Abstract
We assessed the effect of two biological control agents, the mirid Eccritotarsus catarinensis (Carvalho) and the weevil Neochetina eichhorniae (Warner), singly or in combination, on the competitive ability of their host plant, water hyacinth, Eichhornia crassipes (Mart.) Solms-Laub., grown in a screen house, in competition with another aquatic plant (Pistia stratiotes L.). Water hyacinth plant growth characteristics measured included fresh weight, leaf and petiole lengths, number of inflorescences produced, and new shoots. Without herbivory, water hyacinth was 18 times more competitive than water lettuce (across all experimental combinations of initial plant densities), as estimated from fresh weights. Both insect species, singly or in combination, reduced water hyacinth plant growth characteristics. E. catarinensis alone was less damaging than the weevil and under normal conditions, i.e., floating water hyacinth, is not expected to increase control of water hyacinth beyond that of the weevil. When combined with the weevil, half the inoculum of weevils and half the inoculum of mirids produced the same growth reduction as the full inoculum of the weevil. Under conditions where the weevils are not effective because water hyacinths are seasonally rooted in mud, the mirid, which lives entirely on leaves, should become a useful additional biological control agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water hyacinth, Eichhornia crassipes (Mart.) Solms-Laub. (Pontederiaceae) and water lettuce, Pistia stratiotes L. (Araceae) can be found in similar habitats, for example slow-moving or still water bodies (Harley 1990). In the absence of its natural enemies, water hyacinth is the dominant species (Center and Spencer 1981; Wright and Purcell 1995) because of its rapid growth and ability to shade other aquatic plants such as water lettuce (Chadwick and Obeid 1966).
The preferred method of controlling water hyacinth is biological control, because it is environmentally friendly (reviewed in Greathead 2003) and has successfully reduced infestations in many African countries (Hill et al. 1999a; Cilliers et al. 2003; Mbati and Neuenschwander 2005). Among the six arthropod agents released worldwide (Harley 1990; Julien et al. 1999), the two weevils, Neochetina eichhorniae (Warner) and N. bruchi Hustache (Coleoptera: Curculionidae) are the most effective (DeLoach and Cordo 1976; Center and Van 1989; Center et al. 1999). In West Africa, N. eichhorniae is better adapted than N. bruchi (Ajuonu et al. 2003).
Despite the release of several agents against water hyacinth in South Africa, control has been variable, necessitating the search for more agents (Hill et al. 1999a). The most recent is the mirid, Eccritotarsus catarinensis (Carvalho) (Heteroptera: Miridae) first released in South Africa in 1996 (Hill et al. 1999b) and later in Malawi. It was released in Benin in 1999 to complement biological control by the weevil, under ecological conditions where the weevil was not fully effective, but has not become established. There is a possibility that it could be released in the USA (Coetzee et al. 2003).
Water lettuce sometimes re-colonizes water bodies after water hyacinth cover had been reduced by weevils (Ajuonu and Neuenschwander 2003). The response of competing vegetation has therefore been used as an indicator for evaluating the effect of biological control agents against water hyacinth, when measuring their effect on the host plant (Center et al. 2001). This is based on the hypothesis that competition from a second aquatic plant would further increase the effect of herbivory on water hyacinth growth. Center et al. (2005) compared the effect of the two weevils and Coetzee et al. (2005) evaluated the effect of the mirid alone. Using the same methodology, this present study compares the subtle impact of the mirid, E. catarinensis with the more obvious impact of the weevil, N. eichhorniae.
Materials and methods
Location
This experiment was conducted outdoors in a shade screen house (40%) in the centre of a fallow field at the International Institute of Tropical Agriculture (IITA) near Cotonou, Benin. The first replicate lasted from 24 July to 6 October 2004, the second from 11 December 2004 to 26 February 2005, and the third from 9 March to 1 June 2005. The daily minimum and maximum temperatures were 19–22.8 and 25.7–30.9°C, respectively, for the first replicate, 14–25.5 and 29.5–34.7°C for the second, and 20–25 and 30–33.8°C for the third.
Rearing plants and insects
Plants were grown in plastic tubs (30 cm deep; 54 cm diameter) buried 22 cm in soil and covered by a 95 cm high white mosquito net canopy. Heat accumulation in small water containers adversely affected water hyacinth growth; burying the rearing tubs reduced water temperatures by 0.5°C at 8:00 h and by 2°C from 12:00 to 17:00 h compared with surface placement. On the first day, the plastic tubs were filled with 20 l of water. Liquid fertiliser (Fertigofol 737) was used at an application rate of 0.5 ml per litre of water. Thereafter water containing 0.1 ml l−1 Fertigofol 737 was added periodically to maintain the depth of water in each plastic tub between 20 and 30 cm.
