Abstract
A series of experiments in Virginia, USA apple orchards evaluated the temporal effects of natural enemies on Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae) colonies on potted and mature apple trees. Exclusion cage studies using potted trees resulted in increasing colony numbers on fully caged trees and declining colony numbers or colony extinction on trees exposed to natural enemies. Closer examination of the fate of individual colonies on potted trees produced a similar result and revealed that syrphids oviposited in colonies within two days of tree deployment. Heringia calcarata (Loew) (Diptera: Syrphidae) was the predominant predator and had a greater and more rapid impact on colonies than did the parasitoid Aphelinus mali (Haldeman) (Hymenoptera: Aphelinidae). The fate of cohorts of naturally-occurring E. lanigerum colonies on mature trees was examined over two seasons and confirmed that H. calcarata and A. mali were most strongly associated with colony demise.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Woolly apple aphid (WAA), Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae), is a cosmopolitan pest of apple trees, Malus domestica Borkh. (Baker 1915) and is broadly distributed in the USA, where it colonizes apple roots and branches. In the Mid-Atlantic States, root colonies are present year-round (Brown 1986) while aerial colonies are typically most abundant in June and in late summer or early fall (Brown and Schmitt 1994), although significant outbreaks have been infrequent and unpredictable and thought to be due mainly to disruption of biological control. Recently, many apple growers in the Mid-Atlantic region have experienced late-season WAA outbreaks following their use of broad-spectrum insecticides against the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Leskey et al. 2012).
If undisturbed, a guild of natural enemies that includes the parasitoid Aphelinus mali (Haldeman) (Hymenoptera: Aphelinidae) and several species of Syrphidae (Bergh and Short 2008; Brown and Schmitt 1994; Short and Bergh 2004) is thought to effectively suppress WAA in Mid-Atlantic orchards. A. mali has long been considered the main natural enemy of WAA (Asante 1997), likely due in part to its successful introduction to many apple-producing countries in the 1920’s and 1930’s (Howard 1929; Yothers 1953). However, despite many examples of predator associations with WAA colonies (Asante 1997), their contributions have received much less attention until relatively recently.
In Virginia, Short and Bergh (2004) found eggs of three syrphid species associated with WAA colonies, which were identified based on differences in the exochorionic sculpturing reported by Short and Bergh (2005). Larvae of Eupeodes americanus (Weidemann) and Syrphus rectus Osten Sacken are generalist predators, but feeding and egg distribution studies suggested that Heringia calcarata (Loew) is a specialized predator of WAA in orchards (Short and Bergh 2004). Gresham et al. (2013) showed that adult female H. calcarata were present in apple orchards from late April until mid-October, with peaks of abundance in mid-June and from mid-September through early October.
In West Virginia, Brown and Schmitt (1994) reported that unidentified syrphid larvae were associated with WAA colonies from late June through early August 1989, and that they were the predominant natural enemy early in the season. Aphids parasitized by A. mali were present from late June through mid-September, and peak parasitization was in late July. Gontijo et al. (2012) reported that syrphid larvae were the most abundant predators in WAA colonies in Washington, USA but that lacewings and lady beetles also occurred in substantial numbers.
Although recent studies have shown that predators contribute importantly to biological control of WAA (Nicholas et al. 2005; Gontijo et al. 2012, 2015), its natural enemy guild may vary considerably within and among apple-producing countries. Since the impact of predators on WAA in Virginia has not been documented specifically, we report field studies examining the contributions of predators and A. mali to WAA biocontrol in apple Virginia orchards.
Materials and methods
Impact of predator exclusion on WAA colonies
Temporal effects of predator exclusion on arboreal WAA colonies were evaluated via cage experiments in April–May and June–July 2004. ‘Idared and ‘Nittany’ trees (1- to 2-year-old) in 19 l plastic buckets containing soil and peat moss were held in a greenhouse and inoculated with WAA by attaching a ~3 cm long section of infested shoot from other potted trees to the lower trunk of each. Cages constructed of wood frames (1.8 m tall × 0.9 m wide) and amber Lumite® screen (32 × 32 mesh) (BioQuip Products, Inc., Rancho Dominquez, CA, USA) had either fully enclosed top and sides (full cage) or screen on the top and upper and lower thirds of the sides (partial cage) and an open base.
