Abstract
Three species of hover fly commonly prey on woolly apple aphid, Eriosoma lanigerum, in Virginia, USA apple orchards. Larvae of Heringia calcarata are specialized predators of this pest, while Eupeodes americanus and Syrphus rectus are generalist aphid predators. The developmental duration of the immature stages of H. calcarata was determined under laboratory conditions, revealing a generation time of 19–20 d at 25°C. Descriptions of the larval, pupal and adult stages of H. calcarata are reported. Potted apple trees infested with arboreal colonies of woolly apple aphid and deployed in an orchard in Virginia were used as sentinels to measure seasonal changes in the relative abundance of the three syrphid species, based on the number of unhatched eggs deposited during weekly, 48-h exposure intervals from April to October, 2003–2005. Similar trends in the relative abundance of each species were recorded across all years. Eupeodes americanus was recorded first, showing a pronounced peak between mid-April and mid-May, followed by a prolonged period during which it was absent or present in very low numbers and then a much smaller peak in September and October. First records of H. calcarata occurred slightly later than for E. americanus. Early peaks of H. calcarata abundance typically occurred in May and June and tended to be smaller than those of E. americanus. Heringia calcarata eggs were recovered throughout most of each season. The potential role of predation by aphidophagous hover flies on the suppression of woolly apple aphid outbreaks in eastern apple orchards is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The woolly apple aphid, Eriosoma lanigerum (Hausmann) is a cosmopolitan, indirect pest of apple (Baker 1915) that can colonize the roots and arboreal parts of trees and impair their growth, productivity and vigor (Weber and Brown 1988; Brown and Schmitt 1990; Brown et al. 1991, 1995). Following exportation of the specialist, Aphelinid parasitoid of woolly apple aphid, Aphelinus mali (Haldeman) from eastern North America to 50 apple-producing countries (Howard 1929; Yothers 1953), this program became widely regarded as a successful example of classical biological control, despite reports that the establishment of A. mali and its effectiveness at suppressing woolly apple aphid populations varied considerably among regions to which it was introduced (Yothers 1953; DeBach 1964). Different levels of control among regions appeared to be due largely to differing climatic conditions that were more or less favorable for the activity of A. mali against its prey (reviewed in Hagen and van den Bosch 1968). Numerous studies have documented a lower temperature threshold for development and reproduction of woolly apple aphid than of A. mali and a greater number of generations of the aphid than the parasitoid (early research reviewed in Hagen and van den Bosch 1968; Asante and Danthanarayana 1992), leading to a general conclusion that the lag between the onset of activity of the aphid and the parasitoid is primarily responsible for partial control in some regions. Differential susceptibility of the woolly apple aphid and A. mali to the insecticides used to manage orchard pests has also been cited as a factor influencing the effectiveness of the parasitoid (Penman and Chapman 1980; Cohen et al. 1996).
The importance of predation on E. lanigerum early in the apple growing season has long been recognized (Dumbleton and Jeffreys 1938; Bodenheimer 1947) and several studies have concluded that predators exerted more impact on woolly apple aphid than A. mali (Holdsworth 1970; Gruys 1982; Walker 1985; Nicholas et al. 2005). However in general, the role of predators in reducing woolly apple aphid population density is not well understood (Asante 1997; Mols and Boers 2001) and merits further investigation. A review by Asante (1997) revealed that 73 insect species have been reported preying on woolly apple aphid, including Coccinellidae (48%), Syrphidae (21%), Chrysopidae (14%), Cecidomyiidae, Forficulidae and Lygaeidae (17% combined).
Predator exclusion experiments conducted during two consecutive years in Washington State apple orchards (Walker 1985) revealed that a coccinellid, a lacewing and a mirid were important natural enemies of woolly apple aphid in that ecosystem and that A. mali alone did not effect acceptable levels of control. In West Virginia orchards, Brown and Schmitt (1990) found that A. mali and hover fly larvae were the only natural enemies of woolly apple aphid, although the species of Syrphidae were not identified. During a widespread outbreak of woolly apple aphid in the Mid-Atlantic region in 2000, Bergh and Louque (2000) reported that aphidophagous larvae of three species of syrphid flies, Heringia calcarata (Loew), Eupeodes americanus (Weidemann) and Syrphus rectus Osten Sacken, were the most abundant predators of the aphid in apple orchards near Winchester, VA.
Heringia calcarata is a specialized predator of woolly apple aphid (Short and Bergh 2004), while E. americanus (Weidemann) and S. rectus Osten Sacken are common, generalist aphid predators that also feed in colonies of rosy apple aphid, Dysaphis plantaginea (Passerini) and spirea aphid, Aphis spiraecola Patch on apple trees. Eupeodes americanus and S. rectus are widely distributed species that occur respectively, across Canada, south to California, Texas, Mexico and Florida, and in central and eastern Canada, south to Colorado, Texas, Mississippi and North Carolina (Vockeroth 1992). The reported range of H. calcarata [Neocnemodon calcaratus in Wirth et al. (1965)] extends from Quebec to British Columbia, New York and south to Kansas and Virginia.
