Abstract
Ageing is accompanied by alterations in several biochemical processes, highly influenced by its environment. It is controlled by the interactions at various levels of biological hierarchy. To maintain homeostasis, a number of nutrient sensors respond to the nutritional status of the cell and control its energy metabolism. Mitochondrial physiology is influenced by the energy status of the cell. The alterations in mitochondrial physiology and the network of nutrient sensors result in mitochondrial damage leading to age related metabolic degeneration and diseases. Calorie restriction (CR) has proved to be as the most successful intervention to achieve the goal of longevity and healthspan. CR elicits a hormetic response and regulates metabolism by modulating these networks. In this review, the authors summarize the interdependent relationship between mitochondrial physiology and nutrient sensors during the ageing process and their role in regulating metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing, a time-dependent phenomenon, is accompanied with a decline in homeostatic balance and it is mostly inevitable by living organisms. It is a multi-faceted, heterogeneous, progressive process, interacting at different levels of biological hierarchy – molecular, cellular and tissue level, causing various organs to age differently (Jones et al. 2014). A number of age-associated processes have been analyzed, as potential drivers of ageing, as they interact with each other to maintain homeostasis at different levels of organization (Kennedy et al. 2014). Interactions between genetic and epigenetic factors control ageing and modulating these factors extend lifespan. Ageing poses as the primary risk factor to various chronic diseases like type 2 diabetes mellitus (T2DM), Alzheimer’s disease (AD), Parkinson’s disease (PD), cardiovascular disease, cancers, sarcopenia and immune system disorders; and as the most significant cause of human morbidity and mortality (Aunan et al. 2017). Various theories have been proposed in an attempt to explain the phenomenon of ageing (López-Otín et al. 2013; Sharma and Dkhar 2014; Lipsky and Kin 2015).
Ageing in itself is an inclusive term for a number of biological processes with multiple aspects, which have been described by López-Otín et al. (2013) as hallmarks of ageing. Among the hallmarks, the dysregulation of mitochondrial function and a gradual decline in functioning of nutrient sensing pathways act as antagonistic hallmarks of ageing. These hallmarks are interconnected as they encroach upon cellular metabolism. They respond to changes in the environment − nutrient excess or scarcity, physical or chemical stress and exposure to microorganisms. Age-related deterioration of one of the above-mentioned processes has a profound effect on the other. The deregulation of these pathways drives the cell towards a senescent state, which contributes to ageing phenotype and, eventually, drives the organism susceptible to chronic age-related diseases. Thus, targeting metabolic regulation could be an encouraging approach towards extending human healthspan and lifespan.
Dietary interventions, such as calorie restriction, intermittent fasting, usage of CR mimetics, have shown to be of great importance in altering mitochondrial dysregulation and nutrient sensing pathways in ageing organisms, thereby extending their healthspan part of lifespan. This review focuses on two of the hallmarks of ageing, viz., mitochondrial dysfunction and declining function of the nutrient sensing pathways, how nutrient sensors regulate mitochondrial function and how dietary factors alleviate the ill effects of ageing associated with these two hallmarks.
Mitochondrial association during ageing
Mitochondria, as the powerhouse of the cells, provide the energy requirement for aerobic organisms to perform all the activities in the form of adenosine triphosphate (ATP) through the tricarboxylic acid (TCA) cycle and the electron transport chain (ETC), and are the major sources of intracellular reactive oxygen species (ROS). They are also crucial for basic cellular functions, such as redox balance, signalling transduction, metabolic homeostasis, cell differentiation and senescence. Thus, their dysfunction leads to reduction in the ATP production, elevated production of ROS, reduced antioxidant resistance and metabolic disorders, including various age-related neurological, fibrotic and cardiovascular diseases (Boengler et al. 2017; López-Lluch et al. 2018; Zhu et al. 2019; Li et al. 2020). ROS, under normal physiological conditions, have various important functions in regulating cellular metabolism. Hydrogen peroxide (H2O2) and superoxide (O2·−) are two important ROS which act as signalling molecules in regulating cell growth, differentiation and death, activate mitogenic-activated protein and also take part in an organism’s immune response against a number of infections. H2O2 helps in regulating the expression of a number genes, including nuclear factor erythroid-2-related factor 2 (Nrf2), activator protein (AP-1), cAMP response element binding protein (CREB), nuclear factor kappa B (NF-kB), heat shock factor 1(HSF1), hypoxia inducible factor 1(HIF-1), tumour protein p53 (TP53), neurogenic locus notch homolog protein (NOTCH), and specificity protein 1(SP1) (Marinho et al. 2014; Sies 2017). Proteins with sulfhydryl groups can be directly affected by ROS through the oxidation of their thiol moiety. They can also increase the capillary walls’ permeability, thereby stimulating glucose transport into the cells as well as that of serotonin into platelets (Droge 2002; Giorgi et al. 2018). However, uninhibited production of ROS may incite oxidative damages to the major cellular components (proteins, lipids, nucleic acids) due to their high reactivity and are, therefore, likely to be toxic, mutagenic, or carcinogenic. Free radical reaction intermediate products, e.g., peroxynitrite and lipid hydroperoxides, can alter various components of the cell (Giorgi et al. 2018).
In 1954, the free radical theory of ageing (FRTA) was proposed by Denham Harman, linking oxidative stress and ageing (Harman 1956). It was later developed as the mitochondrial free radical theory of ageing (MFRTA), stating that mitochondrial dysfunction and subjection to subsequently heightened ROS production give rise to a vicious loop causing damages to cell and its components, consequently leading to death (Harman 1972). Though the theory initially received not so much enthusiasm, ROS have shown to be of great importance during the process of ageing. Studies have shown the association of oxidative damages to deoxyribonucleic acid (DNA), proteins and lipids with uncontrolled production of ROS, impaired mitochondrial function and untimely cell death or senescence (Sohal and Weindruch 1996; Bokov et al. 2004). Increased production of ROS has also been linked to mitochondrial oxidative damage, accompanied by a decrease in the copy number of mitochondria (Herbener 1976; Yen et al. 1989; Lambert et al. 2007; Cocheme et al. 2011). These changes have shown to be associated with characteristic features of human ageing, such as loss of hair, reduced fat and weight, decrease in the bone density and cardiomyopathy (Trifunovic et al. 2004).
Mitochondrial ROS regulation during ageing
Throughout the life of a cell, there is a constant mitochondrial ROS generation, resulting in an age-associated chronic oxidative stress, accounting for mitochondria being crucial players during ageing (Miquel et al. 1980; Santos et al. 2013). Complexes of the ETC can get assembled into functionally and structurally larger units, also known as ‘respirasomes’, that allow minimizing the diffusion distances of substrates for a more effective electron flow through the ETC. These supercomplexes have been proposed to be linked to an age-dependent destabilization, contributing to the development of an ageing phenotype of the mitochondria, especially in post-mitotic tissues (Gomez and Hagen 2012). The rate at which these ETC complexes are assembled affects the ROS production capacity of the mitochondria (Genova et al., 2015; Moreno-Loshuertos et al. 2016), suggesting that the inability to form large units or their continual degradation might be an important factor in bringing about age-associated heightened ROS production and oxidative stress (Genova et al. 2015). Mitochondrial DNA (mtDNA), mitochondrial enzymes and lipids are the primary targets of ROS. Another factor favouring the adverse consequences of oxidative stress resulting in the accumulation of damaged mtDNA is the close vicinity of the DNA from the sites for ROS production in the ETC (Yen et al. 1989; Lambert et al. 2007; Cocheme et al. 2011). On contrary to their destructive property, ROS, when present lower than a level that can be toxic, are essential for normal physiological functions and involved in many signal transductions, such as regulation of autophagy and inflammatory responses (Whitehall et al. 2020).