On 16 April 2004, ‘Idared’ trees were randomly assigned to three treatments: (1) fully exposed (i.e. not caged), (2) full cage, and (3) partial cage (n = 5 per treatment) and deployed in three rows of a 0.53 ha block of mature ‘Law Rome’ trees at Virginia Tech’s Alson H. Smith, Jr. Agricultural Research and Extension Center (AHS-AREC), Winchester, VA, according to a latin square design. No insecticides were applied during the studies but a routine disease management program was used. Cage were placed between trees in each orchard row, separated by 30.5 m within rows and 7.3 m between rows, and secured with rope to the tree on either side. Earth was mounded around the base and the potted trees sat on the soil surface. WAA colonies on each tree were counted at deployment and then weekly for six weeks. The same design was used with ‘Nittany’ trees deployed from 14 June to 26 July 2004. Colony numbers per tree were intentionally allowed to build to higher numbers at the outset of the late spring than the early spring experiment.
Natural enemies associated with WAA colonies
Since the previous experiments assessed the combined effects of all potential WAA natural enemies, the fate of individual colonies and the specific natural enemies associated with them was evaluated. ‘Gala’ trees planted in 19 l pots in March 2008 were held in a greenhouse and inoculated with WAA in mid-April. On 25 May, the trees supported numerous medium-sized colonies and were randomly divided between two treatments: (1) exposed trees, and (2) caged trees (n = 5 per treatment). Pots were placed in holes in separate areas in a semi-shaded field cage (1.8 m high × 1.8 m wide × 3.6 m long) (BioQuip Products, Inc., Rancho Dominguez, CA, USA) at the AHS-AREC and in holes beneath the canopy of five randomly selected mature ‘Rome’ trees, separated by 25–50 m, in a nearby commercial orchard. The pot rim was flush with the soil surface after the space around it was back-filled. Insecticides used in the orchard just before and during the study included separate alternate-row-middle applications of azinphosmethyl (24 May) and lambda-cyhalothrin (29 May).
Five colonies per tree were tagged and numbered. At two-day intervals for 14 days, each colony was inspected for natural enemies and their impact. WAA colonies are densely packed, enveloped by “wool”, and contain aphids of all stages, precluding accurate determination of aphid numbers by non-destructive means. Thus, each colony was rated for its status relative to that at the outset, when all colonies were enveloped by wool, actively producing wool, honeydew, and crawlers, and when black, parasitized aphid “mummies” were not apparent. Colonies were rated using the scale: 0 (extinct), 1 (a few live aphids), 2 (~25 % of original size), 3 (~50 % of original size), 4 (signs of disturbance, but mostly intact), and 5 (pristine). At each two-day interval, the status of each colony was archived using macro photography. Syrphid eggs found within ~2.5 cm of each colony were counted using a 16× lens. In the field, we did not determine whether these eggs had hatched or identify them to species. Hover fly larvae and other predators on or near each colony were counted and the presence of adult A. mali was noted. After the final evaluation on 8 June, a ~5 cm section of branch containing each colony was pruned and inspected under a microscope. Hatched and unhatched syrphid eggs were identified to species following Short and Bergh (2005). Colonies were then placed individually in plastic cups with a ventilated lid and held at 25 °C and a 14:10 light:dark regime for counts of adult A. mali that emerged over ~three weeks.
Natural enemies associated with WAA colonies on mature trees
WAA colony density in the orchard in which potted trees were deployed in the previous experiment was not quantified, but casual observations indicated much higher densities on the potted trees. To account for the possibility that sensory cues from colonies on the potted trees may have created an artifact by concentrating natural enemy activity, temporal changes in naturally-occurring colonies on mature trees and the natural enemies associated with them were recorded in 2009 and 2010.
