Abstract
The plant matrix influences the performance of omnivorous mirids as biocontrol agents and increasing plant diversity has been hypothesised to enhance pest control. This research aimed to determine the effect of using calabash, Lagenaria siceraria, as a companion plant on the population dynamics and whitefly control efficacy of Dicyphus argensis in tomato greenhouses. The response of D. argensis was also compared with that of Nesidiocoris tenuis. Four treatments were assayed in a complete randomised block design with three replicates each: (1) Bemisia tabaci, (2) B. tabaci + D. argensis, (3) B. tabaci + D. argensis + calabash and (4) B. tabaci + N. tenuis. Calabash harboured high populations of D. argensis, but its abundance on tomato plants was significantly lower in the presence of calabash than in its absence, and in both treatments, it reached lower numbers than N. tenuis. Dicyphus argensis reduced the whitefly density on tomato plants relative to the compartments with no mirids, but the whitefly density was higher in the presence of companion plants, and N. tenuis was more effective in reducing whitefly populations. Calabash served as a host for the multiplication of whitefly and increased the pest density on tomato. In this research, increasing plant diversity in crops did not enhance pest control because: (1) the aggregation of D. argensis in calabash reduced its abundance in tomato plants; (2) the pest populations multiplied. This contrasts with the diversity hypothesis and confirms the importance of the plant context for predatory dicyphines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Increasing plant diversity is considered to enhance natural enemy effectiveness and pest control.
-
Calabash was an optimum host for the omnivorous mirid Dicyphus argensis.
-
Calabash used as a companion plant reduced the density of Dicyphus argensis on tomato plants.
-
Calabash multiplied whitefly and contributed to the increase in the pest on tomato plants.
-
Increasing plant diversity in tomato greenhouses did not enhance pest control.
Introduction
The management of biodiversity for the provision of ecosystem services is a challenging task for applied ecologists, among other reasons because until now no general pattern has emerged, and thus, it depends on the knowledge of the biology and intricate interactions among species for each particular ecosystem (Tscharntke et al. 2016; Lichtenberg et al. 2017; Karp et al. 2018). This is the case of habitat management for the conservation of natural enemies aiming to improve pest control in agroecosystems (Altieri and Letourneau 1982; Landis et al. 2000; Gurr et al. 2017). Increasing plant diversity has been considered one of the key factors for enhancing the activity of natural enemies and reducing pest density in crops (Root 1973; Russell 1989; Altieri 1991; Bianchi et al. 2006). However, this is not a universal principle, and there are many cases where plant diversity has been associated with increased pest incidence (Letourneau et al. 2011). Consequently, the suitable selection of plants is crucial to maximising the positive effects of pest control. Protected agricultural crops are simple systems that may miss many of the requirements for the maintenance of permanent populations of natural enemies, but this may be compensated with the introduction of particular plant species providing alternative host/prey, nectar, pollen, shelter or other vital resources (Landis et al. 2000; Wäckers et al. 2005; Gurr et al. 2017). However, there is also the risk that these plants host pathogens and/or are used by pests to increase their populations (van Rijn et al. 2002; Balzan and Wäckers 2013; Tscharntke et al. 2016).
The order Hemiptera includes many species of natural enemies that play a major role in regulating the populations of phytophagous arthropods in agroecosystems (Wheeler 2001). Many of these hemipterans are omnivores with a broad range of variations in their prey-plant feeding habits and whose biology is highly restricted to particular plant species (Eubanks et al. 2003; Cassis and Schuh 2012). This concerns dicyphines (Hemiptera: Miridae: Bryocorinae), which include several species of high economic importance (van Lenteren 2012). Predatory dicyphines are key species in biological pest control in vegetable crops, especially in tomatoes, because not many natural enemies are as well adapted to live on plants with glandular trichomes as they are (Castañé et al. 2004; Gillespie et al. 2007; Voigt et al. 2007; Perdikis et al. 2008; Calvo et al. 2012a, 2016; Sanchez et al. 2014, 2021). This group includes species such as Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae), which is among the ten most commercialised biological control agents in the world (van Lenteren 2012). Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae) is also extensively used for the control of whitefly and Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in the Mediterranean area (Calvo et al. 2012b; Urbaneja et al. 2012). Apart from the Macrolophus species and N. tenuis, several Palaearctic species of the Dicyphus genus have been tested against the main pests of vegetable crops (Alomar et al. 2002; Ingegno et al. 2013; Abbas et al. 2014).
The zoophytophagous character of predatory dicyphines may cause some trouble when they are used as pest control agents because of the derived damage from plant feeding, but it may also confer some advantage in terms of establishment and persistence in crops when prey is scarce (Sanchez et al. 2003, 2021; Biondi et al. 2016). Zoophytophagy is a common, if not an obligate, feeding strategy in dicyphines, in the sense that these insects need to feed both on plant and animal prey. One of the main functions of plant feeding in zoophytophagous mirids is the acquisition of the required water for prey feeding, metabolism and development (Gillespie and McGregor 2000). Plants may provide some nutrients, but their contribution to their diet is much lower than that of animal food (Gillespie and McGregor 2000; Sanchez et al. 2004; Ingegno et al. 2011). Indeed, phytophagy usually increases as a response to compensate for the scarcity of prey (Sanchez 2008b, 2009; Calvo et al. 2009). However, the fitness of zoophytophagous mirids feeding on plants varies among the species and relative to host plants (Sanchez et al. 2004; Perdikis et al. 2007; Biondi et al. 2016). The behaviour of dicyphines is greatly conditioned by the plant context, and even if the fitness from feeding on plants is much lower than that from feeding on prey, plant preference in dicyphines seems to be mainly driven by their fitness as herbivores (Sanchez et al. 2004; Gillespie et al. 2012). These particular aspects of the biology of dicyphines may limit their performance as biocontrol agents, but it opens the possibility of enhancing their habitats by providing companion plants with a higher nutritional value than that of the crops.