During ageing, along with the increase in the ROS production, there is an associated decline in the antioxidative defense capacity, thereby increasing the deteriorating effects of oxidative stress. This successively leads to the reversible oxidation of the thiol groups, disrupting the antioxidative enzyme activity that can lead to harmful changes in the structure and function of various biomolecules (Freitas et al. 2016). In Drosophila melanogaster, it was observed that overexpression of antioxidative enzymes, such as superoxide dismutase (SOD) and catalase, protects the DNA from the harmful effects of ROS by decreasing their production, ultimately extending the organism’s lifespan (Orr and Sohal 1994; Schriner et al. 2005). It was also observed that there is a higher level of such enzymes with decreased damage to the proteins and lipids, and adaptive mechanism of mitochondrial cysteine depletion in long-lived mice strains and other species (Pamplona et al. 2002; Rebrin and Sohal 2004; Moosmann and Behl 2008).
The negative correlation between ROS production and lifespan has been shown by various studies where hydrogen peroxide diffusing out from mitochondrial respiration was measured. Hydrogen peroxide production rate was much lower in the mitochondria of heart, brain and kidney of long-lived bats than that of short- lived shrew species (Brunet-Rossini 2004). Similar observation was also found in remarkably long-lived naked mole rat as compared to Fisher 344 rats (Csiszar et al. 2007). However, some studies have also shown the opposite results, depicting the positive correlation between ROS production and lifespan extension. In the vascular endothelial cells of long-lived Ames dwarf mice and their normal littermates, the production of hydrogen peroxide was more in the long-lived ones than that of the normal counterpart (Csiszar et al. 2008). In Caenorhabditis elegans, no effect on overall ROS was seen due to mutations in the mitochondria, even though the level of mitochondrial superoxide was elevated. In the mentioned study, unexpectedly, supplementing antioxidants shortened the lifespan of the mutants (Yang et al. 2010). Moreover, when antioxidative enzymes, like mitochondrial SOD2 (manganese superoxide dismutase, MnSOD) and glutathione peroxidase-1 (GPx-1), were silenced in mice, longevity of the organism was not affected despite the rise in level of oxidative stress (Perez et al. 2009; Zhang et al. 2009). Even so, evidence supporting the credibility of the MFRTA outweigh till date. Nonetheless, the contradictory observations made rather supports the theory known as mitohormesis, stating that the mitochondrial ROS in their moderate level have the capacity to activate mechanisms compensating protection of cellular organelles from the harmful effects of ROS and delay the appearance of phenotypes associated with ageing (Ristow and Zarse 2010). For example, in the case of Drosophila and young mice slight increase in the level of ROS have been associated with lifespan extension (Csiszar et al. 2008; Copeland et al. 2009; Basisty et al. 2016). The finding that reducing elevated levels of mitochondrial ROS defends old mice from age-associated decline also suggests that decreasing ROS levels may help in delaying age-related diseases along with lifespan extension in ageing mammals (Schriner et al. 2005; Basisty et al. 2016). In human subjects, administering antioxidative compounds selegeline and vitamin E, singly or in combination, thereby downregulating the ROS concentration, have been seen to slow down the progression of AD (Sano et al. 1997). However, in PD, vitamin E failed to protect or delay the disease progression (Parkinson Study 1993) and was even found to be deleterious in some AD patients (Lloret et al. 2009).
Mitochondrial dysfunction in ageing
Mitochondrial dysfunction is one of the nine classical hallmarks of ageing (López-Otin et al. 2013). It is often linked with a number of processes such as decrease in the respiratory chain activity, fluxes in the TCA cycle, faulty regulation and function of mtDNA, elevated ROS level, intracellular calcium level changes and decline in the ATP level (de Almeida et al. 1989; Petrosillo et al. 2008; Emelyanova et al. 2018; Rottenberg and Hoek 2017; Li et al. 2020). Various studies have shown that mitochondrial quality control maintenance is of pivotal importance in fending off the process of ageing (Weber and Reichert 2010; Romanello et al. 2016). During ageing, there is an accumulation of damaged mitochondria with a mitochondrial turnover decline via mitophagy inhibition. Thus, striking a balance between generating new mitochondria and eliminating impaired ones is crucial for extending longevity (Chistiakov et al. 2014; Denzer et al. 2016). Impaired mitochondria release apoptogens into the cytoplasm due to the rupturing of the outer membrane leading to senescence during ageing (Daum et al. 2013). Mitochondrial dysfunction also adds to a number of metabolic disorders, cancer, neurodegeneration and various other pathologies (Thompson et al. 2015; Vincent et al. 2016; Spinelli et al. 2017; Reeve et al. 2018).
Mitochondria are interconnected dynamically, which enables them to share membranes, metabolites, proteins, solutes and electrochemical gradient as well (Tilokani et al. 2018). Mitochondrial dynamics is crucial in the regulation of mitochondrial function, quality control and senescence (Archer 2013). It comprises of mitochondrial fission, fusion, movement and interconnection with other various cellular organelles, and is regulated by these afore mentioned processes through reconstructing the interconnected structure and function of mitochondria as a result of the nutrient level, signalling molecules and stress (Benard and Rossignol 2008; Sharma et al. 2019). Mitochondrial dynamics are deteriorated during ageing and in various age-associated diseases as seen with the presence of damaged mitochondrial physiology. Mitochondrial fission and fusion are the main modulators of mitochondrial dynamics. Mitochondrial fission is vital for removing aberrant mitochondria through mitophagy, mitochondrial transport and apoptosis via stress-induced hyperfission (Fannjiang et al. 2004; Malena et al. 2009; Thomenius et al. 2011; Mao et al. 2013). During ageing, some proteins associated with mitochondrial fission are found to be dysregulated, possibly leading to the altered mitochondrial dynamics in aged organisms. Cytosolic dynamin-related protein 1 (DRP1), the main protein coordinating mitochondrial fission, was found to be downregulated in aged mice skeletal muscle, and in human vascular endothelial cells (HUVECs) along with mitochondrial fission 1 protein (FIS1) (Mai et al. 2010; Leduc-Gaudet et al. 2015).
Inducing the expression of Drp1 midlife in Drosophila is seen to extend healthspan along with lifespan by enhancing mitochondrial respiration and autophagy. Moreover, mitochondrial fragmentation induction in the intestine of both C. elegans and flies is known for increasing lifespan (Han et al. 2017; Rana et al. 2017). These findings from different models used in ageing studies show that proteins associated mitochondrial fission decline with age and suggest regulating the expression of these proteins might have lifespan extension properties. In contradictory to the above observations, deletion of Drp1 ortholog Dmn1p delays ageing in yeast by inhibiting mitochondrial fission without hindering growth rate or fertility (Scheckhuber et al. 2007). Increasing mitochondrial fission in the skeletal muscle of mouse were also found to be associated with impairment of insulin signalling and mitochondrial dysfunction (Jheng et al. 2012). Therefore, increased or decreased mitochondrial function by elevating mitochondrial fission depends on the type of tissue or the organism (Sharma et al. 2019).
Membrane-bound dynamin related proteins help in attaining fusion of mitochondria. Fusion of mitochondrial outer membrane is mediated by mitofusin 1 (MFN1) and MFN2, and that of inner mitochondrial membrane by optic atrophy 1 (OPA1) (Hall et al. 2014) and loss of any of these proteins contribute to hyperfragmented mitochondrial network. Mitochondrial fusion helps in exchanging metabolites, membrane potential transmission, elevation of the production of ATP, and decreasing ROS production, as well as in reducing mitochondrial-endoplasmic reticulum Ca2+ transfer, preventing Ca2+ induced cell-death (Chen et al. 2005; Buck et al. 2016; Zhao et al. 2017; Jung et al. 2018). It also alleviates mitochondrial stress through contents fusion of partly damaged mitochondria with that of healthy ones. Both fission and fusion allow mitochondria in adapting to severe nutrient level changes allowing more metabolic flexibility. Most studies on mitochondrial dynamics suggest that maintenance of a balance between fusion and fission might prove to be more favourable for an organism to attain a healthier mitochondrial network than separately promoting or inhibiting either of the two processes (Sharma et al. 2019).