Heringia calcarata belongs to the tribe Pipizini, members of which often specialize on hosts that produce flocculent, waxy secretions (Heiss 1938; Evenhuis 1959, 1966). Rojo et al. (2003) recently reviewed the world literature on predatory syrphids and cited numerous reports of Pipizines preying on both arboreal and root colonies of aphids. Pipizine larvae have been collected from arboreal and edaphic colonies of woolly apple aphid in the United States (Walsh and Riley 1869; Metcalf 1916; Holdsworth 1970), Italy (Alfieri 1920), Canada (Evenhuis 1961) and Holland (Evenhuis 1959). Walsh and Riley (1869) reported that Pipiza radicum (Williston) was common on root colonies of woolly apple aphid in Illinois, while Metcalf (1916) identified Pipiza pisticoides (Loew) from arboreal colonies in Maine. Since the original adult specimen described by Walsh and Riley is lost and voucher specimens from Metcalf’s work in Maine have not been found (F.C. Thompson, personal communication), neither of these records can be verified. However, Dr. F.C. Thompson (personal communication), USDA Systematic Entomology Laboratory, Smithsonian Institute, Washington, DC believes that both studies likely refer to H. calcarata.
In Washington State, woolly apple aphid has been increasing in importance as an annual pest of apple over the past few years (E. Beers, personal communication), whereas in the Mid-Atlantic region it is detectable annually, but usually at densities that do not cause economic damage to the arboreal parts of trees or that require intervention. However, for unknown reasons, populations in eastern orchards occasionally reach damaging levels on a large geographical scale, as occurred in 2000, causing premature defoliation and reducing or weakening fruit-bearing wood. Heringia calcarata and other species of hover fly may play an important role in regulating woolly apple aphid populations early in the growing season and the sporadic outbreaks of the pest in Mid-Atlantic apple orchards may be associated with conditions that occasionally prevent adequate suppression of populations by this predator guild. However, much of the basic information necessary to characterize and quantify the contributions of aphidophagous hover flies to the biological control of woolly apple aphid is lacking. This paper describes aspects of the biology and life history of H. calcarata and reports measurements of the seasonal and relative abundance of H. calcarata, E. americanus and S. rectus in Virginia.
Materials and methods
Insects
Woolly apple aphid colonies for rearing H. calcarata were maintained on 1- to 3-year-old apple trees of various cultivars and rootstocks grown in 5 gal plastic pots in a greenhouse or under shaded conditions in screened field cages (1.83 m wide × 1.83 m tall × 3.66 m long) at the Alson H. Smith, Jr. Agricultural Research and Extension Center (AHS-AREC) near Winchester, VA. H. calcarata eggs were collected from arboreal woolly apple aphid colonies on mature apple trees and on young, potted trees placed in orchards. Eggs were identified to species according to pronounced differences in the sculpturing of the exochorion (Short and Bergh 2005).
Developmental duration
A cohort of H. calcarata eggs was obtained from a female fly captured while orienting to arboreal woolly apple aphid colonies in the field. The female was placed in a 1.4 l plastic cage with screened top and a section of apple shoot infested with colonies of the aphid. The cage was held in a controlled environment chamber (Percival Scientific, Inc., Perry, IA) set at 25 ± 1°C and a 15:9 L:D photoperiod. At 2-h intervals during the following 12 h, eggs laid near the colonies were removed using a fine-tipped brush and placed individually in plastic, covered Petri dishes (5 cm diameter × 0.8 cm deep) with tight-locking lids. The dishes were held in the controlled environment chamber and eggs were observed for larval eclosion at 12-h intervals.
A total of 23 neonate (<12-h-old) H. calcarata larvae that had emerged from eggs collected over 2 d from arboreal woolly apple aphid colonies were placed individually in small Petri dishes with sections of apple shoot containing a woolly apple aphid colony. Dishes were held in a controlled environment chamber under the conditions reported above and larval survivorship was assessed at 24-h intervals by gently prodding each insect with a fine-tipped brush. Aphidophagous syrphid larvae void the greasy, black contents of their gut upon cessation of feeding and new sections of apple shoot with woolly apple aphid colonies were provided to each larva daily until gut voidance or death. Upon cessation of feeding, larvae were transferred to 30 ml plastic cups containing damp tissue paper, in which they pupated. Adult emergence was recorded at 24-h intervals and gender determinations were based on adults possessing holoptic or dichoptic eyes.
Adult longevity
Fifteen male and 15 female H. calcarata were reared to maturity on woolly apple aphid. The flies (<12-h-old) were placed individually in cages consisting of a cylindrical, clear plastic tube (16 cm high × 7.5 cm diameter) with screened top that was placed in a flowerpot (10.2 cm diameter) three-fourths full of damp sand and provisioned with a source of sugar water, bee pollen, and a section of apple shoot as a perch. Pollen and sugar water were replaced periodically. The caged flies were held in an environmental chamber at 25°C and a 14:10 L:D photoperiod and were monitored daily until all had died. The longevity of males and females was compared using the two-sample t-test at α = 0.05.
