axyridis before testing on both hosts. Because the level of successful parasitism in natural populations of H. axyridis is very low (Hoogendoorn and Heimpel 2002; Firlej et al. 2005), one can assume that most of the D. coccinellae emerge from C. maculata populations. Our experimental conditions therefore mimic the natural situation where an indigenous parasitoid emerge from an indigenous host and is faced with both indigenous and exotic hosts.
Our results provide additional support to the egg sink hypothesis of Hoogendoorn and Heimpel (2002) and in general to the “evolutionary trap” hypothesis applied to biological invasions (Schlaepfer et al. 2005). The latter hypothesis predicts that indigenous species could be trapped by their evolutionary responses when confronted to exotic species: the set of cues used to recognize a host does not provide an honest evaluation of the quality of the exotic host. Dinocampus coccinellae has shown a preference for large coccinellids (Richerson and DeLoach 1972) and we showed that females spent more time interacting with adult H. axyridis compared to C. maculata with an associated fitness cost as the exotic host is unsuitable for development because of an immunological response which reduces egg hatching success (Firlej et al., unpublished data). Because female D. coccinellae incorrectly assess the suitability of adult H. axyridis as hosts, the parasitoid could experience a decrease in population density when the population of H. axyridis is high compared to other coccinellid host species. However, the defensive behaviors of H. axyridis could decrease the effect of the evolutionary trap by increasing the parasitoid handling time therefore decreasing its oviposition rate and the likelihood of loosing eggs in an unsuitable exotic host.
Populations of H. axyridis and D. coccinellae co-exist in Japan where D. coccinellae can develop successfully on H. axyridis although with a lower success than on other indigenous Japanese coccinellids (Maeta 1969; Koyama and Majerus 2008). This suggests that Canadian D. coccinellae did not yet have enough time to adapt and overcome behavioral and physiological defenses from the newly introduced H. axyridis populations. In the long term, if a sufficient selective pressure is exerted on Canadian D. coccinellae populations, we could observe the selection of isofemale lines able to develop on Canadian H. axyridis populations.
References
Allen GR (1990) Influence of host behavior and host size on the success of oviposition of Cotesia urabae and Dolichogenidea eucalypti (Hymenoptera: Braconidae). J Insect Behav 3:733–750
Balduf WV (1926) The bionomics of Dinocampus coccinellae Schrank. Ann Entomol Soc Am 19:465–498
Barrette M, Wu GM, Brodeur J, Giraldeau LA, Boivin G (2008) Testing competing measures of profitability for mobile resources. Oecologia 158:757–764
Braendle C, Weisser WW (2001) Variation in escape behavior of red and green clones of the pea aphid. J Insect Behav 14:497–509
Chapin JB, Brou VA (1991) Harmonia axyridis (Pallas), the third species of the genus to be found in the United States (Coleoptera: Coccinellidae). Proc Entomol Soc Wash 93:630–635
Chau A, Mackauer M (1997) Dropping of pea aphids from feeding site: consequence of parasitism by the wasp, Monoctonus paulensis. Entomol Exp Appl 83:247–252
Chau A, Mackauer M (2000) Host-instar selection in the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae): a preference for small pea aphids. Eur J Entomol 97:347–353
Collett D (1994) Modeling survival data in medical research. Chapman and Hall, London
Colunga-Garcia M, Gage SH (1998) Arrival, establishment, and habitat use of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) in a Michigan landscape. Environ Entomol 27:1574–1580
Cornell JC, Stamp NE, Bowers MD (1987) Developmental change in aggregation, defense and escape behavior of buckmoth caterpillars, Hemileuca lucina (Saturniidae). Behav Ecol Sociobiol 20:383–388
Cox DR (1972) Regression models and life tables. Biometrics 38:67–77