Both AMP-activated protein kinase (AMPK) and calcineurin-mediated extension of lifespan in C. elegans are suppressed by mitochondrial network fragmentation (Burkewitz et al. 2015). In addition, inactivating dynamin-related protein 1 (Drp1) in C. elegans was seen to significantly enhance the insulin signalling ability to extend lifespan, proposing a correlation between extension of lifespan and mitochondrial fusion/fission ratio (Yang et al. 2011). Therefore, it suggests that mitochondrial dynamics is involved in the regulation of ageing, along with or as a part of ageing-related pathways such as insulin and insulin/insulin-like growth factor-1 (IIS) signalling or AMPK pathway (Giorgi et al. 2018). In fungal models, Podospora anserine and Saccharomyces cerevisiae, deletion of dynamin-related protein 1 (Dnm1p), mediates fission and suppresses ageing, thereby extending lifespan (Scheckhuber et al. 2007). However, upregulating the expression of Drp1 in Drosophila during midlife have shown to extend lifespan, probably through autophagy (Rana et al. 2017). These studies suggest that there is no specific link between either fusion or fission and life expectancy, but rather the regulation of the mitochondrial quality control, controlled by fusion and fission, which determines the process of ageing (Giorgi et al. 2018).
The occurrence of cardiovascular disease increases during ageing and is one of the main factors that ultimately lead to the death of an organism (Rapsomaniki et al. 2014). Hyperfragmented mitochondrial network and low expression level of optic atrophy 1 (Opa1) are associated with failure of the heart in human and mice (Chen et al. 2009). Opa1, along with the mitochondrial assembly regulatory factor (Marf, Drosophila mitofusin), are also necessary for the proper functioning of cardiomyocyte in Drosophila and defects in fusion are linked with cardiomyopathy (Dorn and Scorrano 2010). In mice, Opa1 unbalanced processing and decreased mitochondrial fusion are associated with fragmentation leading to heart failure (Wai et al. 2015). These studies show that fusion, along with Opa1, is essential for the proper functioning of the heart and prevents heart failure, and appear to be conserved throughout the three species, flies, mice and humans (Giorgi et al. 2018).
AD and PD are the most common age-related neurodegenerative diseases and have been associated to mitochondrial dynamics and mitophagy (Chen et al. 2009). In AD, amyloid-β (Aβ) is linked to hyperfragmentation of mitochondrial network induced by Drp1in the disease progression (Reddy et al. 2017). Decreasing the Drp1 level also reduces the production level of soluble Aβ, protecting against mitochondrial and synaptic toxicities caused by Aβ in the progression and pathogenesis of AD (Manczak et al. 2016). PD is also accompanied by fusion/fission ratio alterations. Fragmentation of the mitochondrial network and damaged anterograde axonal transport of mitochondria have been observed in the respiratory–chain deficient dopaminergic neurons (Sterky et al. 2011). Age-associated degenerative effects also affect muscles leading to muscle wasting. In aged mice, it was observed that the intermyofibrillar mitochondria in the skeletal muscle were longer and branched, indicating an increase of mitochondrial fusion and/ or a decrease of fission (Leduc-Gaudet et al. 2015). In case of human, aged subjects had lower Opa1 level in the muscle (Joseph et al. 2012).
Nutrient sensing association during ageing
Organisms are invariably dependent on their ability to utilize nutrients for growth and metabolic functions. Multiple mechanisms and pathways respond to the level of nutrients in order to maintain homeostasis and disruption in these mechanisms disturbs the equilibrium of the cellular environment. These pathways exist in all living organisms, ranging from bacteria (prokaryotes) to yeast and mammals (eukaryotes). Such pathways respond to a range of nutrients and hormones to maintain coordination of coherent responses in the organism as a whole (Rosetti 2000). With an increase in age, many of the proteins involved in nutrient sensing are downregulated, and thus arises a need to actively explore these pathways in order to understand their mechanisms so as to delay the onset of metabolic disorders.
Some of the major pathways involved in nutrient sensing are insulin and insulin/insulin-like growth factor-1(IGF-1) signalling (IIS) (Kaletsky and Murphy 2010), mechanistic target of rapamycin (mTOR) (Hansen et al. 2007), AMP-activated protein kinase (AMPK) (Greer et al. 2007) and sirtuins (Polito et al. 2010). When nutrients are available and stress levels are low, these pathways promote growth and reproduction. However, under low nutrient and high stress conditions, the pathways are altered, and as a consequence the cells are directed towards energy conservation and maintenance, implying that anabolism and catabolism of various metabolites are controlled by the nutrient sensing pathways. The nutrient-sensing pathways use posttranslational phosphorylation and acetylation modification of proteins with modulation of gene expression to regulate energy homeostasis (Steinberg et al. 2009; Wellen et al. 2010; Imai et al. 2010). These pathways regulate several cellular processes such as protein synthesis, autophagy (Klionsky 2000), metabolism (Koubova and Guarente 2003), oxidative stress (Lou et al. 2017), immunity (Iyer et al. 2015) and reproduction (Templeman and Murphy 2018). The downstream signalling cascades of these pathways have been linked to extending lifespan in some lower organisms. These pathways cross-regulate each other, activating or inhibiting one another and their downstream targets. The genes coding for nutrient sensing proteins act as key regulators of lifespan and are also called “nutrient sensing longevity genes” (Mocchegiani 2016). Ageing and longevity are highly influenced by these conserved longevity pathways (Johnson et al. 2015).
IIS pathway
The IIS pathway is a cell-signalling pathway that responds to hormonal signals to control metabolism. It is activated by insulin and insulin-like peptide (ILP) ligands whose levels are responsive to nutrient availability and/or sensory information. It forms part of the somatotropic axis consisting of growth hormone (GH), IGF-1, their receptors and its downstream signalling cascade. It is the most conserved nutrient sensing pathway in a wide spectrum of organisms (Kenyon 2005). The first evidence for its role in ageing was demonstrated in mutant C. elegans with mutations in age-1, phosphatidylinositol 3-kinase (PI3K), a downstream effector of insulin/insulin-like signalling; and daf-2, the insulin/insulin-like receptor (Freidman and Johnson 1988; Kenyon et al. 1993). It was later found that AGE-1, DAF-2 and DAF-16 are homologues of the human PI3K, insulin and IGF-1 transmembrane tyrosine kinase receptor and transcription factor, forkhead box O (FoxO), respectively (Kimura et al. 1997; Lin et al. 1997). Among IIS pathways multiple targets are protein kinase B (Akt), and RAS/MAPK pathway. Akt phosphorylates targets such as FoxO, causing its dispersal outside the nucleus, leading to a decrease in the transcription of its target genes (Siddle 2011). In mammals, Akt indirectly regulates TOR complexes by suppressing tuberous sclerosis complex (TSC) 1/TSC2 (Taniguchi et al. 2006). IIS stimulates the NF-κB pathway, involved in immune-inflammatory processes (Gilmore and Wolenski 2012). IIS expands lifespan through modulating transcription factors like FoxO and heat shock transcription factor-1 (HSF-1) (Tullet et al. 2008).