In 2009, 235 colonies on ‘Yorking’ and ‘Granny Smith’ apple trees in six adjacent rows in a commercial orchard were tagged and numbered between 29 May and 2 June. Colonies were scarce and mainly on larger branches and pruning cuts in the canopy interior. Medium-sized colonies that showed no indication of disturbance by natural enemies and that could be pruned readily were selected and distributed among 167 trees (1.4 ± 0.06 SE colonies per tree). Chlorantraniliprole and fenpyroximate were the only insecticides applied during the study. Starting on 3 June, 27–30 colonies were randomly selected weekly for seven weeks. Each colony was removed with surrounding tissue using a pruner or knife and placed in a lidded jar. Using a microscope at ~15× magnification, counts from each colony were: (1) first instars (“crawlers”), (2) older nymphs and adults (pooled), (3) black aphid “mummies” from which A. mali had emerged, (4) black aphid mummies containing a developing A. mali, (5) hatched and unhatched syrphid eggs by species, (6) syrphid larvae, and (7) other natural enemies. H. calcarata larvae were identified to species (Bergh and Short 2008) while larval E. americanus and S. rectus were pooled.
In spring 2010, colonies were virtually absent in the orchard used in 2009 and in nearby commercial orchards, so two rows of mature trees with 38 heritage apple cultivars lining an access road to the AHS-AREC were selected. The trees were sprayed with acetamiprid, spinetoram plus thiacloprid, and phosmet plus imidacloprid prior to the study, but no insecticides were applied during the experiment. On 7 and 8 June, 152 medium-sized WAA colonies showing no sign of disturbance by natural enemies were tagged and numbered. Colony abundance among the cultivars varied substantially but was generally higher than in the orchard used in 2009 (6.2 ± 0.5 SE colonies tagged per tree). Beginning on 9 June, 25 colonies were sampled and evaluated weekly for six weeks, following the protocols used in 2009.
Statistical analysis
For the exclusion cage studies, treatment effects on the number of WAA colonies through time were compared using a Poisson mixed model. The early and late spring experiments were assigned separate overdispersion and lag−1 autoregressive parameters to account for heterogeneous variance and correlated observations between repeated measurements.
Evaluation of colonies on potted trees deployed in an orchard and in a cage revealed that those on caged trees showed no visually apparent effects of natural enemies during the study and were always assigned a rating of five. Thus, analyses of temporal changes in colony ratings and of the species of hatched syrphid eggs recorded at the end of the study focused only on the trees deployed in an orchard. Changes in colony ratings from those trees were analyzed using a multinomial mixed model with a cumulative probit link. Counts of hatched eggs of E. americanus and S. rectus (pooled) and H. calcarata on colonies from the pruned shoots were compared using a Poisson mixed model, with separate overdispersion parameters for the two species groups. Counts of adult A. mali from colonies pruned from caged and exposed trees were also compared with a Poisson mixed model, with separate overdispersion parameters for the two groups.
Analysis of results from the colony fate study on mature orchard trees examined the relationship between two response variables (live WAA crawlers, live WAA nymphs plus adults) and six predictors (parasitized and previously parasitized WAA, hatched and unhatched H. calcarata eggs, H. calcarata larvae, sample week). Due to their low abundance, eggs and larvae of other syrphid species were not included in the model. Data from 2009 and 2010 were combined so a fixed year effect was also included. Due to the large number of zeros in the dataset, negative binomial (NB) and zero-inflated Poisson (ZIP) models were also considered. The best distribution to model the count data was determined by the Vuong test (Vuong 1989) with a Schwarz adjustment using the %VUONG macro program of SAS (SAS Institute 2014b).