Seasonal and relative abundance of unhatched hover fly eggs
From 2003 to 2005, 1- to 3-year-old potted apple trees of various cultivars, infested with arboreal colonies of woolly apple aphid, were used to examine the seasonal patterns of abundance of unhatched eggs of H. calcarata, E. americanus and S. rectus. Beginning on 16 April, 31 March and 5 April in 2003, 2004 and 2005, respectively, two sentinel trees with ≥5 medium to large aphid colonies on the branches and shoots were transported from the greenhouse or field cages to a 0.53 ha block of ≈20-year-old ‘Rome Beauty’ trees at the AHS-AREC. In 2003, twenty 3-tree plots within the orchard (≈40% of trees) were included in an insecticide evaluation study involving the application of experimental or registered materials at about 14-d intervals from mid-April to late-August. The remaining trees in the orchard were not treated with insecticides in 2003 and the sentinel trees were deployed in those portions of the orchard that were not part of the insecticide test. No insecticides were used in 2004. In 2005, sixteen 3-tree plots within the orchard were included in a season-long insecticide evaluation study. The trees to which the treatments were applied were located at either end of the orchard, and the sentinel trees were deployed in the untreated, middle portion of the block. In all years, a routine maintenance program for disease control was used in the orchard. The potted sentinel trees were positioned next to the trunk of mature trees, so that their branches were within the canopy of, but not in contact with, the larger trees. Sentinel trees were placed next to different trees each week and were not used more than once. Within each year, the trees were deployed during the same 48-h interval each week until about mid-October. This sampling duration was based on the developmental period of H. calcarata eggs and ensured that all eggs deposited during the exposure period were unhatched upon collection of the colonies. At the end of each 48-h period, five short sections of branch or shoot, each with one woolly apple aphid colony, were pruned from each tree. Most of the colonies collected were located on different branches and were distributed throughout the tree. A stereomicroscope, at a magnification of 15×, was used to count the number of unhatched eggs of H. calcarata, E. americanus and S. rectus in each colony. Eggs were identified to species according to Short and Bergh (2005). Periodically, random samples of colonies collected from sentinel trees held in the greenhouse and field cages were examined for hatched and unhatched hover fly eggs to confirm that they had not become contaminated prior to deploying the trees.
Results
Description of life stages
Eggs of H. calcarata, E. americanus, and S. rectus were described in Short and Bergh (2005). Briefly, the dorsal surface of eggs of H. calcarata possessed parallel, longitudinal ribs, those of E. americanus possessed short, longitudinal ribs, and eggs of S. rectus were covered with tubular outgrowths. E. americanus and S. rectus eggs were larger than those of H. calcarata. H. calcarata larvae were flattened dorsoventrally and changed in color from yellow/black in the first and second instars to a pinkish-gray in the third instar. The integument of first and second instars was transparent, while that of the third instar was not. Neonate larvae were 1.0 ± 0.2 SE mm in length (n = 5) and increased in size to 6.5 ± 0.07 SE mm (n = 5) by the third instar. Puparia were 5.0 ± 0.02 SE mm long × 2.0 ± 0.03 SE mm wide (n = 5), light gray, teardrop shaped, becoming mottled prior to adult emergence. Adult H. calcarata are shiny black, 7.0 ± 0.05 SE mm (n = 5) long, with a wingspan of 12.0 ± 0.03 SE mm. Detailed morphological descriptions of adult H. calcarata by F.C. Thompson will be reported elsewhere and are based on voucher specimens deposited with the USDA Systematic Entomology Laboratory, Washington, DC.
Developmental duration and adult longevity
The female fly captured while orienting to arboreal woolly apple aphid colonies laid 24 eggs before dying within the first 24 h of captivity. All of the eggs hatched in 3.0 ± 0.08 SE d. The duration of the larval feeding period, based on when their gut contents were voided, was 7.63 ± 0.20 SE d (n = 16). The total developmental duration of larvae, including the period between feeding cessation and pupation, was 8.22 ± 0.16 SE d (n = 23), and the pupal developmental period was 8.74 ± 0.14 SE d (n = 23). At constant 25°C, H. calcarata completed a generation (egg to adult) in 19.3 ± 0.74 SE d. Under the conditions used, the longevity of virgin, adult female H. calcarata (27.8 ± 2.3 SE d, n = 15) was significantly greater than of males (19.7 ± 2.6 SE d, n = 15) (t = 2.36, df = 28, P = 0.025).