Water hyacinth plants were collected from Sazoué (08°18.32′ N, 001°50.13′ E) on the Mono River where the weed has remained free of weevil infestation. The initial fresh weight per individual plant ranged from 170 to 258 g. Water lettuce plants were taken from a stock maintained at IITA in plastic pools (265 × 67.5 cm). Before placement in the tubs, all plants were washed by spraying with tap water to remove all arthropods, and then covered with the net-canopy. Adult weevils N. eichhorniae, were collected from a field population on the Sô River (06°40.07 N, 002°24.59 E). The mirids E. catarinensis, were reared in the laboratory (Ajuonu et al. 2007).
Experimental set-up
The trial followed an addition series competition experimental design (Spitters 1983), consisting of factorial combination of the two competing species in a randomised complete block design with the plant density ratios nested in species treatment levels.
A mixture of water hyacinth and water lettuce was planted with the following numbers of plants per tub: 3:0, 3:3, 3:9, 9:0, 9:3, 9:9, 0:3, and 0:9. Four sets of each combination were set up; of these, three were each infested either with a single species or a combination of agents and the fourth served as a control (C). The single-species infestations were: E. catarinensis (E) at the rate of 40 adults per plant, or N. eichhorniae (N) at the rate of two pairs of adults per plant. In the combined species treatment (E + N), the infestation rate was 20 adult E. catarinensis and one pair of N. eichhorniae per plant, which is half the inoculum used in the single treatments. This experimental setup was replicated three times on the dates given above.
In contrast with previous studies using the same methodology, which either dealt with two very similar weevils, N. eichhornaie and N. bruchi (Center et al. 2005) or with the impact of a single agent, E. catarinensis (Coetzee et al. 2005), we faced the problem of having to compare the effect of two highly different organisms. The two species N. eichhornaie and E. catarinensis were combined in numbers approximating observed field population levels, and their weights were determined in order to reach a common standard. Ten adult weevils were frozen for 24 h and air dried on a filter paper for 7 h. The weevils were individually weighed (Denver Instrument M-220D, measuring a minimum of 0.0001 mg), yielding an average weight of 0.0077 mg (range 0.0058–0.0094 mg). Forty E. catarinensis adults treated by the same procedure were found to equal one quarter of an adult N. eichhorniae. It would have required 160 mirids to correspond to the two pairs of N. eichhorniae or N. bruchi per plant used in a similar study (Center et al. 2005). For this experiment, this number was reduced to 40 adults per water hyacinth plant, which corresponds to 25% of the weight of the weevil. By comparison, Coetzee et al. (2005) used 15 adult E. catarinensis (50:50 sex ratio), which were considered too few to cause severe damage.
Data collection
Comparing the impact of mirids and weevils was difficult because their damage symptoms are so different. Therefore plant growth characteristics were considered as standard measurable units (Center et al. 1999) and indicators of the effect of both agents (Del Fosse 1978). Measurements taken were:
-
1
length of the second leaf;
-
2
length of the petiole;
-
3
number of daughter plants (only those with three unfurled leaves each were considered);
-
4
number of inflorescences produced per plant by water hyacinth;
-
5
estimates of surface cover of basins (in 5% increments) by water lettuce (Ajuonu et al. 2003); and
-
6
fresh weights of water hyacinth and water lettuce (taken at the beginning on a per plant basis and the end of the experiment as total).
Data were taken at week zero, the date the agents were introduced, and at weeks three, six, and eight. The three-week sampling period corresponds to the generation time of the mirid (Hill et al. 1999b). However, destructive sampling was carried out on week eight instead of week nine, because in an initial trial with the mirid, production of new water hyacinth leaves occurred after eight weeks. At this time most of the leaves were yellowing and brownish and the mirid population collapsed for lack of suitable food.
The numbers of adult and immature mirids were counted on weeks three and eight, on ten leaves in plastic tubs initially containing three plants and on 20 leaves for those initially containing nine water hyacinth plants, to quantify the mirid population. On the same date, the number of adult weevil feeding scars on the second leaf was counted as an indicator of the presence of adults (Wright and Center 1984).