Models were fit using PROC GLIMMIX in SAS 9.4 (SAS Institute 2014a) with random tree and colony effects when appropriate. Analyses involving measurements through time were adequately modeled using either a linear or quadratic time effect. Main effects and interactions between all fixed effects were tested using Type III tests with Kenward-Roger degrees-of-freedom. All statistical comparisons were considered significant at P < 0.05. Vuong test statistics, denoted as Z Poisson and Z ZIP , for comparing the NB model to the Poisson and ZIP models, respectively, are based on asymptotic normality of the likelihood ratio test statistic. Significant effects were further investigated with Tukey-adjusted pairwise comparison tests.
Results
Impact of predator exclusion on WAA colonies
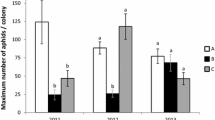
In both the early and late spring experiments, WAA colony numbers declined in the partial cage and fully exposed treatments but increased steadily in full cages (Fig. 1a, b). In the early spring experiment, colonies in the partial cage and fully exposed treatments were essentially extinct within two weeks. In the late spring study, colonies on fully exposed trees declined steadily whereas in partial cages they showed an initial increase and then declined steadily, but neither treatment resulted in their extinction. Both the time × experiment × treatment (F 2,61.94 = 14.15, P < 0.001) and time2 × experiment × treatment interactions (F 2,69.55 = 9.41, P < 0.001) were significant. The late spring study showed greater overdispersion across all treatments than the early experiment. Autocorrelation was modest, being 0.336 ± 0.137 and 0.536 ± 0.110 for the early and late spring studies, respectively. Due to the significant interactions, treatment effects were compared at day 0 and at weeks 3 and 6 for both the early and late studies. On day 0, no significant differences were found. At week 3, the early spring study revealed significant differences between the full cage and the fully exposed (t22.96 = 6.89, P- adj < 0.0001) and partial cage treatments (t23.49 = 6.67, P- adj < 0.0001), while in the late spring only the fully exposed and full cage treatments differed significantly (t 27.09 = 3.50, P- adj < 0.01). At week 6 in both experiments, the full cage treatments differed significantly from the fully exposed (early spring: t 45.89 = 6.38, P- adj < 0.0001; late spring: t 34.97 = 4.61, P- adj < 0.001) and partially caged trees (early spring: t 51.81 = 5.99, P- adj < 0.0001; late spring: t 34.87 = 4.74, P- adj < 0.001). Further comparisons between the fully exposed and partial cage treatments across all weeks for both experiments showed no significant differences.
Exclusion cage effects on woolly apple aphid colony numbers on potted apple trees in an experimental orchard in Virginia, USA in a early spring (16 April through 21 May) and b late spring (14 June through 26 July), 2004. Asterisks indicate significant differences (P < 0.05) among treatments within sample dates
Natural enemies associated with WAA colonies
Based on an initial rating of 5 for pristine colonies, those on the caged trees showed no signs of disturbance during the study. Indeed, many of them increased in size during the 14-day experiment. Colonies on potted trees in the orchard first showed signs of disturbance on day 8 and ratings of their status declined progressively thereafter (Fig. 2a–e). The multinomial model revealed a significant linear time effect (F 1, 169 = 95.77, P < 0.0001). Prior to day 8 the estimated probability of a colony rating of 5 never fell below 95.5 % with standard errors ≤2.93 %. On day 8 this probability dropped to 75.6 % ± 8.17 % and continued to drop until day 14, when it reached 1.03 % ± 0.91 %. By comparison, on day 14 the estimated cumulative probability of a colony rating of 1 or 0 was 84.4 % ± 6.83 %.