Seasonal and relative abundance of unhatched hover fly eggs
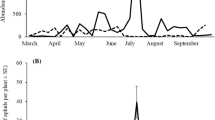
The number of unhatched hover fly eggs collected weekly from colonies on sentinel trees showed seasonal differences among the three species that were quite consistent among the 3 years of sampling (Fig. 1). In all years, E. americanus was the first species recorded. Deployment of the sentinel trees began later in 2003 than in the other years, and a large peak of unhatched E. americanus eggs was recorded during the first 48-h sample interval. In all years, a pronounced peak of E. americanus occurred within the first 6 weeks of sampling (16 April–12 May), followed by a fairly rapid decline in egg abundance and a prolonged period during which it was absent or present in very low numbers. E. americanus abundance showed a second, minor peak between late-August and late-September. First records of unhatched H. calcarata eggs occurred between 19 April and 14 May. Although the weekly abundance of H. calcarata was somewhat variable both within and among years, its eggs were present for most of each growing season. In 2003, a relatively large peak occurred over several weeks in June, followed by a somewhat smaller peak in August, with smaller numbers recorded at other times. Numerous smaller peaks were recorded during the 2004 and 2005 seasons and the latest record of unhatched H. calcarata eggs was on 1 October, 2004. Unhatched eggs of S. rectus were least common in all 3 years. In 2003, its eggs were recorded in only two sample intervals in August, while in 2004 and 2005 S. rectus eggs were found at various times during much of the season and were most abundant and frequently recorded from late-August to September.
Seasonal records of the mean number of unhatched eggs of Heringia calcarata, Eupeodes americanus and Syrphus rectus deposited during 48-h exposure intervals in arboreal woolly apple aphid colonies on potted apple trees deployed in an apple orchard in Virginia. Week 1 corresponds with the earliest date on which sampling was initiated (31 March 2004) during the 3-year study
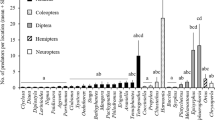
During the 48-h intervals when the infested trees were exposed, hover flies appeared to be quite efficient at locating aphid colonies. In 2003, 2004, and 2005, one or more unhatched eggs were recorded from 46.1, 41.8 and 37.1% of colonies, respectively (Fig. 2). A single egg was found in the majority of colonies with eggs (Fig. 2), although two or more eggs per colony were not uncommon, and in 2004, 17 unhatched E. americanus eggs were recorded from one colony. In 2003 and 2004, more unhatched eggs of H. calcarata than of E. americanus were found, while the total numbers of eggs of both species were nearly identical in 2005 (Fig. 3A and B). Across all years, both species appeared equally likely to deposit multiple eggs in a single colony (Fig. 3A and B). Eggs of S. rectus were far less numerous than of the other species (Fig. 3C). The majority of colonies with eggs contained one or more eggs of a single species, although it was not uncommon to find eggs of two species (Fig. 4), particularly early and late in the season (Fig. 1). Instances of unhatched eggs of all three species in an individual colony did occur, but were very rare (Fig. 4).
Discussion
These data reveal new information about the biology and ecology of H. calcarata and other members of an important guild of aphidophagous hover fly predators in eastern apple orchards and should enhance further studies of their relative roles and effectiveness at suppressing outbreaks of woolly apple aphid.
The 3-d developmental period of H. calcarata eggs at 25°C was similar, although apparently somewhat longer, than of two other Pipizine syrphids (Rojo and Marcos-García 1997). At approximately 20°C, both H. heringii (Zetterstedt) and P. festiva Meigen remained in the egg stage for 2–3 d. Conversely, the period of H. calcarata larval and pupal development at 25°C was shorter than reported for other Pipizine syrphids. At 20°C, the developmental period for H. heringii and P. festiva larvae was 15–20 d, and the pupal developmental period was 13–15 d and 15–20 d, respectively. Similarly, Heeger (1858) reported that the developmental periods of P. vitripennis Meigen and P. varipes Meigen were more than 15 d, although temperature was not reported. Differences in the duration of the developmental periods may be affected by fly species, climate (Bhatia 1939; Lakhanpal and Raj 1998; Soleyman-Nezhadiyan and Laughlin 1998; Michaud and Belliure 2001), aphid host (Sadeghi and Gilbert 2000) and the quantity, mobility, and nutritive value of the prey species (Rüzička 1975; Cornelius and Barlow 1980). In combination with the seasonal abundance data showing newly deposited eggs throughout the apple growing season, the generation time (egg to adult) of H. calcarata indicates that this species is multivoltine in the Mid-Atlantic region and that completion of up to eight generations per year is possible.
Under the laboratory conditions used, the longevity of adult female H. calcarata was greater than that of males, but shorter than reported for other syrphids. Overwintered, gravid female Episyrphus balteatus (De Geer) lived for an average of 42.6 d at 18°C (Kan 1988) and Eristalis tenax (L.) adults survived for up to four months under ambient conditions (Gladis 1994). To date, we have devoted only minimal effort to maintaining and mating H. calcarata in captivity (Short 2003) and do not yet fully understand the optimal conditions required for maintaining this species.