For instance, in the absence of prey, Dicyphus hesperus Knight (Hemiptera: Miridae) has a much higher fitness feeding on mullein (Verbascum thapsus L. -Scrophulariaceae) than on tomato, and the use of mullein as a companion plant in tomato greenhouses enhances its establishment, numerical response and pest control (Sanchez et al. 2003, 2004). Other plants such as tobacco, Calendula officinalis L. (Asteraceae) and Ballota hirsuta Bentham (Lamiaceae) have been used with variable results as alternative hosts for M. pygmaeus in tomato greenhouses (Arnó et al. 2000; Lambion 2014; Balzan 2017; Sanchez et al. 2021). Although, in principle, the idea of increasing crop diversity is appealing, several drawbacks must be considered before adopting this system. In the first place, companion plants may host and multiply pathogens and/or pests, which increase the risk of damage to the target crops (Tscharntke et al. 2016). In the second place, natural enemies may aggregate in highly preferred plant species and disperse at a low rate to the targeted crop (Blitzer et al. 2012).
Against this background, the effect of increasing plant diversity in tomato crops, using calabash Lagenaria siceraria (Molina) Standley (Cucurbitaceae) as a companion plant, was assessed on the establishment, population dynamics and whitefly control efficacy of Dicyphus argensis Sanchez & Cassis 2018. This plant was selected because it was known to be a suitable host for Dicyphus species and because it had been reported as a bad host for Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) (Kishaba et al. 1992; Sanchez and Cassis 2018; López-Gallego et al. 2019). Our working hypothesis was that increasing plant diversity in tomato crops would enhance the numerical and pest control response of D. argensis. Preliminary laboratory experiments showed high predation rates of D. argensis on B. tabaci nymphs (J.A. Sanchez, non-published data). Dicyphus argensis is a recently described species that has never been reported before as a pest control agent (Sanchez and Cassis 2018). Therefore, the efficacy of D. argensis was compared with that of another reputed zoophytophagous mirid, N. tenuis, which is commonly used for pest control in tomato crops in the Mediterranean area.
Materials and methods
Greenhouse
The experiment was conducted in a 40 × 10 m air-inflated, double-layered polyethylene-covered Quonset style greenhouse located at the Koppert facilities in Aguilas (Murcia, Spain). The greenhouse was equipped with a pan and fan cooling system and central heating, and it was divided into 36 compartments of 4 × 2 × 3.5 m each, 12 of which were used for the experiment. Compartment walls and ceilings were constructed of ‘anti-thrips’ polyethylene screening with 220 × 331 µm interstices supported by heavy guy wires connected to the greenhouse superstructure. Floors were covered with woven 2-mm thick polyethylene cloth. Access to the greenhouse was gained through a double, zippered doorway with an additional zippered doorway leading to each compartment from a central corridor. To simplify the terminology, these compartments will also be referred to as greenhouses. Each compartment was provided with a separate duct from the air-conditioning system. Temperature and relative humidity (RH) were monitored in four compartments with HOBO H8 RH⁄ Temp Loggers (Onset Computer, Bourne, MA, USA). The daily average, minimum and maximum temperatures along the assay period were 18.1 °C, 12.4 °C and 22.7 °C, respectively. The daily average, minimum and maximum RH along the assay were 68.1, 31.9 and 87.6, respectively.
Whitefly and predatory mirids
Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) adults to infest the tomato plants were collected from a colony maintained on tobacco plants, Nicotiana tabacum L. (Solanaceae), and originally obtained from field samples from several locations in the Region of Murcia, Spain (37°59′10″ N, 1°7′49″ W) and that were identified with polymerase chain reaction (PCR) as biotype ‘Q’. Nesidiocoris tenuis was provided in bottles containing 500 2–3-day-old adults (Nesibug™; Koppert Biological Systems, Berkel en Rodenrijs, The Netherlands). Dicyphus argensis adults were collected from a colony maintained on N. tabacum and fed with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs. The original colony was collected on pumpkin, Cucurbita maxima Duchesne (Cucurbitaceae), in the northwest Murcia Region (Spain) (38°6′32″ N, 1°47′1″ W). A sample of adults was collected from the rearing colonies and kept as voucher specimens at the IMIDA collection. These specimens were later identified as D. argensis, according to Sanchez and Cassis (2018).
Experimental design and procedure
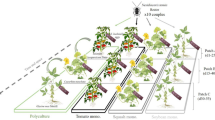
Four treatments were assayed in a complete randomised block design with three replicates each: (1) B. tabaci, (2) B. tabaci + D. argensis, (3) B. tabaci + D. argensis + companion plant -calabash and (4) B. tabaci + N. tenuis. Each block/replicate was a group of four adjacent greenhouse compartments, each being randomly designated for each treatment.
Seeds of tomato, Solanum lycopersicum L. (Solanaceae) cv. Razymo (Rijk Zwaan, De Lier, The Netherlands), were first sown into 5.4-cm2 peat moss root cubes. When seedlings reached the five-leaf stage, 12 plants were transplanted inside each compartment on 27 January 2005 into 10-L pots with coco peat fibre as substrate. During the experiment, tomato plants were trained by the main stem to a black polyethylene string tied to a stainless-steel overhead wire. Secondary shoots were removed and water and fertilisers were supplied as required through the drip irrigation system (Mithra™; Novedades Agrícolas). In each compartment designated for ‘B. tabaci + D. argensis + companion plant’, two calabash plants were also transplanted the same day as the tomato plants. Calabash plants were approximately 20 cm tall at transplant. None of the plants was sprayed with pesticides during the experiments.