Temporal changes in ratings of woolly apple aphid colonies on potted apple trees deployed between 25 May and 8 June, 2008 in a commercial orchard in Virginia, USA and the number of syrphid eggs associated with them. Ratings of colony appearance or status were: 0 = extinct, 1 = a few live aphids, 2 = ~25 % of original size, 3 = ~50 % of original size, 4 = signs of disturbance, but mostly intact, and 5 = pristine, with no signs of disturbance by natural enemies
Adult A. mali were observed on colonies on trees in the orchard and cage on each sample date, possibly due in part to their exposure to A. mali from other trees in the greenhouse prior to deployment. Syrphid eggs also were recorded near colonies on all trees in the orchard on each date (Fig. 2a–e), but never from colonies on caged trees. Syrphid larvae were first observed on trees in the orchard on day 6. In total, 15 H. calcarata larvae and two E. americanus or S. rectus larva were recorded. No other predators or evidence of their presence (e.g. eggs, rapid colony disruption/decimation) was observed on colonies on trees in the orchard or cage.
Examination of colonies pruned from the trees on day 14 revealed H. calcarata eggs associated with 88 % of colonies on trees in the orchard, most of which had hatched prior to the evaluation (Table 1). Hatched eggs of E. americanus or S. rectus were associated with 40 and 16 % of colonies, respectively. The overdispered Poisson model based on hatched egg counts showed that, on average, there were 3.316 ± 0.723 more H. calcarata eggs than eggs from the other two species combined (t 41.62 = 5.51, P < 0.0001). Estimated means per colony were 2.54 ± 0.785 and 0.765 ± 0.188 for H. calcarata and E. americanus plus S. rectus, respectively. Hatched eggs of both H. calcarata and E. americanus or H. calcarata and S. rectus were in 24 and 4 % of colonies, respectively, and hatched eggs of all three species were in 12 % of colonies. No other predators or evidence of their presence was observed on trees from either treatment. Significantly fewer adult A. mali emerged from colonies pruned from trees deployed in the orchard (7.3 ± 1.8 per colony) than from caged trees (74.7 ± 7.1 per colony) (t 8 = 8.99, P < 0.0001).
Natural enemies associated with WAA colonies on mature trees
In 2009 (Fig. 3a, b) and 2010 (Fig. 4a, b), counts of WAA crawlers and nymphs plus adults showed a marked declined within 2–3 weeks and most colonies were nearly extinct by week 4. In both years, some aphids that were currently or previously parasitized by A. mali were recorded on the first sample date (Figs. 3c, d, 4c, d). Similarly, hatched and unhatched H. calcarata eggs were also found in the first samples (Figs. 3e f, 4e, f). The number of currently parasitized aphids declined progressively in both studies, whereas the numbers of previously parasitized aphids remained relatively constant. Counts of hatched H. calcarata eggs increased over time, but unhatched eggs decreased markedly after the first 2–3 weeks. Total syrphid egg counts were 288 in 2009 and 238 in 2010, of which 97.9 and 92.0 % were H. calcarata in the respective years. In 2009 and 2010, E. americanus eggs represented 1.4 and 6.3 % and S. rectus eggs represented 0.7 and 1.7 %, respectively. Total syrphid larvae counts were 26 in 2009 and 32 in 2010, of which 96.2 and 96.9 % were H. calcarata in the respective years, with the remainder either E. americanus or S. rectus. The only other predators or evidence of predators associated with colonies were green lacewing eggs (n = 2 and 3 in 2009 and 2010, respectively) and one unidentified lacewing larva in 2010.