In mammals, levels of IGF-1, predominantly produced by liver cells, are regulated by GH and a decline in the level of circulating IGF-1 and GH is associated with ageing (Sun et al. 2013). In long-lived dwarf Ames (Prop1df) and Snell (Pitdw) mice, decrease in levels of IIS is observed correlating it with longevity (Brown-Borg et al. 1996). It has been found that mice with deficiency of GH and reduced levels of IGF-1 demonstrate an increase in lifespan (Coschigano et al. 2000; Lorenzini et al. 2014). Similar observations on expansion of lifespan due to decrease in IIS has been found in Drosophila (Clancy et al. 2001). Consequently, mutations and downregulation of targets in these pathways have been associated with increased longevity (Hadem et al. 2020). Among humans, various pockets of population like Ashkenazi Jewish centenarians and their Japanese peers have exhibited variants in the insulin receptor gene (Kojima et al. 2004; Suh et al. 2008). Moreover, predictions have been made about the relation between low IGF-1 levels and survival in humans with exceptional lifespan (Milman et al. 2014). Also, a deficiency in growth hormone receptors is linked to lower risk of age-associated conditions like cancer and diabetes, and a reduction in proteins that play a role in ageing (Guevara-Aguirre et al. 2011). Another study in Dutch nonagenarians also showed a positive correlation between IGF-1 and longevity (van der Spoel et al. 2015).
mTOR
The serine/threonine kinase, mTOR belongs to the phosphoinositide 3-kinase-related family thatis highly conserved among eukaryotes and that can be inhibited by the immunosuppressive drug rapamycin (Sabatini et al. 1994). It plays an integral role in regulating growth and metabolism in response to several upstream cues, including signals from IIS and other growth factors, the cellular energy sensor AMPK, amino acids, lipids, cholesterol and oxygen levels (Saxton and Sabatini 2017). mTOR functions in two structurally and functionally distinct multiprotein complexes termed TOR complex 1 (mTORC1) and TOR complex 2 (mTORC2) (Loewith and Hall 2011). mTORC1, along with its downstream effectors ribosomal protein S6 kinase β1 (S6K1) and eukaryotic translation initiation factor 4E(e1F4E)-binding protein 1(4E-BP), plays a major role in nutrient sensing by regulating cell growth (accumulation of cell mass) through coordination of protein anabolism (Ma and Blenis 2009), glycolysis (Shi et al. 2018), autophagy (Ganley et al. 2009; Kim et al. 2011) promoting lipid and nucleotide synthesis (Ben-Sahra et al. 2013) modulation of the senescence-associated secretory phenotype (Laberge et al. 2015).
mTORC1 activity increases during ageing contributing to age-related obesity supporting the idea that intense anabolic activity is responsible for accelerating ageing and age-related diseases (Yang et al. 2012). Enhanced 4E-BP increases translation of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), increasing mitochondrial respiration and inhibiting autophagy, thereby accelerating ageing (Tsai et al. 2015). However, various studies have shown that the mTOR pathway regulates lifespan in simple organisms such as yeast, worms and flies where mTORC1 was inhibited (Vellai et al. 2003; Kapahi et al. 2004; Kaeberlin et al. 2005; Arriola Apelo and Lamming 2016). The mTOR complexes are inhibited by rapamycin and rapalogs. Rapamycin, an immunosuppressive, anti-fungal drug inhibits mTORC1, by forming a complex by binding with the small endogenous protein FK506-binding protein (FKBP) and inhibiting mTORC1 (Shimobayashi and Hall 2014). mTORC1 activity is reduced in vital organs of Snell dwarf and global GH receptor (GHR) gene-disrupted mice (Dominick et al. 2015). In elder humans, the inhibition of mTOR has led to improvement in immunoscence and immune functions (Mannick et al. 2014).
mTORC2 controls growth by regulating lipogenesis, glucose metabolism (García-Martínez and Alessi 2008; Yuan et al. 2012) and the actin cytoskeleton (Cybulski and Hall 2009). In mammals, an interaction between mTOR and IIS signalling occurs via Akt, as it gets activated upon phosphorylation by mTORC2 (Sarbassov et al. 2005) leading to inhibition of Foxo transcription factors (Guertin et al. 2006). mTORC2 is also inhibited upon long exposure to rapamycin (Sarbassov et al. 2006). Although not much is known about the role of mTORC2 in ageing, but in some studies it was found that inhibiting mTORC2 may play a role in extending lifespan (Yu et al. 2019). In male mice, pharmaceutical treatment with acarbose and 17α-estradiol extends lifespan due to a boost in hepatic mTORC2 signalling (Garatt et al. 2017).
AMPK
A critical sensor of energy status is AMPK, which is activated in response to cellular energy depletion. AMPK is activated by direct AMP binding or due to elevated cellular levels of AMP, ADP, and/or calcium (Hardie et al. 2012, 2016). It is a key regulator of energy homeostasis and mitochondrial metabolism (Herzig and Shaw 2018). AMPK is a heterotrimeric kinase composed of one catalytic subunit α and two regulatory subunits β and γ (Steinberg and Kemp 2009). The catalytic α-subunit contains an important residue, Thr-172 located in the N-terminus of the kinase domain (Hawley et al. 1996). In mammals, AMPK is activated by upstream kinases, liver kinase B1 (LKB1) (Woods et al. 2003; Shaw et al. 2004) and calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) in response to intracellular Ca2+ (Hurley et al. 2005).
Under prolonged conditions of reduced energy, AMPK phosphorylatesPGC-1α, class IIA histone deacetylases (HDACs) co-activating cAMP response element-binding protein (CREB), CREB-regulated transcription coactivator-2 (CRTC2) and FoxO pathways. AMPK activates processes that results in breakdown of nutrients and produces energy by activating the uptake of glucose and fatty acids. AMPK reprogrammes metabolism through transcriptional regulation of biosynthetic pathways and use of mitochondrial substrates as an energy source (Mihaylova and Shaw 2011). AMPK controls metabolism at the transcriptional level by phosphorylating sterol regulatory element-binding protein 1 (SREBP1) (Li et al. 2011), carbohydrate-responsive element-binding protein (ChREBP) (Kawaguchi et al. 2002) and hepatocyte nuclear factor 4α (HNF4α) (Hong et al. 2003) which are key transcriptional regulators of lipid and glucose metabolism. AMPK activates autophagy by inhibiting mTORC1 indirectly by activating TSC2 and raptor (Inoki et al. 2003; Gwinn et al. response pathways like AMPK, SIRT).
AMPK plays a role in increasing healthspan by regulating inflammation by inhibiting a pro-inflammatory transcription factor NF-κB through its downstream signalling cascade like SIRT1, PGC-1α, p53, and Forkhead box O (FoxO) factors (Salminen et al. 2011). AMPK extends lifespan by activating ULK1, a kinase needed for autophagy and suppressing mTOR (Kim et al. 2011). The activation of AMPK has been found to be beneficial in age-related pathologies like atherosclerosis, T2DM, obesity and other metabolic diseases (Day et al. 2017). The role of AMPK in lifespan expansion by anti-ageing small molecules like metformin, resveratrol, aspirin and rapamycin has also been established as reviewed elsewhere (Burkewitz et al. 2014).
Sirtuins
Another class of energy sensors are sirtuins, a family of NAD+-dependent enzymes that catalyze post-translational modification of both histone and non-histone proteins. Since sirtuins are dependent on NAD+ for its activity, they are activated during the state of fasting or exercise. Sirtuins comprise a universally conserved family of NAD+-dependent deacetylases and/or ADP-ribosyltransferases found in all three domains of life (Frye 2000; Imai et al. 2000; Landry et al. 2000). Sirtuins regulate energy metabolism and are modulated by diet. In mammals, the family is represented by seven member SIRT1-7, variably located in different cellular compartments like nucleus (SIRT1,2,6,7), mitochondria (SIRT 3,4,5), cytoplasm (SIRT 1,2) (Frye 2000). Its targets for deacetylation include p53, NF-κB, PGC-1α, eNOS, mTORC2 and FoxO3a.
SIRT1 is the most studied among its counterparts. SIRT1 deacetylates many of the proteins and enzymes involved in metabolic processes (Zhao et al. 2010). SIRT1 regulates gluconeogenesis and glycolysis via PGC- 1αby promoting its nuclear localization, in laboratory animals and in vitro (Nemoto et al. 2005). In addition, it inhibits glycolysis while activating gluconeogenesis and fatty acid oxidation in most tissues (Silva and Wahlestedt 2010). PGC-1α lies at the center of the energy sensing pathway as it is phosphorylated and deacetylated by both AMPK and sirtuins, respectively and is a key controller of transcription factors to activate gene expression and control mitochondrial biogenesis (Rodgers et al. 2005). Sirtuins play a role in a number of age-related degenerative conditions like cardiovascular diseases, neurodegenerative diseases, cancer and diabetes and can serve as a therapeutic target (Hall et al. 2013).