Each compartment was infested with 72 adult females of B. tabaci plus an unknown quantity of males on 3 February 2005. This infestation rate was chosen to simulate a strong and early whitefly attack. Two weeks later, on 17 February 2005, N. tenuis and D. argensis were released into designated compartments at a rate of 1 adult per plant and a sex ratio of 1:1 (male: female). This release was repeated 1 week later at the same rate. This release schedule for the mirids was chosen following the recommended release rate for N. tenuis in commercial tomato crops (Calvo and Urbaneja 2004) and to assure the presence of food (first whitefly nymphs) when first mirids were released. Mirid and whitefly adults to be released were cooled briefly in a cold room at 8 °C for counting and sexing before being released into the designated experimental compartments.
Sampling of insects
Plants were monitored weekly for 13 weeks after transplanting, beginning on 3 February 2005, just before the whitefly release, and finishing on 28 April 2005. Six tomato plants were randomly selected on each sampling date for each compartment, and in compartments with calabash, two plants were also selected. Whitefly and mirid nymphs and adults were counted on three leaves from each of the selected plants: one leaf was randomly selected from the upper, one from the middle and one from the bottom third of the plant. In each case, leaves were turned carefully to count first whitefly and mirid adults and then the other insect stages using a 15X hand lens. In addition, the number of nymphs and adults of mirids was counted on the whole plant during the first eight weeks of the experiment. This was done to increase the accuracy and precision of the estimates of the mirid numbers in the first weeks, when the populations were small and densities on leaves were very low. To reduce the risk of accidental contamination among treatments, special care was always taken to enter the B. tabaci-only compartments first and then the compartments with mirid release.
For the assessment of mirid damage, the number of necrotic rings was counted on each of the leaves sampled. In addition, the number of aborted and viable flowers was counted in one truss of flowers from each plant.
Statistical analysis
The abundance of mirids, whiteflies nymphs and adults expressed as the number of individuals per leaf was compared among treatments using generalised linear mixed models (GLMM). In the case of mirids, only the data from the weeks after their release were used for the analyses because no mirids were observed in the previous weeks. The date of sampling, plant code and strata of the plant from where leaves were taken (upper, middle and bottom) were introduced in the models as random factors. GLMMs were run using the function “glmmPQL” (“MASS package”) (Venables and Ripley 2002) set to the negative binomial, as it was found to be the distribution that best fit the experimental data for all the groups of insects (R-Development-Core-Team 2017). The χ2 and p-values for the fixed factors were obtained by the Wald test using the “Anova” function in the R “car” package (R-Development-Core-Team, 2017). The post hoc pairwise multiple comparisons among treatments was run using Tukey´s test with the function “glht” in the “multcomp” package in R (Hothorn et al. 2006). The same approach was followed to compare the number of mirids (nymphs + adults) per plant among treatments. In this model, the only two differences were that the control treatment was excluded from these analyses, as no mirids were observed along the 5 weeks that the whole plant was sampled and only date and plant code were used as random factors.
The above-explained procedure for the abundance of insects on leaves was also followed to compare the number of necrotic rings per leaf and the proportion of aborted flowers per truss among treatments. In the GLMM that explained the variation in the proportion of aborted flowers the data were transformed by the arcsine of the square root to account for the deviation from normality, and only the date was used as a random factor. All the statistical analyses were run using the R software (R-Development-Core-Team 2017).
Results
Population dynamics of mirids and plant damages
The number of mirids per tomato plant over the 5 weeks after the release differed significantly among treatments (χ2 = 15.4; df = 2; P < 0.001). Significant differences in the abundance of mirids in tomato plants were found between the compartments with D. argensis plus companion plants and those where N. tenuis and D. argensis were released (Tukey's test, P < 0.01). In contrast, no significant differences were found between the compartments with N. tenuis and D. argensis (Tukey's test, P = 0.836). The number of mirids per tomato plant started to increase in the third week after the release to reach density peaks of 3.39 ± 1.33, 2.22 ± 0.98 and 0.56 ± 0.40 (mean ± SE) individuals per plant in the compartments with N. tenuis, D. argensis and D. argensis plus companion plants, respectively (Fig. 1A). In calabash plants, the number of D. argensis reached much higher abundances (17.5 ± 4.3 individuals per plant) in the fifth week after its release (Fig. 1B).
(A) Number of mirids per tomato plant + SE in greenhouses compartments with four different treatments: (1) B. tabaci releases -Control; (2) B. tabaci + D. argensis releases; (3) B. tabaci + D. argensis + calabash as companion plant; and (4) B. tabaci + N. tenuis releases. (B) Number of D. argensis per plant + SE in calabash
Significant differences in the number of mirids per leaf over the ten weeks after the release were found among all the treatments (χ2 = 588.3; df = 3; P < 0.001). The highest number of mirids was reached in the compartments where N. tenuis was released, followed in descending order by those with D. argensis, D. argensis plus companion plants and the control (Fig. 2A). Nesidioscoris tenuis reached its highest density (3.72 ± 0.36 individuals per leaf) in the last week of the experiment. In the compartments where D. argensis was released, the abundance of this mirid over the thirteen weeks of the experiment was much lower than that of N. tenuis. Dicyphus argensis reached density peaks of 0.44 ± 0.28 and 0.41 ± 0.33 individuals per leaf in the last weeks in the compartments with and without companion plants, respectively (Fig. 2A). The population dynamics of D. argensis in the compartments with and without calabash were very similar, but the density of this mirid in tomato plants was higher in the compartments without companion plants until the eleventh week. The control compartments were free of mirid during most of the experiment, with just a few N. tenuis (0.08 ± 0.04 individuals per plant) found in two of the compartments in the last two weeks of the experiment (Fig. 2A). In calabash, the density of D. argensis increased progressively to reach a peak of 0.72 ± 0.24 individuals per leaf in the eleventh week (Fig. 2B).