Data analysis
Analysis of variance by the GLM procedure (SAS/STAT Software version 2002) was used to assess differences in species treatments (single agent, species combination, and the control), followed by Student–Newman–Keuls test at P = 0.05. To achieve normality, count data were log10(x + 1) transformed before analyses. The means for fresh weight (water hyacinth and water lettuce), number of daughter plants, and inflorescences of water hyacinth were calculated on the basis of the initial planting densities. Means of leaf and petiole lengths of water hyacinth (on the second leaf position) were based on the number of mature plants (plants with at least five unfurled leaves) per plastic tub on each sampling date.
Multiple regression analysis was carried out, using an inverse linear model, \( 1/w_{{\text{h}}} = a_{{{\text{h}}0}} + a_{{{\text{hh}}}} d_{{\text{h}}} + a_{{{\text{hi}}}} d_{ 1} \) (Spitters 1983). Here, 1/w h is the inverse biomass yield of individual water hyacinth plants, while d h and d 1 represent water hyacinth and water lettuce planting densities, respectively. The coefficient a hh estimates intraspecific competition, while the coefficient a hi estimates interspecific competition. The ratio a hh/a hi measures the effect of intraspecific competition by water hyacinth on its own yield, relative to the effect of interspecific competition by water lettuce.
Results
Numbers of mirids and adult weevil feeding scars
Three weeks after introducing the agents, the mean numbers (±SE) of adults and immatures were 15.1 ± 1.3 per leaf in the treatment (E) with the mirid alone, where 40 adults (20 females: 20 males) per plant had been used. In the treatment (E + N) with both species (with half the inoculation rate of single species), the mean was 9.2 ± 0.7 adult and immature mirids. At week eight the mean counts (adults and immatures) per leaf declined to 7.1 ± 0.6 (53%) in treatment E and to 1.9 ± 0.2 (79%) in treatment E + N, when plants became less suitable because of declining vigour resulting from the effect of the weevil.
The mean numbers (±SE) of adult weevil feeding scars in treatment with the weevil alone (N) were 62 ± 6 per leaf at week three and 56 ± 6 at week eight, indicating that some of the adults used for inoculation survived the period of the experiment. In treatment with both species (E + N) inoculated with two adult weevils, which is half the rate in treatment with weevil alone, feeding scars numbered 42 ± 4 at week three and 36 ± 4 at week eight. During the destructive sampling (week eight), several weevil pupae were observed in treatments with weevil alone and the combination of weevil and mirid, indicating that full larval development had occurred.
Plant growth characteristics
Data on plant growth characteristics from the first replicate (24 July to 6 October 2004) and the third replicate (9 March to 1 June 2005) were generally higher in value than those from the second replicate (11 December 2004 to 26 February 2005). This showed that the periods of the first and third replicates, which corresponded to the short and long rainy periods, supported plant and insect population growth better than the second replicate conducted during the dry season and the harmattan, a dry dusty wind originating in the Sahara desert. The block effect, created by repeating the experiment three times, was therefore removed and replication was introduced as a factor in the ANOVA model.
The results of ANOVA (Table 1) show that fresh weight (F 1,62 = 34.60, P < 0.01) and number of shoots produced (F 1,39 = 94.86, P < 0.01) were significantly greater at low initial planting density of water hyacinth (3H:0L, 3H:3L, 3H:9L) than at high density (9H:0L, 9H:3L, 9H:9L), while the other characteristics were not, but followed similar pattern. The subsequent discussion therefore focuses on plant weight and new shoots, with less emphasis on the other variables.
Plant competition
Regression results (Table 2) show that yield in fresh weight was affected by intraspecific competition (a hh > 0) in all treatments, and in some cases (where a hi > 0) by interspecific competition. Without herbivores, the competition ratio a hh/a hi for water hyacinth was 18 times (P = 0.04) more than water lettuce. The ratio declined to 2.64 (P = 0.27) in the treatment with the weevil N. eichhorniae and to −0.328 (P = 0.28) in the treatment with E. catarinensis. It further declined to −23.87 (P = 0.01) for both species together.
Effect of treatments
The effects of treatments varied according to species, and species combination, and on the growth characteristics measured. Water hyacinth fresh weight in the control (C) increased significantly and differed from the treatments with agents (F 1,48 = 62.85, P < 0.01) (i.e. E, N, and E + N combined). In Table 3, the percentage changes (increase or decrease) in growth characteristics were based on the initial means and final (eighth week) values and, except for treatment with mirid alone, with increased number of shoots, all water hyacinth growth characteristics declined compared with the initial values. The effect on the production of inflorescences was highest (−100%), indicating reduced reproductive capacity of the water hyacinth.