Sirtuins role in lifespan extension was only discovered due to overexpression of Sir2 genes in C.elegans (Tissenbaum and Guarente 2001). In mice, the role of sirtuins in longevity has been much explored. Overexpression of Sirt1 in transgenic mice showed features resembling calorie restricted mice like lean body, reduced blood cholesterol levels and metabolically active (Bordone et al. 2007). On the other hand, a high fat diet led to loss of Sirt1 in adipose tissues in mice (Chalkiadaki and Guarente 2012). In humans, undergoing dietary restriction the levels of SIRT1 increases in the muscles (Civitarese et al. 2007). Sirt3 is required for the reduction of oxidative damage in cochlear cell in calorie restricted mice (Someya et al. 2010).
There is an important correlation between the nutrient and energy sensors. The energy sensors (AMPK and Sirtuins) function in the opposite direction of nutrient sensors (mTOR). Overall, AMPK and sirtuins regulate the catabolism of metabolites and inhibit their anabolism. These signalling pathways are interconnected as they regulate each other by its downstream signalling cascades. Energy sensors play a central role in the metabolism of glucose, lipid, protein and lastly autophagy and mitochondrial homeostasis (Guarente 2011; Houtkeeperet al. 2012; Herzig and Shaw 2018). It has been found that the upregulation of these pathways aids towards healthy ageing.
Metabolic dysfunctions associated with mitochondrial and nutrient sensing deregulation
Visceral fat accumulation or obesity during ageing poses higher risk of morbidity due to surge in possible risk for cardiovascular diseases, T2DM and hypertension. In obesity-associated diseases like T2DM and insulin resistance, lesser expression of mtDNA and reduced levels of mitochondrial complexes have been observed, indicating dysfunction of mitochondria. This affects organs such as liver, muscle and adipose tissue, thereby developing metabolic disorders (Patti and Corvera 2010). Fatty acid oxidation capacity is also reduced due to mitochondrial dysfunction and has been proposed to induce liver, muscle and adipose tissue to accumulate lipids, particularly long-chain fatty acids, which increases resistance to insulin (Lowell and Shulman 2005; Hafizi Abu Bakar et al. 2015). Increased level of ROS has been observed in skeletal muscle of obese individuals due to the accumulation of impaired mitochondria during ageing, leading to muscle deterioration (Barbieri et al. 2013). Mitochondrial network fragmentation and reduction in the mitochondrial membrane potential has been linked to reduced mitofusin 2 (Mfn2) expression in the skeletal muscle of obese individuals and in T2DM patients. Mitofusin proteins are located on the surface of the outer mitochondrial membrane and act in the membrane fusion process of mitochondria. The reduced expression of Mfn2 plays a crucial role in the impairment of mitochondria linked with accumulation of fat (López-Lluch et al. 2018).
In the white adipose tissue, AMPK, among the several metabolic regulators, has a critical role in metabolic syndrome progression and resistance to insulin. Various downstream substrates required for the increase in oxidation of lipids and glucose uptake are regulated by phosphorylated AMPK, thereby resulting in glucose intolerance, obesity and lower physical capability in case of insufficient AMPK (Steinberg et al. 2010). As regards to its crucial role in regulating metabolism and mitochondrial activity, a decline in AMPK levels and activity during ageing can lead to physiological decline of adipose and other tissues in old age (Ruiz et al. 2016).
Regulation of mitochondrial physiology by nutrient sensors
Mitochondrial activity, dynamics, biogenesis and turnover respond to nutrient availability i.e., nutrient excess stimulates fission and nutrient deficiency stimulates fusion (Rambold et al. 2011). It is regulated by nutrient sensors such as mTOR, AMPK and sirtuins, maintaining an equilibrium of activity according to the requirement of the cells (López-Lluch 2017). During ageing, an imbalance in this process contributes to the loss of mitochondrial homeostasis in sarcopenic muscles, associated with the upregulation of mitochondrial fusion in skeletalmuscle (Joseph et al. 2013).
In conditions of high calorie consumption, there is activation of mTOR. Various cellular processes − insulin resistance, adipogenesis, inflammation, tumor formation and angiogenesis also play a role in the activation of mTOR (Lee et al. 2007; Laplante and Sabatini 2009). mTOR signalling pathway is downregulated by AMPK, thereby regulating cell bioenergetics, and sirtuins are activated in conditions when there is low amount of energy suchas calorie restriction (CR) (López-Lluch et al. 2018). Mitophagy, essential for cell survival is regulated by AMPK, mTOR and FoxO3 transcription factors (Zhao et al. 2007; Hirota et al. 2012). Downregulation of mTOR stimulates mitophagy and upregulation of AMPK activates mitochondrial biogenesis, oxidative metabolism, and resistance to oxidative stress (Guarente 2014). By modulating autophagy and mitophagy, mTORC1 regulates mitochondrial turnover. Through the phosphorylation of inhibitory sites of initial mediators unc-51-like kinase 1/2 (ULK-1 and ULK-2), mTORC1 blocks autophagy, while it is induced by AMPK by phosphorylating the activation sites of the same initiators (Alers et al. 2012).
A metabolic shift to β-oxidation, lower respiration rate, and more OXPHOS proteins were found in Ames dwarf and GH deficient mice, linking IIS signalling and mitochondrial metabolism (Brown-Borg et al. 2012). The expression of genes involved in mitochondrial processes like β-oxidation, TCA cycle and glycolysis were found to be higher in Fat-specific insulin receptor knockout mice (FIRKO) with respect to the control mice of same age (Katic et al. 2007), implying the role of IIS signalling and somatotropic axis in mitochondrial metabolism.
Sirtuins are associated to different important features of cell physiology, from the control of cell cycle to metabolism and antioxidant defense. Their effect on the physiology of mitochondria and metabolism of cell has been linked to their impact on extension of lifespan. Sirtuins activation and mitochondrial biogenesis helps in part of the overall effect of CR (Guarente 2013). The aforesaid nutrient sensors also regulate the activity of the crucial transcription factor, PGC1α, involved in the activation of mitochondrial biogenesis.PGC1α modulates mitochondrial biogenesis and oxidative phosphorylation, balances mitochondrial dynamics, and controls the copy number of the mitochondrial genome (Gouspillou et al. 2014). It also controls the expression of transcription factors involved in mitochondrial biogenesis and respiration control, like the nuclear respiratory factors 1 and 2 (NRF1 and 2), mitochondrial transpiration factor A (TFAM) or peroxisome-proliferator activated receptor-γ (PPARγ) (Martin-Montalvo and de Cabo 2013). SIRT3, present in mitochondria having deacetylase activity, is stimulated after CR in the skeletal muscle and under a high-fat diet, its level decreases suggesting a calorie-dependent modulation (Palacios et al. 2009). It takes part in the breaking down of lipids and regulation of complexes II, III and IV activities of the ETC by deacetylation. It is also involved in the modulation of autophagy by FoxO3, regulation of Mn-SODactivity and mitochondrial dynamics through autosomal dominant optic atrophy-1(OPA1) activation (López-Lluch et al. 2018). FoxO proteins take part in the removal of mitochondria and are involved in modulating cell inducing several stress-induced genes. Preventing mitochondrial dysfunction and improving mitochondrial functional quality by CR, polyphenols or exercise are associated with FoxO proteins regulation during ageing and their modulation by mTOR, AMPK and sirtuins (López-Lluch and Navas 2016).