(A) Number of mirids per tomato leaf + SE in greenhouses compartments with four different treatments: (1) B. tabaci releases -Control; (2) B. tabaci + D. argensis releases; (3) B. tabaci + D. argensis + calabash as companion plant; and (4) B. tabaci + N. tenuis releases. (B) Number of D. argensis per leaf + SE in calabash
Significant differences were found in the proportion of aborted flowers among treatments (χ2 = 26.6; df = 3; P < 0.001), as the proportion of aborted flowers in the compartments with N. tenuis was significantly higher than that observed in the other compartments (Tukey's test, P < 0.05). The proportion of aborted flowers increased progressively to reach peaks of 0.33 ± 0.31, 0.19 ± 0.17, 0.18 ± 0.02 and 0.15 ± 0.05 in the compartments with N. tenuis, D. argensis plus companion plants, D. argensis and the control, respectively (Fig. 3A). In the compartments where N. tenuis was released, the leaf damage increased progressively to reach a peak of 0.78 ± 0.39 necrotic rings per leaf in the last week of the experiment (Fig. 3B). No leaf damage was found in the rest of the treatments; however, the differences in leaf damage among treatments were not significant (χ2 = 4.17; df = 3; P = 0.243).
(A) Proportion of aborted flowers + SE in tomato plants in greenhouses compartments with four different treatments: (1) B. tabaci releases -Control; (2) B. tabaci + D. argensis releases; (3) B. tabaci + D. argensis + calabash as companion plant; and (4) B. tabaci + N. tenuis releases. (B) Number of necrotic rings per tomato leaf + SE in the same four treatments as in graph A
Whitefly population dynamics
The density of B. tabaci nymphs differed significantly among all the treatments (χ2 = 4,246,923; df = 3; P < 0.001). The highest number of whitefly nymphs was observed in the control compartments, followed by those where D. argensis was released and calabash used as a companion plant, those with D. argensis and no companion plants and, finally, those where N. tenuis was released (Fig. 4A). The density of whitefly nymphs increased progressively from its release until the last week of the experiment to reach peaks of 493.6 ± 116.3 nymphs per leaf in the compartments with D. argensis and companion plants, 316.9 ± 87.8 nymphs per leaf in the controls and 161.1 ± 77.7 nymphs per leaf in the compartments with only D. argensis (Fig. 4A). In the compartments with N. tenuis, the density of whitefly nymphs increased to reach a maximum of 72.5 ± 21.6 nymphs per leaf in the tenth week and decreased thereafter to 20.0 ± 17.3 nymphs per leaf in the last week (Fig. 4A). Significant differences were also found in the density of B. tabaci adults among treatments (χ2 = 41.4; df = 3; P < 0.001). The abundance of whitefly adults differed among all the treatments (P < 0.01), except for that between the control and the D. argensis plus companion plant compartments (Tukey's test, P = 0.716) and between the treatments with N. tenuis and D. argensis (Tukey's test, P = 0.960) (Fig. 4B). The density of whitefly adults increased progressively to reach peaks of 493.6 ± 116.3, 316 ± 87.8, 161.1 ± 77.7 and 43.1 ± 39.7 adults per leaf in the compartments with D. argensis plus calabash, the controls, the compartments with D. argensis and those with N. tenuis, respectively (Fig. 4 B). On 15 April, an unexpected increase took place in the number of whitefly adults in tomato plants in the compartments with calabash (Fig. 4B). This, in turn, produced a sudden increase in the number of nymphs in the following weeks, which was likely due to the reproduction of adults immigrating from calabash. The density of whitefly in calabash started to increase at the beginning of April to reach a peak of 13.5 ± 10.2 and 8.1 ± 4.0 nymphs and adults per leaf in the last week of the experiment, respectively (Fig. 5).
(A) Number of whitefly nymphs per tomato leaf + SE in greenhouses compartments with four different treatments: (1) B. tabaci releases -Control; (2) B. tabaci + D. argensis releases; (3) B. tabaci + D. argensis + calabash as companion plant; and (4) B. tabaci + N. tenuis releases. (B) Number of whitefly adults per tomato leaf + SE in the same four treatments as in graph A
Discussion
Increasing plant diversity in tomato crops using calabash as a companion plant did not enhance the pest control efficacy of the omnivorous mirids D. argensis. This is in contrast with our working hypothesis and with the theories that predict an enhancement in the effectivity of natural enemies in regulating the populations of herbivores when plant diversity increases (Root 1973; Altieri and Letourneau 1982; Russell 1989). Calabash was an optimum host for the multiplication of D. argensis; in fact, this mirid reached populations of several orders of magnitude higher in this host than in tomato plants. However, the presence of calabash did not enhance the numerical response of D. argensis in the crop plants. On the contrary, the abundance of this mirid on tomato was significantly lower in the presence than in the absence of the companion plant. This lack of spillover of the D. argensis populations built up in calabash to tomato plants could be due to the relatively lower preference of this mirid for tomato than for calabash. Another parallel assay found that when calabash plants were cut to force D. argensis to disperse to tomato plants, it chose to leave the greenhouses they were in and tried to immigrate to others where calabash was present (Sanchez 2008a). Similar behaviour was observed in D. hesperus (Hemiptera: Miridae), which showed a low preference for pepper and it did not increase its population on pepper plants, even when mullein plants hosting a high population of this mirid were cut to force its dispersal (Sanchez 2008a). In a similar context, strips of Calendula officinalis L. (Asteraceae) hosting several zoophytophagous dicyphine species did not significantly affect their abundance in tomato crops (Balzan 2017).