Our use of the easily recognizable differences in the exochorionic sculpturing of eggs among these three species (Short and Bergh 2005) has proven invaluable to field studies of this predator guild. Our previous attempts (Short 2003) to monitor the presence and abundance of adult hover flies employed yellow water pan traps, sticky traps and adult emergence traps, but yielded inconsistent results and low captures. Monitoring the abundance of unhatched eggs has provided a reliable and repeatable measure of adult activity under natural conditions, although our use of a fixed, 48-h sample interval likely provided a conservative indication of the potential impact of predation by these flies on woolly apple aphid populations. Given that hover flies are most active during sunny, warm weather (Metcalf 1916; Maier and Waldbauer 1979) and that the sentinel trees were deployed during the same 48-h period each week, without regard to forecasted weather conditions, many of the weekly fluctuations recorded during each season (Fig. 1) may have been due to the effects of inclement weather on the foraging of flies and each season contained weeks when no eggs were deposited. Furthermore, despite the use of a relatively brief and fixed sample interval, flies found and oviposited in a fairly large percentage of all colonies evaluated and it appears probable that exposing sentinel trees to the predators for longer periods and during favorable weather would reveal a much larger impact than what these data show.
It must be noted that the measurements of hover fly activity were collected from an orchard that was either unsprayed (2004) or only partially sprayed with pesticides (2003 and 2005) and that the sentinel trees were much more heavily infested with aphid colonies than the mature trees in the orchard. However, Short and Bergh (2004) reported that unhatched eggs of all three hover fly species were collected from colonies of rosy apple aphid, spirea aphid and/or woolly apple aphid in a commercial apple orchard in which pesticides were applied routinely. With respect to the generalist predators, E. americanus and S. rectus, it is likely that these highly vagile insects move into orchards from surrounding habitats in response to the availability of aphid pests of apple.
Although the use of alternate prey by H. calcarata remains unknown, this species may reside primarily within apple orchards. Given that it can develop on both arboreal and edaphic colonies of woolly apple aphid (discussed below), it may be that populations of H. calcarata are at least somewhat resilient to foliar pesticide applications. Further, since most woolly apple aphid colonies are found in the interior of the canopy and are covered by a layer of hydrophobic, waxy filaments, hover fly larvae feeding within these colonies may be afforded some protection from exposure to pesticide sprays. Indeed, woolly apple aphid is considered a difficult pest to control with contact pesticides. Finally, it appears that the density of arboreal woolly apple aphid colonies is not an impediment to their location and exploitation by H. calcarata. We have commonly observed small, isolated woolly apple aphid colonies in commercial apple orchards that contain eggs and/or larvae of this predator.
Given that E. americanus, H. calcarata and S. rectus are spatially and temporally sympatric at various points in the growing season in Virginia orchards and that it was not uncommon to find unhatched eggs of more than one species per colony, questions about the extent and effects of intraguild predation arise. The generalist feeding habits of E. americanus and S. rectus contrast with the prey-specific feeding of H. calcarata (Short and Bergh 2004). Differences in their behavior may influence the nature and direction of their intra- and interspecific interactions, and consequently their relative contributions to woolly apple aphid suppression. Larval E. americanus and S. rectus are considerably larger than H. calcarata, and both cannibalism and interspecific aggression may be more pronounced in the generalists than the specialist species. Furthermore, larvae of generalist and specialist species may differ in their propensity to prey on aphids parasitized by A. mali and therefore could differentially influence the size and impact of the parasitoid population (Rosenheim 1998; Brodeur and Rosenheim 2000). More information on the life history and predator–prey associations of the two species of generalist hover flies is needed, especially E. americanus, given the abundance of its unhatched eggs recorded during the early part of each season.
To date, the only quantitative assessment of the impact of predation on woolly apple populations in North America was provided by Walker (1985). Predator exclusion studies, employing both cages and insecticides in an unsprayed section of an apple orchard in Washington, revealed that the actions of three predators suppressed colonies and that A. mali alone was not able to effect acceptable levels of control. Although the hover fly, Eupeodes (Metasyrphus) fumipennis (Thompson), was observed feeding in colonies late in the season, syrphid larvae were not abundant and did not contribute substantially to aphid suppression in either year of the study. Bergh (unpublished data) used exclusion cages and direct observations in Virginia and, like Walker (1985), found that A. mali alone was not effective at preventing woolly apple aphid outbreaks. However, unlike the Washington study, hover fly larvae appeared to be the dominant predators in Virginia, concurring with earlier observations by Brown and Schmitt (1990) and Bergh and Louque (2000). While earwigs have been shown to control woolly apple aphid populations in Australia (Nicholas et al. 2005) and Holland (Mueller et al. 1988), they have not been considered a significant predator of any apple pest in the Mid-Atlantic region of the United States and it is likely that the predator complex and their relative impacts vary considerably among geographic locations.