Sestrins, a highly conserved stress-inducible small protein, regulate mTORC1 activity through the activation of AMPK. Inactivation of sestrin gene expression, in invertebrates, gives rise to various metabolic dysfunctions such as oxidative damage, mitochondrial dysfunction, accumulation of fat and deterioration of muscle favouring the acceleration of ageing (Lee et al. 2013). Sestrins have been linked to ageing through their effect on the rate of mitochondrial turnover (Wang et al. 2017). They are also associated with the metabolic regulation in mice aiding prolongevity effect. Sestrin proteins are regulated by oxidative stress through p53, NRF-2, AP-1 and FoxO and they modulate various components that help in decreasing the production of ROS, inducing mitochondrial biogenesis and mitophagy, thereby ameliorating oxidative metabolism (Lee et al. 2013). Through activating AMPK, the inactivation of sestrins-dependent mTORC1 plays a key role in maintaining autophagy and in defective mitochondria elimination (Ishihara et al. 2013). The levels of sestrins in muscle decline during ageing, but the level of sestrin 2 increases in mouse muscle in response to insulin and autophagy (Lenhare et al. 2017). Modulated by a number of stress factors, sestrins family of proteins, are emerging as essential metabolic regulators for mitochondrial turnover and various age-related complications by hormetic response to a mild stress (Wang et al. 2017).
Improving healthspan through dietary interventions
Impaired mitochondrial function and nutrient sensing are the common denominator in age-related fat accumulation leading to metabolic dysfunction. It can be said that the contribution of these processes is the major contributors in pathogenesis of age-related diseases. Over the period of a century, various methods have been used to alleviate the effects of ageing. Dietary interventions like calorie restriction (CR), intermittent fasting (IF), physical exercise, and the use of certain chemical/nutraceutical agents referred to as CR mimetics, are known to alleviate the effects of ageing. Several studies have shown that CR increases the capacity to extend longevity, affecting both, median and maximum lifespan in different organisms from yeasts to mammals (Sharma 2004; Testa et al. 2014, Madeo et al. 2015).CR is a nutritional intervention where one is subjected to a reduced calorie intake without resulting into malnutrition. Different kinds of dietary interventions regimens are followed in studies to analyse and understand the effects in various animal models as per their lifespan. There is also variation in the duration of the time followed for the regimen. These include calorie restriction (CR), intermittent fasting (IF) or alternate day fasting, ketogenic diet (KD), time restricted feeding (TRF) and diet with specific amount of limited nutrients.
Calorie restriction
CR is a dietary restriction regimen where there is a 10–50% decrease in the overall calorie intake without malnutrition for a particular duration. CR increases lifespan when calories are limited by a certain proportion but can have deleterious effect when the restriction percentage is increased beyond 50%. Variations in the effects of CR on ageing have been reported depending on the time of initiation, dietary composition, the duration of regimen, sex and species (Weindruch and Sohal 1997). CR has shown to have varying effects on different tissues (Arslan-Ergul et al. 2016).
The beneficial of CR are modulated through the mechanisms of nutrient sensing pathways and functions of mitochondria. Reducing oxidative stress is one of the major effects of CR on cellular physiology. This can be attained through various mechanisms, including a reduction in the rate of ROS generation, upregulation in the removal of ROS, thereby minimizing their harmful effects, and stimulating the repair process to lessen the impact of the damages. Various studies have shown that CR regulates redox homeostasis through these mentioned processes. There is also a lesser production of superoxide radical generation by CR (Speakman and Mitchell 2011).
CR helps in removing visceral fats, which is harmful due to its pro-inflammatory and diabetogenic activity, for healthy ageing (Finkel 2015). It also induces autophagy in human tissues, providing various anti-ageing effects by stimulation of better-quality control on organelles, stem cell optimum activity promotion, amelioration of immunological activities, and malignant transformation prevention (Galluzzi et al. 2015). Efficient autophagic reaction also improves various features of disorders associated with ageing, such as neurodegeneration, T2DM and arteriosclerosis (Levine and Kroemer 2008). CR activates autophagic responses through nutrient sensors such as SIRT1, AMPK, and mTORC1 and combines such responses to FoxO1 activation, which maintains telomerase activity as well (Makino et al. 2016).
Intermittent fasting
IF is a dietary restriction regime in which organisms undergo long hours of fasting and refeedingthat progressively reduces the total food intake by roughly 30% in model organisms (Suchiang and Sharma 2011). It has served as one of the alternatives for traditional CR. There are various modifications of IF including 12 h eating and 12 h fasting, alternate day feeding, 5:2 IF (5 days eating followed by 2 days fasting in a week). IF resembles the prehistoric times, where food was scarce, and the organisms needed to starve until food was available. IF in mice reduces inflammation and enhances autophagy by the activation of transcriptional factors induced by nutrient and hormonal signals (Yang et al. 2016; Bagherniya et al. 2018). IF also enhances long-term memory consolidation and improves physiological health (Dias et al. 2021; Zhang et al. 2022). IF upregulates brain-derived neurotrophic factor (Bdnf) expression in various brain regions of rodents (Mattson and Arumugam 2018; Liu et al. 2019). This can possibly regulate systemic and local anti-ageing effects as Bdnf stimulates synaptic plasticity, enhances neurogenesis, promotes neurons’ resistance to cell death, modulates appetite, improves cardiovascular and gastrointestinal autonomic control systems, and elevates glucose metabolism. In addition, IF alleviates various organs from ischemic damage, delays neurodegeneration in several rodent models, and counteracts or improves the after-effect of epileptic seizures and neuro-trauma (Longo and Mattson 2014; López-Otín et al. 2016). IF shows similar effects in humans in other age-related diseases (de Cabo and Mattson 2019).
Time restricted feeding
In TRF, the organisms are allowed to feed ad libitum during a limited period of 8 h and fasted for the next 16 h. This cycle of feeding and fasting continues for a longer duration in order to determine its impact on the organism. The concept of TRF arose within the context of circadian rhythms. In mice, TRF reduces adiposity and pro-inflammatory cytokines (Sundaram and Yan 2016), improves insulin resistance and hepatic steatosis (Ryan et al. 2013), gives protection against obesity, fatty liver disease, insulin resistance, hyperinsulinaemia and inflammation (Hatori et al. 2012). These positive metabolic effects are mediated by metabolic modulation of mTOR, CREB and AMPK pathway (Chaix et al. 2014). Recent TRF trials in humans have allowed a feeding window between 4 and 12 h, although a window of < 10 h is thought to be optimal based on glycogenolysis, fatty acid oxidation, and gluconeogenesis modulations occurring in the absence of dietary glucose availability. In a recent study where sedentary adults with overweight and obesity restricted their eating to an 8-hour ad libitum flexible feeding window; study volunteers experienced significant weight reduction (Anton et al. 2019).
Ketogenic diet
KD is a dietary regimen, which represents a group of diets, comprising of high fat and low carbohydrate content. After the application of a KD the body switches into starvation mode. In this situation the body uses fat as the energy source, and produces ketones-β-hydroxybutyrate, acetoacetate and acetone. Ketone bodies are lipid driven molecules (beta-oxidation), and they act as alternative substrates for energy production during fasting and low-carb diets (Jensen et al. 2020). In KD fed mice, the cardiac cells were found to abate oxidative stress, improve mitochondrial function and promote autophagic flux (Yu et al. 2020). KD improves mitochondrial biogenesis via the PGC-1α-Sirt3-UCP2 axis (Hasan-Olive et al. 2019) and protein acetylation levels and regulated mTORC1 signalling, demonstrating that KD extends longevity and healthspan in mice (Roberts et al. 2017). Also in rats, KDs change the gene expression levels in the CA3 region of hippocampus, which is known to impact cognitive abilities during brain ageing (Hernandez et al. 2019). These data suggest that KDs have the potential to impact the ageing.