In contrast, other authors have reported a positive effect when increasing plant diversity in crops. For instance, the use of mullein as a companion plant enhanced the establishment of D. hesperus and the control of the whitefly Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) in tomato glasshouses (Sanchez et al. 2003). In the same way, tobacco used as a banker plant for M. pygmaeus reduced the density of T. vaporariorum in tomato and hosted populations of this mirid during free crop periods (Arnó et al. 2000; Fischer and Terrettaz 2003; Bresch et al. 2014). Annual strips of C. officinalis near greenhouses increased the occurrence of M. pygmaeus in tomato crops (Lambion 2014; Ardanuy et al. 2022). Finally, B. hirsuta has been reported to enhance the establishment, numerical and pest control response of M. pygmaeus in tomato greenhouses (Sanchez et al. 2021).
Dicyphus argensis was found to significantly reduce the abundance of the whitefly B. tabaci compared to the greenhouses where mirids were not released. Predatory mirids are reputed predators of small arthropod pests such as whiteflies, thrips, aphids, dipterans, leafminers, psyllids and lepidopterans (Riudavets and Castañé 1998; Perdikis et al. 2008; Calvo et al. 2009, 2012b, 2016; Urbaneja et al. 2012; Ingegno et al. 2013; Sanchez et al. 2014, 2021; Abbas et al. 2014). In particular, other Dicyphus species such as D. hesperus, Dicyphus tamanini Wagner and Dicyphus hyalinipennis (Burmeister) (Heteroptera: Miridae) have been reported as effective biocontrol agents of whitefly in tomato greenhouses (Ceglarska 1999; Sanchez et al. 2003; Gillespie et al. 2007; Castañé et al. 2011). This research also confirmed that N. tenuis can quickly build up its population and effectively reduce the density of whiteflies in tomato crops (Sanchez 2008b). In contrast, D. argensis reached lower population levels and was less effective in controlling whiteflies than N. tenuis. The slower population increase of D. argensis could be due to its lower fitness than N. tenuis when feeding on B. tabaci on tomato plants (Sanchez J.A., unpublished data). However, it could also arise from high emigration rates because of the low preference of D. argensis for tomato plants compared to calabash (Sanchez 2008a). Plant preferences are known to strongly affect the population dynamics of predatory dicyphines (Gillespie et al. 2012). For example, emigration rates in D. hesperus were found to vary with plant species, with higher patch-leaving times registered in the less-preferred plant species (Sanchez et al. 2004; VanLaerhoven et al. 2006).
Dicyphus argensis was found to produce less damage to tomato plants than N. tenuis. However, these differences could have been just due to the lower abundance of the former species. Omnivorous mirids are known to produce blemishing in vegetative plant parts and flower abortion, in particular when prey is scarce (Sanchez 2008b, 2009; Calvo et al. 2009). The presence of companion plants did not significantly affect crop damage. In contrast, the use of Dittrichia viscosa L. (Asteraceae) and Sesamum indicum L. (Pedaliaceae) as an alternative host for N. tenuis was found to reduce damage in tomato plants (Biondi et al. 2016). In the same way, D. hesperus produced less damage to tomato plants in association with mullein than to tomatoes in monoculture (Gillespie et al. 2012).
The use of calabash as a companion plant had the undesirable effect of increasing B. tabaci density in tomato plants. Plant diversity may not only benefit natural enemies but also phytophagous arthropods that attack crop plants (van Rijn et al. 2002; Balzan and Wäckers 2013; Tscharntke et al. 2016). Many arthropod pests are polyphagous and feed on a wide range of cultivated and wild plants, thus, pests may multiply in wild plants and disperse to crops (Tillman et al. 2009; Blitzer et al. 2012). The higher density of whiteflies in the greenhouses with companion plants in comparison to the controls could have been due to several reasons. In the first instance, whiteflies on tomatoes in the greenhouses with the companion plants were likely exposed to lower predation because of the lower abundance of D. argensis on tomato plants. Other authors have also reported disruption in biological control when providing alternative plant resources to omnivorous natural enemies (Frank et al. 2011). In the second instance, calabash served as a host for the multiplication of B. tabaci that dispersed to tomato plants. This is in contrast with previous works, where it was found that L. siceraria acted as a trap plant that reduced the immigration of B. tabaci when placed outside tomato greenhouse (López-Gallego et al. 2019). The different results could have been due to variations in the density of glandular trichomes between the calabash plants used in the two experiments, as pubescence seems to be an ovipositional deterrent for B. tabaci (Kishaba et al. 1992). However, the trichome density was not measured and this statement cannot be corroborated. In spite of D. argensis being present in calabash in high numbers and the high predation rates observed in previous laboratory assays (J.A. Sanchez, non-published data), this mirid was unable to prevent the whitefly outbreak on calabash. As stated above, it is likely that the food resources provided by calabash reduced whitefly predation.