An intriguing aspect of predation by H. calcarata, and one that remains poorly understood, is its potential role as a predator of edaphic colonies of woolly apple aphid. Reports of predation by other Pipizine syrphid larvae on edaphic aphid colonies were reviewed by Rojo et al. (2003). Anecdotal evidence (Walsh and Riley 1869) and preliminary data from laboratory studies (Short, unpublished data) indicate that H. calcarata larvae can burrow down through soil to find, feed and develop on buried woolly apple aphid colonies. Recent field observations further support the existence of predation on edaphic colonies. In September 2004, we discovered that female H. calcarata were abundant in a young orchard at the AHS-AREC that showed signs of infestation by woolly apple aphid (e.g., galls on exposed roots). Prior to that finding, we had observed adult H. calcarata only rarely in the field. Between 7 and 11 October, walking surveys at hourly intervals in the orchard revealed flies commonly flying and walking near the base of trees, with peak periods of activity between 13:00 and 16:00 EST. The maximum number of flies observed during one survey interval was 39. Based on observations of fly abundance recorded between 15:00 and 15:30 over 12 d, we calculated a lower temperature threshold for activity of about 18°C. During this period, 27 flies were sub-sampled from the orchard and all were identified as female. Ten females were dissected and counts of the fully and partially developed ovarioles in each revealed an average of 38.2 (range = 22–60) per individual. Since these initial sightings, foraging H. calcarata females have been observed at various times throughout the growing season. Observations of this foraging behavior revealed that the flies spent considerable time searching relatively small areas of soil around the base of trees and would return to these areas repeatedly. Several examples of what appeared to be oviposition in the soil were observed, involving females backing into cracks in the soil surface where they remained still for several seconds and then resumed searching. In early October 2006, careful excavation of a small area where this behavior had been observed revealed the presence of four, unhatched H. calcarata eggs in the soil and an active woolly apple aphid colony approximately 2 cm below the soil surface. In combination, these observations suggest that location of edaphic colonies of woolly apple aphid by female H. calcarata may be facilitated by a kairomone.
It appears increasingly evident that aphidophagous hover flies play an important role in maintaining woolly apple aphid populations below economically damaging densities in Virginia and likely other Mid-Atlantic States. In this region, arboreal colonies of woolly apple aphid typically appear in April or May, show peak abundance in June, decline through July and then exhibit a second, smaller peak of abundance in September and October (Schoene and Underhill 1935; Brown and Schmitt 1994). Seasonal peaks of abundance of unhatched eggs of H. calcarata and E. americanus coincided with the seasonal population dynamics of woolly apple aphid, and it is likely that they have an especially large impact early in the growing season, when they are most numerous and when cooler temperatures are more favorable for the development and reproduction of woolly apple aphid than of A. mali. Brown and Schmitt (1994) showed that a greater percentage of colonies contained syrphid larvae than were parasitized by A. mali in June, and that the percentage of parasitized colonies exceeded those with syrphid larvae in July and August. However, as mentioned previously, sporadic, region-wide outbreaks of woolly apple aphid populations do occur in eastern orchards, causing significant damage to bearing and non-bearing trees, and neither the proximate nor ultimate causes underlying this phenomenon are known. While such outbreaks have occurred on a local scale in response to the use of pyrethroids (Penman and Chapman 1980) regional outbreaks cannot be explained solely by pesticide use patterns. Most commercial apple growers in the Mid-Atlantic States typically do not vary their pesticide programs significantly from year to year. Furthermore, the spray schedules and combinations of pesticides used within a given season can differ considerably among growers. Brown and Schmitt (1994) provided evidence that the use of pyrethroids in orchards in West Virginia had only a limited affect on parasitism by A. mali and predation by syrphid larvae and concluded that this natural enemy guild showed resilience to the use of a broad spectrum pesticide. Recent evidence lends further support to this contention. Conventional wisdom suggested that woolly apple aphid populations tended to reach damaging levels in the year following emergence of the 17-year periodical cicada, Magicicada spp. Explanations for this included the creation of many favorable sites for colony establishment caused by damage from cicada oviposition and disruption of biological control through the use of quick knockdown, broad-spectrum pesticides for cicada control. Following the massive emergence of Brood X of the cicada in this area in 2004, no such increase in the pest status of woolly apple aphid was observed in 2005, despite widespread use of pyrethroids and carbamates against cicada during the previous spring.
The fact that woolly apple aphid heavily infests apple orchards in some years, regardless of current or past pesticide programs, suggests that some combination of abiotic and/or biotic factors may occasionally disrupt its suppression by natural enemies early in the season, allowing populations to increase to damaging levels during the period when A. mali is not considered to be effective. Studies designed to quantify the impact of predators and to determine the factors that influence their effectiveness will provide growers with valuable insights into the importance of natural enemies and their conservation. Further, such research should improve our predictive capabilities and help to alleviate the likelihood or impact of future outbreaks of woolly apple aphid.