Box-plot distributions (R Core Team 2015) showing temporal changes in a live woolly apple aphid crawlers, b live aphid nymphs plus adults, c aphids currently parasitized by Aphelinus mali, d aphids previously parasitized by A. mali, e hatched H. calcarata eggs, and f unhatched H. calcarata eggs, based on data collected weekly from arboreal woolly apple aphid colonies in a Virginia, USA apple orchard beginning 3 June, 2009. Medians (line through rectangles), interquartile range (rectangles), minimal and maximal values, and outliers are shown
Box-plots distributions (R Core Team 2015) showing temporal changes in a live woolly apple aphid crawlers, b live aphid nymphs plus adults, c aphids currently parasitized by Aphelinus mali, d aphid previously parasitized by A. mali, e hatched Heringia calcarata eggs, and f unhatched. H. calcarata eggs, based on data collected weekly from arboreal woolly apple aphid colonies in a Virginia, USA apple orchard beginning 9 June, 2010. Medians (line through rectangles), interquartile range (rectangles), minimal and maximal values, and outliers are shown
For both response variables, the Vuong test indicated that the negative binomial model provided a better fit to the data than the Poisson and ZIP models (crawlers: Z Poisson = 3.10 and Z ZIP = 2.98; nymphs plus adults: Z Poisson = 4.90 and Z ZIP = 4.40; P < 0.01 for all tests). Summary coefficients and Type III fixed effect tests based on this model are in Table 2. Negative and positive parameter estimates indicate declining and increasing counts, respectively. Coefficients associated with the interactions between the predictors and year effect served as an adjustment term to the overall predictor coefficient for observations collected in 2009. Insignificant interactions implied consistent relationships between years.
Counts of crawlers and nymphs plus adults were significantly correlated with sample week and with currently and previously parasitized aphids. Crawler counts were also correlated with unhatched H. calcarata eggs. Most interactions between year and the six predictors were not significant, implying consistent relationships between years. Exceptions were those between H. calcarata larvae and crawlers and sample week and nymphs plus adults (Table 2). The overall relationship between H. calcarata larvae and crawlers was not significant, implying that this relationship was only observed in 2009. The negative relationship between H. calcarata larvae and crawlers for 2009, as indicated by the negative interaction coefficient, implied that, for this year, larger counts of H. calcarata larvae were correlated with fewer WAA crawlers. The positive interaction between year and sample week for nymphs plus adults implied that the decline in this response variable was slower in 2009 than in 2010.
Discussion
Extending upon previous reports that H. calcarata was commonly associated with WAA in Virginia orchards (Bergh and Short 2008; Gresham et al. 2013; Short and Bergh 2004), combined results from the present experiments confirm that H. calcarata and A. mali are key agents that usually suppress WAA populations in Virginia. Initial cage experiments showed pronounced effects of natural enemy exclusion or access on WAA colony numbers and the results were very similar to those of Gontijo et al. (2015) in Washington, who deployed WAA-infested trees in cages enabling access by A. mali only, A. mali and predators, or neither. They showed that aphid populations increased rapidly when all natural enemies were excluded, that A. mali alone only suppressed the rate of aphid population growth, and that most colonies were quickly decimated when both A. mali and predators were allowed access.
Despite single alternate-row-middle applications of azinphosmethyl and lambda-cyhalothrin in the orchard during the study in 2008, natural enemies impacted WAA colonies progressively during the experiment, conforming to Brown and Schmitt’s (1994) observations. Frequent inspection of individual colonies on potted trees in the orchard revealed that syrphids were the predominant predators detected, that they oviposited in colonies within two days, and that H. calcarata was most common. Gontijo et al. (2012) reported that syrphids represented 62–81 % of all predators associated with WAA colonies in Washington and that, of the five species recorded, E. americanus and E. fumipennis Thompson were most common. Our results also concur with the finding that H. calcarata often oviposited on WAA colonies on excised apple shoots within ≤12-h of deployment at the base of apple trees (Gresham et al. 2013). The greater abundance of H. calcarata eggs and the presence of eggs from more than one syrphid species in some colonies conforms to Bergh and Short (2008).
Bergh and Short (2008) reported a three-days developmental duration of H. calcarata eggs at 25 °C and a larval feeding duration of 7–8 days. Larvae consumed 105.3 ± 1.9 WAA during their development (Short and Bergh 2004) and showed highest rates of consumption between the 1st and 5th day after hatch (Short 2003). Assuming that H. calcarata oviposited in many colonies on potted trees during the first two-day interval, these life-history traits may explain much about the temporal changes in the size and appearance of individual colonies recorded over 14 days. While the effects of other potential predators was not explicitly recorded, we assume that predation by larger generalist predators, such as nocturnally-feeding earwigs, would have had much more apparent and rapid disruptive effects on colony appearance than the gradual decline recorded.