Other cases where introducing alternative host plants increased pest density have been reported in the literature (Letourneau et al. 2011; Tscharntke et al. 2016). For example, in the absence of predators, tomato with tobacco as a banker plant strongly increased the abundance densities of the whitefly T. vaporariorum (Bresch et al. 2014). Stink bugs were found to disperse from peanuts to the neighbouring cotton fields and fed on cotton bolls (Tillman et al. 2009). The abundance of Pieris rapae (L.) (Lepidoptera: Pieridae) and Plutella xilostella (L.) (Lepidoptera: Plutellideae) was higher in broccoli interplanted with nectar-producing plants than in broccoli monocultures (Zhao et al. 1992).
In conclusion, this research adds to the list of cases where increasing plant diversity in crops did not enhance pest control. Calabash was an optimum companion plant for the multiplication of D. argensis, but it contributed neither to increasing its abundance in the tomato plants nor to enhancing whitefly control. On the contrary, the aggregation of D. argensis in calabash reduced its abundance in tomato plants. These results outline the importance of the plant context for dicyphine species and that of selecting the right companion plant to enhance the performance of natural enemies as biocontrol agents (Wäckers et al. 2005; Gillespie et al. 2012). In addition, the pest situation in tomatoes worsened because of the multiplication of B. tabaci on calabash. This has been identified as one of the possible causes of the failure of biological control when increasing plant diversity (Tscharntke et al. 2016). In the present research, we add another cause, that is, when alternative host plants attract and reduce the abundance of natural enemies in crops.
Although the lower efficiency of D. argensis compared to N. tenuis may not justify augmentative releases, it could nonetheless provide a valid biocontrol service when it naturally colonises tomato crops and, thus, it would be worth its inclusion in conservation biocontrol programmes. This species has been naturally found in crops such as tomato and pumpkin, as well as on some Solanaceae, Scrophulariaceae and Fagaceae wild host plants (Sanchez and Cassis 2018). Enhancing the habitat by providing host plants for these mirids may also benefit other dicyphine species, as they share many of their host plant species (Alomar et al. 2002; Sanchez and Cassis 2018). Nonetheless, further studies will be required to properly manage habitats to ensure the spillover of predatory mirids to crops and enhancement of pest control.
Data availability
Data and material will be available upon request.
References
Abbas S, Pérez-Hedo M, Colazza S, Urbaneja A (2014) The predatory mirid Dicyphus maroccanus as a new potential biological control agent in tomato crops. Biocontrol 59:565–574. https://doi.org/10.1007/s10526-014-9587-6
Alomar Ò, Goula M, Albajes R (2002) Colonisation of tomato fields by predatory mirid bugs (Hemiptera: Heteroptera) in northern Spain. Agric Ecosyst Environ 89:105–115. https://doi.org/10.1016/S0167-8809(01)00322-X
Altieri MA (1991) How best can we use biodiversity in agroecosystems. Outlook Agric 20:15–23. https://doi.org/10.1177/003072709102000105
Altieri MA, Letourneau DK (1982) Vegetation management and biological control in agroecosystems. Crop Prot 1:405–430. https://doi.org/10.1016/0261-2194(82)90023-0
Ardanuy A, Figueras M, Matas M, Arnó J, Agustí N, Alomar Ò, Albajes R, Gabarra R (2022) Banker plants and landscape composition influence colonisation precocity of tomato greenhouses by mirid predators. J Pest Sci 95:447–459. https://doi.org/10.1007/s10340-021-01387-y
Arnó J, Ariño J, Español R, Marta, M, Alomar, O (2000) Conservation of Macrolophus caliginosus Wagner (Het. Miridae) in commercial greenhouses during tomato crop-free periods. IOBC wprs Bull 23:241–246.
Balzan MV (2017) Flowering banker plants for the delivery of multiple agroecosystem services. Arthropod Plant Interact 11:743–754. https://doi.org/10.1007/s11829-017-9544-2
Balzan MV, Wäckers FL (2013) Flowers to selectively enhance the fitness of a host-feeding parasitoid: adult feeding by Tuta absoluta and its parasitoid Necremnus artynes. Biol Control 67:21–31. https://doi.org/10.1016/j.biocontrol.2013.06.006
Bianchi FJJ, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc Biol Sci 273:1715–1727. https://doi.org/10.1098/rspb.2006.3530
Biondi A, Zappalà L, Di Mauro A, Tropea-Garzia G, Russo A, Desneux N, Siscaro G (2016) Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? Biocontrol 61:79–90. https://doi.org/10.1007/s10526-015-9700-5
Blitzer EJ, Dormann CF, Holzschuh A, Klein AM, Rand TA, Tscharntke T (2012) Spillover of functionally important organisms between managed and natural habitats. Agric Ecosyst Environ 146:34–43. https://doi.org/10.1016/j.agee.2011.09.005
Bresch C, Ottenwalder L, Poncet C, Parolin P (2014) Tobacco as banker plant for Macrolophus pygmaeus to control Trialeurodes vaporariorum in tomato crops. Univers J Agric Res 2:297–304. https://doi.org/10.13189/ujar.2014.020803
Calvo FJ, Urbaneja A (2004) Nesidiocoris tenuis un aliado para el control biológico de mosca blanca. Hortic Int 44:20–25