Diet with limited amount of specific nutrients
The alternative to aforesaid dietary interventions is also gaining popularity as these effects seem to have a temporal impact on the organisms (Austad and Hoffman 2021). The idea that nutrients and not calories were responsible for the beneficial effects of dietary restriction has also been studied. Restriction of specific components like proteins or rather specific amino acids have also shown to impart similar results independent of CR (Soultoukis and Patridge 2016). Diets restricted with essential amino acids like methionine, tryptophan or threonine and other branched chained amino acids (BCAAS) like leucine, isoleucine and valine have also been studied in model organisms (Xiao et al. 2011; Perrone et al. 2013; Fontana et al. 2016). Such diets also impart its effects via the nutrient sensing proteins like mTOR, AMPK and GCN2.
CR Mimetics
The implementation of such dietary interventions in humans comes with some side effects like lowering of body temperature and a decrease in circulating sex hormones (Cangemi et al. 2010; Soare et al. 2011) and/or slow wound healing (Hunt et al. 2012). This led to the development of a newer strategy as CR mimetics. CR mimetics (CRM) is defined as substances that mimic the metabolic, hormonal and physiological effects of CR (Ingram and Roth 2015). In principle, a successful CRM would increase healthspan and lifespan by targeting the metabolic and stress response pathways affected by CR, but without markedly restricting caloric intake. Interestingly, some of the known CR mimetics compounds like resveratrol and metformin act as activators of energy sensors (AMPK and sirtuins) or drugs like Rapamycin inhibit the nutrient sensor mTOR.
Various other compounds also act as CR mimetics and help to increase healthspan in humans. Polyphenolic compounds, such as curcumin and resveratrol, and nutritional mushrooms like Coriolus versicolor and Hericium have strong redox-active properties which when ingested in small doses were found to be non-toxic in numerous experimental models, including humans. However, at high concentrations, these compounds exhibited toxic properties, which are harmful to the organism, thereby showing biphasic dose response when ingested in hormetic range (Calabrese et al. 2010; Trovato et al. 2018). These nutraceuticals induce adaptive cellular stress response pathways like AMPK, SIRT, FoxO, PGC1α, Nrf2, antioxidant response elements (AREs) and heat shock response (HSR) (Calabrese et al. 2010; Martel et al. 2019). Activation of the Nrf2/ARE and HSR together reduces amyloid formation in AD, induces cytoprotective enzyme heme oxygenase 1(HO-1) and mediates anti-inflammatory effects via the downregulation of nuclear factor kappa B (NF-kB), thereby, inducing protection against oxidative stresses that cause a wide range of chronic pathologies, including neurodegenerative conditions (Yang et al. 2005; Calabrese et al. 2010; Miquel et al. 2018). Across species, the longevity network detects nutrient availability and energy status, mitochondrial functional status and the production rate of ROS in the mitochondria for the regulation of lifespan, by coordinating the information with various signalling pathways, including the vitagenes. Vitagenes encode for genes, viz. heat shock proteins (Hsp) Hsp32, Hsp 70, the thioredoxin and the sirtuin protein systems that are involved in regulating cellular homeostasis (Calabrese et al. 2012). Activation of the vitagenes through hormetic CR-mimetics produces molecules like heat shock proteins, glutathione and bilirubin, having anti-oxidative and anti-apoptotic activities (Mancuso et al. 2006; Calabrese et al. 2007, 2010; Cornelius et al. 2013).
Conclusions
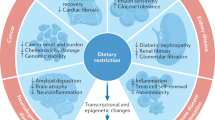
The intricacy of ageing process is highly complex and can only be understood upon analyzing its various aspects in a biological network. In the course of ageing, the role of mitochondria is regulated by various nutrient sensors directly, as these sensors control mitochondrial functions such as biogenesis, oxidative phosphorylation, autophagy, and mitochondrial turnover. Nutrient sensors such as mTOR, AMPK and sirtuins influence the expression of their downstream target transcription factors to reprogramme the signalling network and change the machinery and metabolism of the cell (Fig. 1). The identification of both deregulated nutrient sensing and mitochondrial dysfunction as network hallmark of ageing, opens up new avenues in future research in the area of biomedical gerontology. These processes are interdependent and form a network that regulates cellular metabolism and homeostasis during ageing. However, the core idea of all ageing research is to attain longevity with an extended healthspan. Also, nutrition serves as the most convenient way to act upon ageing. Dietary interventions act upon both these processes maintaining the metabolic homeostasis as a result of hormetic response by various signalling pathways and reducing the generation of ROS. Thus, it may prove to be effective in delaying the onset of age-related disorders. A big challenge that lies ahead of researchers is to find out the effect of the various interventions on different individuals and the optimum level of intervention to be followed to attain the goal of extended healthspan and lifespan in humans.
An overview of mitochondrial regulation by nutrient sensors. Various nutrient sensors regulate mitochondrial biogenesis, function and mito/autophagy to avoid the accumulation of impaired mitochondria. Impaired mitochondria accumulation is one of the main factors for mitochondrial dysfunction during ageing, resulting in a number of age-related metabolic disorders. Interventions such as CR and CR mimetics help in alleviating the ill-effects of dysfunctional mitochondria by stimulating mitochondrial biogenesis and mito/autophagy. [AMPK, AMP-activated protein kinase; Sirt, sirtuins; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1α; NRF, nuclear respiratory factors; PPARγ, peroxisome-proliferator activated receptor-γ; FoxO, forkhead box O; mTOR, mechanistic target of rapamycin]
References
Alers S, Löffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1):2–11. https://doi.org/10.1128/MCB.06159-11
Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M (2019) The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 11(7):1500. https://doi.org/10.3390/nu11071500
Archer SL (2013) Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369(3):2236–2251. https://doi.org/10.1056/NEJMra1215233
ArriolaApelo SI, Lamming DW (2016) Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci 71(7):841–849. https://doi.org/10.1093/gerona/glw090
Arslan-Ergul A, Erbaba B, Karoglu ET, Halim DO, Adams MM (2016) Short-term dietary restriction in old zebrafish changes cell senescence mechanisms. Neuroscience 334:64–75. https://doi.org/10.1016/j.neuroscience.2016.07.033
Aunan JR, Cho WC, Søreide K (2017) The biology of ageing and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis 8(5):628–642. https://doi.org/10.14336/ad.2017.0103
Austad SN, Hoffman JM (2021) Beyond calorie restriction: aging as a biological target for nutrient therapies. Curr Opin Biotechnol 70:56–60. https://doi.org/10.1016/j.copbio.2020.11.008
Barbieri E, Sestili P, Vallorani L, Guescini M, Calcabrini C, Gioacchini AM, Annibalini G, Lucertini F, Piccoli G, Stocchi V (2013) Mitohormesis in muscle cells: a morphological, molecular, and proteomic approach. Muscles Ligaments Tendons J 3(4):254–266
Bagherniya M, Butler AE, Barreto GE, Sahebkar A (2018) The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 47:183–197. https://doi.org/10.1016/j.arr.2018.08.004
Basisty N, Dai DF, Gagnidze A, Gitari L, Fredrickson J, Maina Y, Beyer RP, Emond MJ, Hsieh EJ, MacCoss MJ, Martin GM, Rabinovitch PS (2016) Mitochondrial-targeted catalase is good for the old mouse proteome, but not for the young: ‘reverse’ antagonistic pleiotropy? Aging Cell 15(4):634–645. https://doi.org/10.1111/acel.12472
Benard G, Rossignol R (2008) Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal 10(8):1313–1342. https://doi.org/10.1089/ars.2007.2000
Ben-Sahra I, Howell JJ, Asara JM, Manning BD (2013) Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339(6125):1323–1328. https://doi.org/10.1126/science.1228792
Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S (2017) Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle 8(3):349–369. https://doi.org/10.1002/jcsm.12178
Bokov A, Chaudhuri A, Richardson A (2004) The role of oxidative damage and stress in aging. Mech Ageing Dev 25(10–11):811–826. https://doi.org/10.1016/j.mad.2004.07.009
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L (2007) SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6(6):759–767. https://doi.org/10.1111/j.1474-9726.2007.00335.x