Davis D, Stewart SL, Manica A, Majerus MEN (2006) Adaptative preferential selection of female coccinellid hosts by the parasitoid wasp Dinocampus coccinellae (Hymenoptera: Braconidae). Eur J Entomol 103:41–45
de Farias AMI, Hopper KR (1999) Oviposition behavior of Aphelinus asychis (Hymenoptera: Aphelinidae) and Aphidius matricariae (Hymenoptera: Aphidiidae) and defense behavior of their host Diuraphis noxia (Homoptera: Aphididae). Environ Entomol 28:858–862
Dill LM, Fraser AHG, Roitberg BD (1990) The economics of escape behaviour in the pea aphid, Acyrtosiphon pisum. Oecologia 83:473–478
Firlej A, Boivin G, Lucas E, Coderre D (2005) First report of parasitism of Harmonia axyridis parasitism by Dinocampus coccinellae Schrank in Canada. Biol Invasions 7:553–556
Firlej A, Chouinard G, Coderre D (2006) Selection and optimization of a meridic diet for the rearing of Hyaliodes vitripennis Say (Hemiptera: Miridae), a predatory of mites in apple orchard. Biocontrol Sci Tech 16:743–751
Firlej A, Lucas E, Coderre D, Boivin G (2007) Teratocytes growth pattern reflects host suitability in a host––parasitoid assemblage. Physiol Entomol 32:181–187
Gentry GL, Dyer LA (2002) On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 83:3108–3119
Geoghegan IE, Majerus TMO, Majerus MEN (1998) Differential parasitisation of adult and pre-imaginal Coccinella septempunctata (Coleoptera: Coccinellidae) by Dinocampus coccinellae (Hymenoptera: Braconidae). Eur J Entomol 95:571–579
Gerling D, Roitberg BD, Mackauer M (1990) Instar-specific defense of the pea-aphid, Acyrtosiphon pisum: influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelinidae). J Insect Behav 3:501–514
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Gordon RD (1985) The Coccinellidae (Coleoptera) of America north of Mexico. J NY Entomol Soc 93:1–912
Gross P (1993) Insect behavioral and morphological defenses against parasitoids. Annu Rev Entomol 38:251–273
Hodek I, Honěk A (1996) Ecology of coccinellidae. Kluwer Academic Publishers, Dordrecht
Hoogendoorn M, Heimpel GE (2002) Indirect interactions between an introduced and a native ladybird beetle species mediated by a shared parasitoid. Biol Control 25:224–230
Hoogendoorn M, Heimpel GE (2004) Competitive interactions between an exotic and a native ladybeetle: a field cage study. Entomol Exp Appl 111:19–28
Keane RM, Crawley MJ (2002) Exotic plants invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–171
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J Insect Sci 3:1–16
Koyama S, Majerus MEN (2008) Interactions between the parasitoid wasp Dinocampus coccinellae and two species of coccinellid from Japan and Britain. BioControl 53:253–264
Lederhouse RC (1990) Avoiding the hunt: primary defenses of Lepidopteran caterpillars. In: Evans DL, Schmidt JO (eds) Insect defenses: adaptative mechanisms and strategies of prey and predators. SUNY Press, New York, pp 175–189
Losey JE, Denno RF (1998) The escape response of the pea aphids to foliar-foraging predators: factor affecting dropping behaviour. Ecol Entomol 23:53–61
Mackauer M, Michaud JP, Völkl W (1996) Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): host recognition, host quality, and host value. Can Entomol 12:959–980
Maeta Y (1969) Biological studies on the natural enemies of some Coccinellid beetles. I. On Perilitus coccinellae (Schrank). Kontyû 37:147–166
Mansfield S, Mills NJ (2004) A comparison of methodologies for the assessment of host preference of the gregarious egg parasitoid Trichogramma platneri. Biol Control 29:332–340
Martel V, Wajnberg E, Boivin G (2008) Patch time allocation in male parasitoids. Ecol Entomol 33:608–613
Michaud JP (2002) Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environ Entomol 31:827–835
Musser FR, Shelton AM (2003) Factors altering the temporal and within-plant distribution of coccinellids in corn and their impact on potential intra-guild predation. Environ Entomol 32:575–583
Noldus LPJJ (1991) The observer: a software for collection and analysis of observational data. Behav Res Meth Instrum Comput 23:415–429