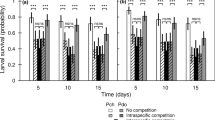
Leaf and petiole lengths varied according to treatments (Fig. 1). At the third week although leaf length in the treatment with both species did not differ statistically, it was lower than the treatment with the weevil alone. By the sixth and eighth week, leaf and petiole length declined in all treatments with agents compared with the control, except in the treatment with mirid alone, where no statistical difference was found in petiole lengths.
Leaf length (n = 18) and petiole length (n = 12) on week 0, before introducing agents and weeks 3, 6, and 8, after introducing agents. Treatments are: E. catarinensis (E), N. eichhorniae (N) and the combination of both species (E + N; at half the inoculum of weevils and half the inoculum of mirids used in single treatments). Bars represents means ± SE. Means with the same letter are not significantly different (P < 0.05)
Planting density and herbivory
Initial water hyacinth planting densities and herbivory affected one another, as indicated by fresh weights and the numbers of new shoots (Fig. 2). At low densities (3H) there was no statistical difference among treatments with agents, although each differed significantly from the control. At high water hyacinth planting densities (9H) fresh weights decreased and differed depending on treatment.
Effect of initial water hyacinth planting densities (3H and 9H) and herbivory on fresh weight and production of new shoots eight weeks after introducing agents (C = the control without agent, E = E. catarinensis, N = N. eichhorniae, and E + N = combination both species). Means with the same letter are not significantly different (P < 0.05) and bars represent means ± SE
Discussion
With similar methodology, two closely related weevils, Neochetina eichhorniae and N. bruchi, were used as single treatments and together (Center et al. 2005) and Coetzee et al. (2005) used the mirid, E. catarinensis alone. This experiment assessed, for the first time, the impact of the mirid and the weevil (singly and both together), from different insect orders with very different modes of feeding. The reduction in growth of the host plant, water hyacinth, which was experimentally subjected to competition by another floating water weed, water lettuce, was measured.
Water hyacinth is generally assumed to be a superior competitor over water lettuce (Wright and Purcell 1995). This study quantifies this competitive advantage (18.5 times), which is, however, only shown when the plant is not attacked by biological control agents as indicated by the competition ratio a hh/a hi (Table 2). This confirms the displacement of water lettuce where it already exists before the introduction of water hyacinth (Center et al. 2001; Ajuonu and Neuenschwander 2003). With agents, the ratio declined to −0.32 in the presence of the mirid, to 2.6 with the weevil only, while both species reduced the ratio to −23.87. In similar studies, without agents, the ratio was 23.6 times but declined to 10 in the presence of the mirid (Coetzee et al. 2005) while Center et al. (2005) reported a ratio of 41 times without agents which declined to 1.4 in the presence of the weevil N. eichhorniae.
In the single-species treatments, the differences in damage severity of the two biocontrol species are reflected in the impact on plant growth characteristics, with the weevil having greater effect. The mirid, with a short life cycle of three weeks, lives entirely on the leaf of water hyacinth (Hill et al. 1999b) and damage is usually mild (Coetzee et al. 2003). For the weevil, with a longer life cycle of 120 days, the primary damage is due to the larval stages that bore into the crown of the water hyacinth (DeLoach and Cordo 1976) leading to shoot mortality (Center and Van 1989). In this experiment, although the mirid inoculation rate was 25% of the weight of the weevil, it reduced water hyacinth growth by an amount similar to that by the weevil when compared with the control (Fig. 2).
The mean number (±SE) of adults and immature mirids per leaf after one generation in the single treatment (E) was 15.1 ± 1.3 compared with 9.2 ± 0.7 in the mixed treatment with half the inoculum of mirids. This relatively better performance in the mixed treatment may be because of access to fresh feeding holes made by the weevils, as reported in a laboratory study (Ajuonu et al. 2007).