References
Alfieri E (1920) A probably new species of gallicolous aphid of the elm and its symbionts. Boll Lab Zool Gen Agrar R Scuola Sup Agric, Portici 14:18–32
Asante SK (1997) Natural enemies of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae): a review of the world literature. Plant Prot Quart 12:166–172
Asante SK, Danthanarayana W (1992) Development of Aphelinus mali an endoparasitoid of woolly apple aphid, Eriosoma lanigerum at different temperatures. Entomol Exp Appl 65:31–37
Baker AC (1915) The woolly apple aphis. USDA Rept. No. 101, 56 pp
Bhatia ML (1939) Biology, morphology and anatomy of aphidophagous syrphid larvae. Parasitology 31:78–129
Bergh JC, Louque RL (2000) Heringia (Neocnemodon) calcarata: A specialist predator of the woolly apple aphid? Proc 76th Cumberland-Shenandoah Fruit Workers Conf. Winchester, VA
Bodenheimer FS (1947) Studies on the physical ecology of the woolly apple aphis (Eriosoma lanigerum) and its parasite Aphelinus mali in Palestine. Bull. Rehovot. Agric. Res. Stn. No. 43, 20 pp
Brodeur J, Rosenheim JA (2000) Intraguild interactions in aphid parasitoids. Entomol Exp Appl 97:93–108
Brown MW, Schmitt JJ (1990) Growth reduction in nonbearing apple trees by woolly apple aphids (Homoptera: Aphididae) on roots. J Econ Entomol 83:1526–1530
Brown MW, Schmitt JJ (1994) Population dynamics of woolly apple aphid (Homoptera: Aphididae) in West Virginia apple orchards. Environ Entomol 23:1182–1188
Brown MW, Glenn DM, Wisniewski ME (1991) Functional and anatomical disruption of apple roots by the woolly apple aphid (Homoptera: Aphididae). J Econ Entomol 84:1823–1826
Brown MW, Schmitt JJ, Range S, Hogmire HW (1995) Yield reduction in apple by edaphic woolly apple aphid (Homoptera: Aphididae) populations. J Econ Entomol 88:127–133
Cohen H, Horowitz AR, Nestel D, Rosen D (1996) Susceptibility of the woolly apple aphid parasitoid, Aphelinus mali (Hym.: Aphelinidae), to common pesticides used in apple orchards in Israel. Entomophaga 41:225–233
Cornelius M, Barlow CA (1980) Effect of aphid consumption by larvae on development and reproductive efficiency of a flower fly, Syrphus corollae (Diptera: Syrphidae). Can Entomol 112:989–992
DeBach P (1964) Biological control of insect pests and weeds. Chapman Hall, London, pp 844
Dumbleton LJ, Jeffreys FJ (1938) The control of the woolly aphis by Aphelinus mali. N Z J Sci Technol 20:183–192
Evenhuis HH (1959) Cnemodon vitripennis (Meig.) as a predator of the woolly apple aphid, Eriosoma lanigerum (Hausm.). Entomol Ber 19:238–240
Evenhuis HH (1961) Some notes on the Dipterous enemies of aphids harmful for apple growing in Nova Scotia. Can Entomol 93:1020–1021
Evenhuis HH (1966) Syrphid predators of apple aphids and their parasites, pp. 191–193. Proc. Symp. on Ecology of Aphidophagous Insects, Liblice near Prague, 1965
Gladis T (1994) Establishment and utilization of a mass rearing of Eristalis tenax (Diptera, Syrphidae) in the Gatersleben genebank. Insecta 1:287–294
Gruys P (1982) Hits and misses. The ecological approach to pest control in orchards. Ent Exp Appl 31:70–87
Hagen KS, van den Bosch R (1968) Impact of pathogens, parasites and predators on aphids. Annu Rev Entomol 13:325–338
Heeger VE (1858) Neue metamorphosen einiger dipteren. Sitzungsber Akad Wiss Wien Nat Kl 31:295–309
Heiss EM (1938) A classification of the larvae and puparia of the Syrphidae of Illinois exclusive of aquatic forms. Ill Biol Monogr 4:1–142
Holdsworth RP (1970) Aphids and aphid enemies: Effect of integrated control in an Ohio apple orchard. J Econ Entomol 63:530–535
Howard LO (1929) Aphelinus mali and its travels. Ann Entomol Soc Am 22:341–368
Kan E (1988) Assessment of aphid colonies by hoverflies. II Pea aphids and 3 syrphid species; Betasyrphus serarius (Wiedemann), Metasyrphus frequens Matsumura and Syrphus vitripennis (Meigen) (Diptera: Syrphidae). J Ethol 6:135–142
Lakhanpal GC, Raj D (1998) Predation potential of coccinellid and syrphid on important aphid species infesting rapeseed in Himachal Pradesh. J Entomol Res 2:181–190
Maier CT, Waldbauer GP (1979) Diurnal activity patterns of flower flies (Diptera: Syrphidae) in an Illinois sand area. Ann Entomol Soc Am 72:237–245
Metcalf CL (1916) Syrphidae of Maine. ME Agric Exp Stn Bull 253:193–264