Calvo J, Bolckmans K, Stansly PA, Urbaneja A (2009) Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. Biocontrol 54:237–246. https://doi.org/10.1007/s10526-008-9164-y
Calvo FJ, Bolckmans K, Belda JE (2012a) Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. Biocontrol 57:809–817. https://doi.org/10.1007/s10526-012-9455-1
Calvo FJ, Lorente MJ, Stansly PA, Belda JE (2012b) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp Appl 143:111–119. https://doi.org/10.1111/j.1570-7458.2012.01238.x
Calvo FJ, Torres-Ruiz A, Velázquez-González JC, Rodríguez-Leyva E, Lomeli-Flores JR (2016) Evaluation of Dicyphus hesperus for biological control of sweet potato whitefly and potato psyllid on greenhouse tomato. Biocontrol 61:415–424. https://doi.org/10.1007/s10526-016-9719-2
Cassis G, Schuh RT (2012) Systematics, biodiversity, biogeography, and host associations of the miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annu Rev Entomol 57:377–404. https://doi.org/10.1146/annurev-ento-121510-133533
Castañé C, Alomar O, Goula M, Gabarra R (2004) Colonization of tomato greenhouses by the predatory mirid bugs Macrolophus caliginosus and Dicyphus tamaninii. Biol Control 30:591–597. https://doi.org/10.1016/j.biocontrol.2004.02.012
Castañé C, Arnó J, Gabarra R, Alomar O (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29. https://doi.org/10.1016/j.biocontrol.2011.03.007
Ceglarska EB (1999) Dicyphus hyalinipennis Burm. (Heteroptera: Miridae): a potential biological control agent for glasshouse pests in Hungary. IOBC wprs Bull 22:33–36
Eubanks MD, Styrsky JD, Denno RF (2003) The evolution of omnivory in heteropteran insects. Ecology 84:2549–2556. https://doi.org/10.1890/02-0396
Fischer S, Terrettaz C (2003) Release Strategies of the Mirid Macrolophus caliginosus in Protected Tomato Crops. Rev suisse Vitic Arboric Hortic 35:191–196
Frank SD, Shrewsbury PM, Denno RF (2011) Plant versus prey resources: Influence on omnivore behavior and herbivore suppression. Biol Control 57:229–235. https://doi.org/10.1016/j.biocontrol.2011.03.004
Gillespie DR, McGregor RR (2000) The functions of plant feeding in the omnivorous predator Dicyphus hesperus: water places limits on predation. Ecol Entomol 25:380–386. https://doi.org/10.1046/j.1365-2311.2000.00285.x
Gillespie D, McGregor R, Sanchez JA, VanLaerhoven S, Quiring D, Roitberg B, Foottit R, Schwartz M, Shipp L (2007) An endemic omnivorous predator for control of greenhouse pests. In: Vicent C, Goettel MS, Lazarovits G (eds) Biological control: a global perspective. CABI, Agassiz, pp 128–135. https://doi.org/10.1079/9781845932657.0128
Gillespie DR, Vanlaerhoven SL, McGregor RR, Chan S, Roitberg BD (2012) Plant feeding in an Omnivorous mirid Dicyphus hesperus: why plant context matters. Psyche. https://doi.org/10.1155/2012/495805
Gurr GM, Wratten SD, Landis DA, You M (2017) Habitat management to suppress pest populations: progress and prospects. Annu Rev Entomol 62:91–109. https://doi.org/10.1146/annurev-ento-031616-035050
Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 15:651–674. https://doi.org/10.1198/106186006X133933
Ingegno BL, Pansa MG, Tavella L (2011) Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol Control 58:174–181. https://doi.org/10.1016/j.biocontrol.2011.06.003
Ingegno BL, Ferracini C, Gallinotti D, Alma A, Tavella L (2013) Evaluation of the effectiveness of Dicyphus errans (Wolff) as predator of Tuta absoluta (Meyrick). Biol Control 67:246–252. https://doi.org/10.1016/j.biocontrol.2013.08.002
Karp DS, Chaplin-Kramer R, Meehan TD, Martin EA, DeClerck F, Grab H et al (2018) Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc Natl Acad Sci U S A 115:E7863–E7870. https://doi.org/10.1073/pnas.1800042115
Kishaba AN, Castle S, McCreight JD, Desjardins PR (1992) Resistance of white-flowered gourd to sweetpotato whitefly. HortScience 11:1217–1221. https://doi.org/10.21273/HORTSCI.27.11.1217
Lambion J (2014) Flower strips as winter shelters for predatory miridae bugs. Acta Hortic 1041:149–156. https://doi.org/10.17660/ActaHortic.2014.1041.16
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201. https://doi.org/10.1146/annurev.ento.45.1.175
Letourneau DK, Armbrecht I, Rivera BS, Lerma J, Carmona EJ, Daza MC et al (2011) Does plant diversity benefit agroecosystems? a synthetic review. Ecol Appl 21:9–21. https://doi.org/10.1890/09-2026.1
Lichtenberg EM, Kennedy CM, Kremen C, Batáry P, Berendse F, Bommarco R et al (2017) A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob Chang Biol 23:4946–4957. https://doi.org/10.1111/gcb.13714
López-Gallego E, Cerezuela-Serrano J, Ramírez-Soria M, Pérez-Marcos M, Sanchez JA (2019) Pumpkin as a barrier-trap plant to reduce whitefly immigration into tomato greenhouses. IOBC-WPRS Bull 147: 125–127
Perdikis DC, Favas C, Lykouressis DP, Fantinou A (2007) Ecological relationships between non-cultivated plants and insect predators in agroecosystems: the case of Dittrichia viscosa (Asteraceae) and Macrolophus melanotoma (Hemiptera: Miridae). Acta Oecologica 31:299–306. https://doi.org/10.1016/j.actao.2006.12.005