In treatments with both species, the impact of agents is expected to occur in an overlapping sequence according to their respective life cycles, i.e., three weeks for the mirid (Hill et al. 1999b) and 120 days for the weevil (DeLoach and Cordo 1976). At the third week of this study, the increased mirid population caused severe leaf damage, added to damage on young leaves and petioles by adult weevils (DeLoach and Cordo 1976). By the sixth and eighth week (while the mirid population dropped by 79% due to reduced food value of the leaves), weevil larvae had developed into second/third instars and were old enough to cause severe damage (DeLoach and Cordo 1976) in addition to the damage by mirids. This explains why, despite the 79% decrease in the mirid population at week eight, effects of treatment with both agents (E + N) was statistically similar to those for the weevils alone (N) (Fig. 1) and also produced the lowest number of inflorescences (Table 3).
Initial planting density affected water hyacinth turnover and impact by agents. At the end of the experiment, fresh weights at low water hyacinth densities (3H) were 1.5 times higher, while the numbers of new shoots were 27.3-fold those at high densities (9H) (Table 1). Also, other plant growth characteristics, leaf/petiole length and number of flowers produced, had higher turnover at low water hyacinth planting densities. Center and Van (1989) have shown that at low plant densities leaf production is accelerated. This explains the small effect of agents recorded at low planting densities in contrast with high planting densities where effects of herbivory were severe (Fig. 2). The better performance of the agents at high planting densities can be attributed to the combined effects of intraspecific interaction among plants at high densities and interspecific interactions with water lettuce when affected by biological control agents, similar to the results of Center et al. (2005).
The addition series competition design (Spitters 1983) used in the two studies (Center et al. 2005; Coetzee et al. 2005) shows that herbivory can reduce the competitive ability of water hyacinth grown with another aquatic plant, water lettuce, similar to this study that combined the mirid and the weevil for the first time. Although there is uncertainty about whether one species of agent is better than multiple agents (Denoth et al. 2002), this experiment has demonstrated that both species are compatible with each other. E. catarinensis alone is less damaging than the weevil and under normal conditions, i.e., floating water hyacinth; the mirid is not expected to increase control of water hyacinth beyond that of the weevil. However, when combined with the weevil, half the inoculum of weevils and half the inoculum of mirids still produced the same growth reduction as the full inoculum of the weevil. Under conditions where the weevils are not effective because water hyacinths are seasonally rooted in mud (Ajuonu et al. 2003), the mirid, which lives entirely on leaves (Hill et al. 1999b), should become a useful additional biological control agent.
References
Ajuonu O, Neuenschwander P (2003) Release, establishment, spread and impact of the weevil Neohydronomus affinis (Coleoptera: Curculionidae) on water lettuce (Pistia stratiotes) in Benin, West Africa. Afr Entomol 11:205–211
Ajuonu O, Schade V, Veltman B, Sedjro K, Neuenschwander P (2003) Impact of the exotic weevils Neochetina spp.: (Coleoptera: Curculionidae) on water hyacinth, Eichhornia crassipes (Lil: Pontederiaceae) in Benin, West Africa. Afr Entomol 11:153–161
Ajuonu O, Byrne M, Hill P, Neuenschwander P, Korie S (2007) Survival of the mirid Eccritotarsus catarinensis as influenced by Neochetina eichhorniae and Neochetina bruchi feeding scars on leaves of water hyacinth Eichhornia crassipes. BioControl 52:193–205
Center TD, Spencer NR (1981) The phenology and growth of water hyacinth (Eichhornia crassipes (Mart.) Solms) in a euthrophic North-Central Florida Lake. Aquat Bot 10:1–32
Center TD, Van TK (1989) Alteration of water hyacinth (Eichhornia crassipes (Mart.) Solms) leaf dynamics and phytochemistry by insect damage and plant density. Aquat Bot 35:181–195
Center TD, Dray FA Jr, Jubinsky GP, Leslie AJ (1999) Water hyacinth weevils (Neochina eichhorniae and N. bruchi) inhibit water hyacinth (Eichhornia crassipes) colony development. Biol Control 15:39–50