Michaud JP, Belliure B (2001) Impact of syrphid predation on production of migrants in colonies of the brown citrus aphid, Toxoptera citricida (Homoptera: Aphididae). Biol Control 1:91–95
Mols PJM, Boers JM (2001) Comparison of a Canadian and a Dutch strain of the parasitoid Aphelinus mali (Hald.) (Hym., Aphelinidae) for control of woolly apple aphid Eriosoma lanigerum (Haussmann) (Hom., Aphididae) in the Netherlands: a simulation approach. J Appl Entomol 125:255–262
Mueller IE, Blommers LHM, Mols PJM (1988) Earwig (Forficula auricularis) predation on the woolly apple aphid, Eriosoma lanigerum. Entomol Exp Appl 47:145–152
Nicholas AH, Spooner-Hart RN, Vickers RA (2005) Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program. BioControl 50:271–291
Penman DR, Chapman RB (1980) Woolly apple aphid outbreak following use of fenvalerate in apples in Canterbury, New Zealand. J Econ Entomol 73:49–51
Rojo S, Marcos-García MA (1997) Syrphid predators (Dipt.: Syrphidae) of gall forming aphids (Hom.: Aphididae) in Mediterranean areas: Implications for biological control of fruit trees pests. Entomophaga 42:269–276
Rojo S, Gilbert FS, Marcos-Garcia MA, Nieto JM, Mier MP (2003) A world review of predatory hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. CIBIO ediciones, Alicante, Spain, 319 pp
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rüzička Z (1975) The effects of various aphids as larval prey on the development of Metasyrphus corollae (Dipt.: Syrphidae). Entomophaga 20:393–402
Sadeghi H, Gilbert F (2000) Aphid suitability and its relationship to oviposition preference in predatory hoverflies. J Animal Ecol 69:771–784
Schoene WJ, Underhill GW (1935) Life history and migration of the apple woolly aphis. Va. Exp. Stn. Tech. Bull. No. 57. 31 pp
Short BD (2003) Inaugural studies of the life history and predator/prey associations of Heringia calcarata (Loew) (Diptera: Syrphidae), a specialist predator of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Homoptera: Eriosomatidae). M.Sc. thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA
Short BD, Bergh JC (2004) Feeding and egg distribution studies of Heringia calcarata (Loew) (Diptera: Syrphidae), a specialized predator of the woolly apple aphid (Homoptera: Eriosomatidae) in Virginia apple orchards. J Econ Entomol 97:813–819
Short BD, Bergh JC (2005) Separation of three common hover fly predators of woolly apple aphid based on the exochorionic sculpturing of eggs. Can Entomol 137:67–70
Soleyman-Nezhadiyan E, Laughlin R (1998) Voracity of larvae, rate of development in eggs, larvae and pupae, and flight seasons of adults of the hoverflies Melangyna viridiceps Macquart and Symosyrphus grandicornis Macquart (Diptera: Syrphidae). Aust J Entomol 37:243–248
Vockeroth JR (1992) The flower flies of the subfamily Syrphinae of Canada, Alaska and Greenland. Diptera: Syrphidae. The insects and arachnids of Canada, Part 18. Agriculture Canada publication 1867, 456 pp
Walker JTS (1985) The influence of temperature and natural enemies on population development of woolly apple aphid, Eriosoma lanigerum (Hausmann). Ph.D. dissertation, Washington State University, Pullman, WA, 88 pp
Walsh BD, Riley CV (1869) The apple-root plant-louse. Am Entomol 5:81–84
Weber DC, Brown MW (1988) Impact on woolly apple aphid (Homoptera: Aphididae) on the growth of potted apple trees. J Econ Entomol 81:1170–1177
Wirth WW, Sedman YS, Weems Jr HV (1965) Family Syrphidae. In: Stone A, Sabrosky C, Wirth W, Foote R, Coulson J (eds) A Catalog of the Diptera of America North of Mexico. United States Department of Agriculture Handbook Number, 276 pp. 557–625
Yothers MA (1953) An annotated bibliography on Aphelinus mali (Hald.) a parasite of the woolly apple aphid 1851–1950. USDA
Acknowledgments
We thank B. Mackintosh for donating sentinel apple trees, C. Thompson and M. Brown for valuable insights and consultation, J. Engleman for assistance with data collection, A. Zhang for photographs of H. calcarata and the Virginia Apple Research Program for partial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergh, J.C., Short, B.D. Ecological and life-history notes on syrphid predators of woolly apple aphid in Virginia, with emphasis on Heringia calcarata . BioControl 53, 773–786 (2008). https://doi.org/10.1007/s10526-007-9114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9114-0