Many more adult A. mali emerged from colonies pruned from caged trees than from trees in the orchard, indicating that parasitism alone did not result in observable changes in the caged colonies over 14 days. This conforms to previous findings (Gontijo et al. 2015) that A. mali only slowed WAA colony growth and suggests that predation was primarily responsible for colony decline on potted trees in the orchard. The comparatively low numbers of adult A. mali that emerged from colonies on orchard trees suggested an impact of intraguild predation on A. mali (Gresham et al. 2013). Gontijo et al. (2015) also provided evidence for some degree of intraguild predation by generalist predators on parasitism of WAA colonies by A. mali in Washington, USA.
When cohorts of naturally-occurring WAA colonies in the canopy of mature apple trees were evaluated in two consecutive years, temporal changes in aphid numbers and the natural enemies associated with them corroborated the results from potted tree studies. Aphid numbers declined quickly following the initiation of the study, colonies were brought to extinction or near extinction within the first four weeks of sampling, and H. calcarata and A. mali were the predominant natural enemies associated with this response.
Clearly, both H. calcarata and A. mali contributed to WAA colony suppression in Virginia orchards. Given that arboreal WAA populations and H. calcarata show initial annual peaks in May and June (Gresham et al. 2013) and that highest parasitism by A. mali tends to occur later (Brown and Schmitt 1994), H. calcarata may have a greater impact on WAA than A. mali early in the season, as our results suggest. Gontijo et al. (2012) found that syrphids predominated in WAA colonies in June and July in Washington and Brown and Schmitt (1994) showed that unidentified syrphid larvae were most common in colonies from West Virginia orchards in June. Beers et al. (2010) also suggested that WAA populations in Washington orchards may be more impacted by predation early in the season than later.
Following a period of very low H. calcarata abundance in mid-summer, Gresham et al. (2013) recorded large numbers of females foraging beneath apple trees between September and early October, coinciding with the period when WAA colonies often resurge (Brown and Schmitt 1994). However, female H. calcarata also oviposit in the soil (Bergh and Short 2008), where larvae are presumed to develop on WAA colonies on apple roots (Walsh and Riley 1869). Given that adult H. calcarata were captured in emergence traps beneath apple trees in the spring (Short 2003), larvae are also presumed overwinter belowground. Thus, oviposition in late summer may be predominantly in the soil, precluding an impact on aerial colonies. Indeed, Bergh (unpublished data) found that the majority of syrphid eggs and larvae associated with WAA colonies in late summer were E. americanus and S. rectus.
Recent WAA outbreaks in Virginia orchards treated with multiple, post-bloom applications of broad-spectrum insecticides against brown marmorated stink bug have usually occurred between late July and September. Our data suggest that these outbreaks are due primarily to the disruption of A. mali, H. calcarata, or both. Aggressive intervention against brown marmorated stink bug in Mid-Atlantic apple orchards begins in late June or July and may continue through August, during which the impact of A. mali on WAA colonies may be greatest. Many orchard insecticides are known to be highly toxic to A. mali (Cohen et al. 1996; Gontijo 2011), but their effects on H. calcarata have not been examined. These results provide new insights into the importance of predation to WAA biocontrol in Virginia apple orchards and relate directly to an improved capacity to elucidate and mitigate the specific underlying causes of recent WAA outbreaks in the Mid-Atlantic region.