Center TD, Van TK, Hill MP (2001) Can competition experiments be used to evaluate the potential efficacy of new water hyacinth biological control agents? In: Julien MH, Hill MP, Jianqing D (eds) Proceedings of the second meeting of global working group for the biological and integrated control of water hyacinth, ACIAR proceeding number 102. Beijing, China, 9–12 October 2000, pp 77–81
Center TD, Van TK, Dray AF Jr, Franks JS, Rebelo TM, Pratt DP, Rayamajhi BM (2005) Herbivory alters competitive interactions between two invasive aquatic plants. Biol Control 33:173–185
Chadwick MJ, Obeid M (1966) A competitive study of the growth of Eichhornia crassipes Solms. and Pistia stratiotes L. in water-culture. J Ecol 54:563–575
Cilliers CJ, Hill MP, Ogwang JA, Ajuonu O (2003) Aquatic weeds in Africa and their control. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in IPM systems in Africa. CABI Publishing, Wallingford, pp 161–178
Coetzee JA, Byrne M, Hill M (2003) Failure of Eccritotarsus catarinesis, a biological control agent of water hyacinth, to persist on pickerelweed, a non-target host in South Africa, after forced establishment. Biol Control 28:229–236
Coetzee JA, Center TD, Byrne MB, Hill MP (2005) Impact of the biological control agent Eccritotarsus catarinesis, a sap-feeding mirid, on the competitive performance of water hyacinth, Eichhornia crassipes. Biol Control 32:90–96
Del Fosse ES (1978) Effect on water hyacinth of Neochetina eichhorniae (Col.: Curculionidae) combined with Orthogalumna terebrantis (Acari: Galumnidae). Entomophaga 23:379–387
DeLoach CJ, Cordo HA (1976) Life cycle and biology of Neochetina bruchi, a weevil attacking water hyacinth in Argentina, with notes on N. eichhorniae. Ann Entomol Soc Am 69:643–652
Denoth M, Frid L, Myers JH (2002) Multiple agents in biological control: improving the odds? Biol Control 24:20–30
Greathead JD (2003) Historical overview of biological control in Africa. In: Neuenschwander P, Borgemeister C, Langewald J (eds) Biological control in IPM systems in Africa. CABI Publishing, Wallingford, pp 1–26
Harley KLS (1990) The role of biological control in the management of water hyacinth, Eichhornia crassipes. Biol News Inform 11:11–22
Hill MP, Julien MH, Center TD (eds) (1999a) Proceedings of the first IOBC global working group meeting for the biological and integrated control of water hyacinth. Harare, Zimbabwe, November 1998, 182 pp
Hill MP, Cilliers CJ, Neser S (1999b) Life history and laboratory host range of Eccritotarsus catarinensis (Carvalho) (Heteroptera: Miridae), a new natural enemy released on water hyacinth (Eichhornia crassipes (Mart.) Solms-Laub.) (Pontederiaceae) in South Africa. Biol Control 14:127–133
Julien MH, Griffiths MW, Wright AD (1999) Biological control of water hyacinth. The weevils Neochetina bruchi and N. eichhorniae: biologies, host ranges, and rearing, releasing and monitoring techniques for biological control of Eichhornia crassipes. AClAR Monograph No. 60, 87 pp
Mbati G, Neuenschwander P (2005) Biological control of three floating water weeds, Eichhornia crassipes, Pistia stratiotes, and Salvinia molesta in the Republic of Congo. BioControl 50:635–645
SAS/STAT Software version 9.1 (2002) SAS Institute Inc. Cary, North Carolina
Spitters CJT (1983) An alternative approach to the analysis of cropping experiments.1. Estimation of competition effects. Neth J Agric Sci 31:1–11
Wright AD, Center DT (1984) Predicting population intensity of adult Neochetina eichhorniae (Coleoptera: Curculionidae) from incidence of feeding on leaves of water hyacinth, Eichhornia crassipes. Environ Entomol 13:1478–1482
Wright AD, Purcell MF (1995) Eichhornia crassipes (Mart.) Solms-Laubach. In: Groves RH, Shepherd RCH, Richardson RG (eds) The biology of Australian weeds. R. G. and F. J. Richardson, Melbourne, pp 111–121
Acknowledgements
The authors thank IITA for providing funding and facilities, staff of Plant Protection Research Institute in Pretoria, South Africa (ARC-PPRI), particularly the late H. Oberholzer, for supplying the starter colony of mirids, and Fen Beed, Manuele Tamò (IITA), and Alana Den Breeyen, Plant Pathology Department, University of Florida for comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: John Scott.
Rights and permissions
About this article
Cite this article
Ajuonu, O., Byrne, M., Hill, M. et al. The effect of two biological control agents, the weevil Neochetina eichhorniae and the mirid Eccritotarsus catarinensis on water hyacinth, Eichhornia crassipes, grown in culture with water lettuce, Pistia stratiotes . BioControl 54, 155–162 (2009). https://doi.org/10.1007/s10526-008-9185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-008-9185-6