Obrycki JJ (1989) Parasitization of native and exotic coccinellids by Dinocampus coccinellae (Schrank) (Hymenoptera: Braconidae). J Kansas Entomol Soc 62:211–218
Obrycki JJ, Tauber MJ, Tauber CA (1985) Perilitus coccinellae (Hymenoptera: Braconidae): parasitization and development in relation to host-stage attacked. Ann Entomol Soc Am 78:852–854
Orr CJ, Obrycki JJ, Flanders RV (1992) Host-acceptance behavior of Dinocampus coccinellae (Hymenoptera: Braconidae). Ann Entomol Soc Am 85:722–730
Potting RPJ, Vermeulen NE, Conlong DE (1999) Active defense of herbivorous hosts against parasitism: adult parasitoid mortality risk involved in attacking a concealed stemboring host. Entomol Exp Appl 91:143–148
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390
Richerson JV, DeLoach CJ (1972) Some aspects of host selection by Perilitus coccinellae. Ann Entomol Soc Am 65:834–839
Sall J, Lehman A (1996) JMP start statistics: A guide to statistical and data analysis using JMP® and JMP IN® software. Duxbury Press, Toronto
Sato S, Yasuda H, Evans EW (2005) Dropping behavior of larvae of aphidophagous ladybirds and its effects on incidence of intrigued predation: interactions between the intrigued prey. Adalia bipunctata (L.) and Coccinella septempunctata (L.) and the intraguild predator, Harmonia axyridis pallas. Ecol Entomol 30:220–224
Schlaepfer MA, Sherman PW, Blossey B, Runge MC (2005) Introduced species as evolutionary traps. Ecol Lett 8:241–246
Singer MS, Stireman JO (2003) Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 100:554–562
Sloggett JJ, Webberley KM, Majerus MEN (2004) Low parasitoid success on a myrmecophilous host is maintained in the absence of ants. Ecol Entomol 29:123–127
Snyder WE, Clevenger GM, Eigenbrode SD (2004) Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia 140:559–565
Sokal RR, Rohlf FJ (1981) Biometry: The principles and practice of statistics in biological research. W.H. Freeman and company, New York
Strand MR, Pech LL (1995) Immunological basis for compatibility in parasitoid-host relationship. Annu Rev Entomol 40:31–56
Tedders WL, Schaefer PW (1994) Release and establishment of Harmonia axyridis (Coleoptera: Coccinellidae) in the southern United States. Entomol News 105:228–243
Völkl W, Stadler B (1996) Colony orientation and successful defense behavior in the conifer aphid, Schizolachnus pineti. Entomol Exp Appl 78:197–200
Wajnberg E (2006) Time allocation strategies in insect parasitoids: from ultimate predictions to proximate behavioral mechanisms. Behav Ecol Sociobiol 60:589–611
Wajnberg E, Berhnard P, Hamelin F, Boivin G (2006) Optimal patch time allocationfor time-limited foragers. Behav Ecol Sociobiol 60:1–10
Walker AM, Hoy MA (2003) Responses of Lipoplexis oregmae (Hymenoptera: Aphidiidae) to different instars of Toxoptera citricida (homoptera: Aphididae). J Econ Entomol 96:1685–1692
Yasuda H, Kikuchi T, Kindlmann P, Sato S (2001) Relationships between attack and escape rates cannibalism, and intraguild predation in larvae of two predatory ladybirds. J Insect Behav 14:373–383
Yasuda H, Evans EW, Kajita Y, Urakawa K, Takizama T (2004) Asymmetric larval interactions between introduced and indigenous ladybirds in North-America. Oecologia 141:722–731
Acknowledgments
We thank Danielle Thibodeau, Josiane Vaillancourt, Julie Frenette, Mathilde Lheureux for technical help in insect rearing and experiments and Mick Wu for help in statistics. We also thank two anonymous reviewers for valuable comments on this manuscript. This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to Dr. D. Coderre and a grant from Hydro-Québec to A. Firlej.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Torsten Meiners.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10526-010-9274-1
Rights and permissions
About this article
Cite this article
Firlej, A., Lucas, É., Coderre, D. et al. Impact of host behavioral defenses on parasitization efficacy of a larval and adult parasitoid. BioControl 55, 339–348 (2010). https://doi.org/10.1007/s10526-009-9262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-009-9262-5