Perdikis DC, Kapaxidi E, Papadoulis G (2008) Biological control of insect and mite pests in greenhouse solanaceous crops. Eur J Plant Sci Biotechnol 2:125–144
R-Development-Core-Team (2017) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Riudavets J, Castañe C (1998) Identification and evaluation of native predators of Frankliniella occidentalis (Thysanoptera: Thripidae) in the mediterranean. Environ Entomol 27:86–93. https://doi.org/10.1093/ee/27.1.86
Root RB (1973) Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol Monogr 1:95–124. https://doi.org/10.2307/1942161
Russell EP (1989) Enemies hypothesis: a Review of the Effect of vegetational diversity on predatory insects and parasitoids. Environ Entomol 18:590–599. https://doi.org/10.1093/ee/18.4.590
Sanchez JA (2008b) Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric For Entomol 10:75–80. https://doi.org/10.1111/j.1461-9563.2007.00357.x
Sanchez JA (2009) Density thresholds for Nesidiocoris tenuis (Heteroptera: Miridae) in tomato crops. Biol Control 51:493–498. https://doi.org/10.1016/j.biocontrol.2009.09.006
Sanchez JA, Cassis G (2018) Towards solving the taxonomic impasse of the biocontrol plant bug subgenus Dicyphus (Dicyphus) (Insecta : Heteroptera : Miridae) using molecular, morphometric and morphological partitions. Zool J Linn Soc 184:330–406. https://doi.org/10.1093/zoolinnean/zly005/5003105
Sanchez JA, Gillespie DR, McGregor RR (2003) The effects of mullein (Verbascum thapsus ) on the population dynamics of Dycyphus hesperus (Heteroptera: Miridae) in tomato greenhouses. Biol Control 28:313–319. https://doi.org/10.1016/S1049-9644(03)00116-6
Sanchez JA, Gillespie DR, McGregor RR (2004) Plant preference in relation to life history traits in the zoophytophagous predator Dicyphus hesperus. Entomol Exp Appl 112:7–19. https://doi.org/10.1111/j.0013-8703.2004.00174.x
Sanchez JA, La-Spina M, Lacasa A (2014) Numerical response of Nesidiocoris tenuis (Hemiptera: Miridae) preying on Tuta absoluta (Lepidoptera: Gelechiidae) in tomato crops. Eur J Entomol 111:387–395. https://doi.org/10.14411/eje.2014.041
Sanchez JA, López-Gallego E, Pérez-Marcos M, Perera-Fernández L (2021) The effect of banker plants and pre-plant release on the establishment and pest control of Macrolophus pygmaeus in tomato greenhouses. J Pest Sci 94:297–307. https://doi.org/10.1007/s10340-020-01257-z
Sanchez JA (2008a) Population dynamics constraints in an omnivore dicyphine. In: Mason PG, Gillespie DR, Vincent C (eds) Procceedings of the third international symposium on biological control of arthropods. Christchurch, New Zealand. pp. 268–271. https://doi.org/10.1073/pnas.2002557117
Tillman PG, Northfield TD, Mizell RF, Riddle TC (2009) Spatiotemporal patterns and dispersal of stink bugs (Heteroptera: Pentatomidae) in peanut-cotton farmscapes. Environ Entomol 38:1038–1052. https://doi.org/10.1603/022.038.0411
Tscharntke T, Karp DS, Chaplin-Kramer R et al (2016) When natural habitat fails to enhance biological pest control—Five hypotheses. Biol Conserv 204:449–458. https://doi.org/10.1016/j.biocon.2016.10.001
Urbaneja A, Gonzalez-Cabrera J, Arno J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222. https://doi.org/10.1002/ps.3344
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
van Rijn PCJ, Van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679. https://doi.org/10.1890/0012-9658(2002)083[2664:HPBFPF]2.0.CO;2
VanLaerhoven SL, Gillespie DR, Roitberg BD (2006) Patch retention time in an omnivore, Dicyphus hesperus is dependent on both host plant and prey type. J Insect Behav 19:613–621. https://doi.org/10.1007/s10905-006-9047-y
Venables WN, Ripley BD (2002) Statistic and computing. Modern applied statistic with S. Springer-Verlag, New York Inc, New York. https://doi.org/10.1007/978-0-387-21706-2
Voigt D, Gorb E, Gorb S (2007) Plant surface-bug interactions: Dicyphus errans stalking along trichomes. Arthropod Plant Interact 1:221–243. https://doi.org/10.1007/s11829-007-9021-4
Wäckers FL, van Rijn PCJ, Bruin J (eds) (2005) Plant-Provided Food for Carnivorous Insects. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511542220
Wheeler AG (2001) Biology of the plant bugs (Hemiptera: Miridae). Cornell University Press, Ithaca, New York
Zhao J, Ayers G, Grafius E, Stehr F (1992) Effects of neighboring nectar-producing plants on populations of pest Lepidoptera and their parasitoids in broccoli plantings. Gt Lakes Entomol 25:253–258
Acknowledgements
This work was funded by research project AGL2003-07532-C03-03.
Funding
Project AGL2003-07532-C03-03.
Author information
Authors and Affiliations
Contributions
JAS, MdP and FJC conceived and designed the research; JAS analysed the data and wrote the manuscript. MdP and FJC conducted the experiments as well as revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Alberto Urbaneja.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanchez, J.A., del Pino, M. & Calvo, F.J. Increasing plant diversity does not always enhance the efficacy of omnivorous mirids as biocontrol agents. J Pest Sci 95, 1557–1566 (2022). https://doi.org/10.1007/s10340-022-01526-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01526-z