References
Asante SK (1997) Natural enemies of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae): a review of the world literature. Plant Prot Q 12:166–172
Baker AC (1915) The woolly apple aphis. USDA Rept. No. 101
Beers EH, Cockfield SD, Gontijo LM (2010) Seasonal phenology of woolly apple aphid (Hemiptera: Aphididae) in central Washington. Environ Entomol 39:286–294
Bergh JC, Short BD (2008) Ecological and life-history notes on syrphid predators of woolly apple aphid in Virginia, with emphasis on Heringia calcarata. BioControl 53:773–786
Brown MW (1986) Observations of woolly apple aphid, Eriosoma lanigerum (Hausmann) (Homoptera: Aphididae), root infestation in eastern West Virginia. Melsheimer Entomol Ser 36:5–8
Brown MW, Schmitt JJ (1994) Population dynamics of woolly apple aphid (Homoptera: Aphididae) in West Virginia apple orchards. Environ Entomol 23:1182–1188
Cohen H, Horowitz AR, Nestel D, Rosen D (1996) Susceptibility of the woolly apple aphid parasitoid, Aphelinus mali (Hym: Aphelinidae), to common pesticides used in apple orchards in Israel. Entomophaga 41:225–233
Gontijo LM (2011) Integrated biological control of woolly apple aphid in Washington State. Ph.D. Dissertation, Washington State University, Pullman
Gontijo LM, Cockfield SD, Beers EH (2012) Natural enemies of woolly apple aphid (Hemiptera: Aphididae) in Washington State. Environ Entomol 41:1364–1371
Gontijo LM, Beers EH, Snyder WE (2015) Complementary suppression of aphids by predators and parasitoids. Biol Control 90:83–91
Gresham SDM, Charles JG, Sandanayaka WRM, Bergh JC (2013) Laboratory and field studies supporting the development of Heringia calcarata as a candidate biological control agent for Eriosoma lanigerum in New Zealand. BioControl 58:645–656
Howard LO (1929) Aphelinus mali and its travels. Ann Entomol Soc Am 22:341–368
Leskey TC, Short BD, Butler BB, Wright SE (2012) Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) in mid-Atlantic tree fruit orchards in the United States: case studies of commercial management. Psyche, Article ID 535062, doi:10.1155/2012/535062
Nicholas AH, Spooner-Hart RN, Vickers RA (2005) Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program. BioControl 50:271–291
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
SAS Institute (2014a) SAS/Stat 9.4 User’s Guide 9, SAS Institute Inc., Cary, NC
SAS Institute (2014b) Tests for comparing nested and nonnested models. http://support.sas.com/kb/42/514.html
Short BD (2003) Inaugural studies of the life history and predator/prey associations of Heringia calcarata (Loew) (Diptera: Syrphidae), a specialist predator of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Homoptera: Eriosomatidae). Masters: Entomology, Virginia Tech, Blacksburg
Short BD, Bergh JC (2004) Feeding and egg distribution studies of Heringia calcarata (Diptera: Syrphidae), a specialized predator of woolly apple aphid (Homoptera: Eriosomatidae) in Virginia apple orchards. J Econ Entomol 97:813–819
Short BD, Bergh JC (2005) Separation of three common hover fly predators of woolly apple aphid based on the exochorionic sculpturing of eggs. Can Entomol 137:67–70
Vuong QH (1989) Likelihood ratio tests for model selection and non-tested hypotheses. Econometrica 57:307–333
Walsh BD, Riley CV (1869) The apple-root plant-louse. Am Entomol 5:81–84
Yothers MA (1953) An annotated bibliography on Aphelinus mali (Hald.) a parasite of the woolly apple aphid 1851–1950. USDA Bureau of Entomology and Plant Quarantine E-861, p 61
Acknowledgments
We thank Jean P. Engelman for her technical assistance with this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Marta Montserrat.
Rights and permissions
About this article
Cite this article
Bergh, J.C., Stallings, J.W. Field evaluations of the contribution of predators and the parasitoid, Aphelinus mali, to biological control of woolly apple aphid, Eriosoma lanigerum, in Virginia, USA. BioControl 61, 155–165 (2016). https://doi.org/10.1007/s10526-016-9714-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-016-9714-7