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A (1996) Dwarf mice and the ageing process. Nature 384(6604):33. https://doi.org/10.1038/384033a0
Brown-Borg HM, Johnson WT, Rakoczy SG (2012) Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age (Dordr) 34(1):43–57. https://doi.org/10.1007/s11357-011-9212-x
Brunet-Rossinni AK (2004) Reduced free-radical production and extreme longevity in the little brown bat (Myotislucifugus) versus two non-flying mammals. Mech Ageing Dev 125(1):11–20. https://doi.org/10.1016/j.mad.2003.09.003
Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang C-H, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJW, Chen Q, Huang S, C-C, O’Neill C, Edelson BT, Pearce EJ, Sesaki H, Huber TB, Rambold AS, Pearce EL (2016) Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166(1):63–76. https://doi.org/10.1016/j.cell.2016.05.035
Burkewitz K, Zhang Y, Mair WB (2014) AMPK at the nexus of energetics and aging. Cell Metab 20(1):10–25. https://doi.org/10.1016/j.cmet.2014.03.002
Burkewitz K, Morantte I, Weir HJM, Yeo R, Zhang Y, Huynh FK, Ilkayeva OR, Hirschey MD, Grant AR, Mair WB (2015) Neuronal CRTC-1governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 160(5):842–855. https://doi.org/10.1016/j.cell.2015.02.004
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Giuffrida Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8(10):766–775. https://doi.org/10.1038/nrn2214
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13(11):1763–1811. https://doi.org/10.1089/ars.2009.3074
Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ (2012) Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta Mol Basis Dis 1822(5):753–783. https://doi.org/10.1016/j.bbadis.2011.11.002
Cangemi R, Friedmann AJ, Holloszy JO, Fontana L (2010) Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 9(2):236–242. https://doi.org/10.1111/j.1474-9726.2010.00553.x
Chaix A, Zarrinpar A, Miu P, Panda S (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20(6):991–1005. https://doi.org/10.1016/j.cmet.2014.11.001
Chalkiadaki A, Guarente L (2012) High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 16:180–188. https://doi.org/10.1016/j.cmet.2012.07.003
Chen Y, Cai J, Murphy TJ, Jones DP (2002) Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem 277(36):33242–33248. https://doi.org/10.1074/jbc.M202026200
Chen H, Chomyn A, Chan DC (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280(28):26185–26192. https://doi.org/10.1074/jbc.M503062200
Chen L, Gong Q, Stice JP, Knowlton AA (2009) Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84(1):91–99. https://doi.org/10.1093/cvr/cvp181
Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV (2014) Mitochondrial aging and age-related dysfunction of mitochondria. Bio Med Res Int 2014:238463. https://doi.org/10.1155/2014/238463
Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, CALERIE Pennington Team (2007) Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4(3):e76. https://doi.org/10.1371/journal.pmed.0040076
Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L (2001) Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292(5514):104–106. https://doi.org/10.1126/science.1057991
Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, belenky RA, Saeed S, Carre JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP (2011) Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab 13(3):340–350. https://doi.org/10.1016/j.cmet.2011.02.003
Copeland JM, Cho J, Lo T Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW (2009) Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol 19(19):1591–1598. https://doi.org/10.1016/j.cub.2009.08.016
Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V (2013) Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: Mitochondria as a “chi”. Immun Ageing 10(1):1–3. https://doi.org/10.1186/1742-4933-10-15
Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ (2000) Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141(7):2608–2613. https://doi.org/10.1210/endo.141.7.7586
Cox KH, Pipingas A, Scholey AB (2015) Investigation of the effects of solid lipid curcumin on cognition and mood in ahealthy older population. J Psychopharmacol 29:642–651. https://doi.org/10.1177/0269881114552744
Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z (2007) Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart CircPhysiol 293(2):H919–H927. https://doi.org/10.1152/ajpheart.01287.2006
Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z (2008) Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart CircPhysiol 295(5):H1882–H1894. https://doi.org/10.1152/ajpheart.412.2008
Cybulski N, Hall MN (2009) TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34(12):620–627. https://doi.org/10.1016/j.tibs.2009.09.004
Daum B, Walter A, Horst A, Osiewacz HD, Kühlbrandt W (2013) Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc Natl Acad Sci 110(38):15301–15306. https://doi.org/10.1073/pnas.1305462110
Day EA, Ford RJ, Steinberg GR (2017) AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab 28(8):545–560. https://doi.org/10.1016/j.tem.2017.05.004
de Almeida AF, Curi R, Newsholme P, Newsholme EA (1989) Maximal activities of key enzymes of glutaminolysis, glycolysis, Krebs cycle and pentose-phosphate pathway of several tissues in mature and aged rats. Int J Biochem 21(8):937–940. https://doi.org/10.1016/0020-711x(89)90295-4
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381(26):2541–2551. https://doi.org/10.1056/nejmc2001176
Denzer I, Muench G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103:80–94. https://doi.org/10.1016/j.phrs.2015.11.019
Dias GP, Murphy T, Stangl D, Ahmet S, Morisse B, Nix A, Aimone LJ, Aimone JB, Kuro-O M, Gage FH, Thuret S (2021) Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol Psychiatry 26(11):6365–6379. https://doi.org/10.1038/s41380-021-01102-4
Dominick G, Berryman DE, List EO, Kopchick JJ, Li X, Miller RA, Garcia GG (2015) Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology 156(2):565–575. https://doi.org/10.1210/en.2014-1690
Dorn GW, Scorrano L (2010) Two close, too close: sarcoplasmic reticulum-mitochondrial cross-talk and cardiomyocyte fate. Circulation Res 107(6):689–699. https://doi.org/10.1161/CIRCRESAHA.110.225714
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Emelyanova L, Preston C, Gupta A, Viqar M, Negmadjanov U, Edwards S, Kraft K, Devana K, Holmuhamedov E, O’Hair D, Tajik AJ, Jahangir A (2018) Effect of aging on mitochondrial energetics in the human atria. J Gerontol A Biol Sci Med Sci 73(5):608–616. https://doi.org/10.1093/gerona/glx160
Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA (2017) NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med 23(10):899–916. https://doi.org/10.1016/j.molmed.2017.08.001
Fannjiang Y, Cheng W-C, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basañez G, Hardwick JM (2004) Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev 18:2785–2797. https://doi.org/10.1101/gad.1247904
Feng J, Bussière F, Hekimi S (2001) Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell 1(5):633–644. https://doi.org/10.1016/s1534-5807(01)00071-5
Finkel T (2015) The metabolic regulation of aging. Nat Med 21(12):1416–1423. https://doi.org/10.1038/nm.3998
Fontana L, Cummings NE, ArriolaApelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME, lchokersahebkar DW (2016) Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep 16(2):520–530. https://doi.org/10.1016/j.celrep.2016.05.092
Freitas I, Boncompagni E, Tarantola E, Gruppi C, Bertone V, Ferrigno A, Milanesi G, Vaccarone R, Tira ME, Vairetti M (2016) In situ evaluation of oxidative stress in rat fatty liver induced by a methionine- and choline-deficient diet. Oxid Med Cell Longev. https://doi.org/10.1155/2016/9307064
Friedman DB, Johnson TE (1988) A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118(1):75–86. https://doi.org/10.1093/genetics/118.1.75
Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. BiochemBiophys Res Commun 273(2):793–798. https://doi.org/10.1006/bbrc.2000.3000
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, Mauiri MC, Martin SJ, Penninger J, Piacentini M, Rubinsztein DC, Simon H-U, Simonsen A, Thorburn AM, Velasco G, Ryan KM, Kroemer G (2015) Autophagy in malignant transformation and cancer progression. EMBO J 34(7):856–880. https://doi.org/10.15252/embj.201490784
Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X (2009) ULK1.ATG13.FIP200 complex mediates mTOR signalling and is essential for autophagy. J BiolChem 284(18):12297–12305. https://doi.org/10.1074/jbc.M900573200
García-Martínez JM, Alessi DR (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416(3):375–385. https://doi.org/10.1042/bj20081668
Garratt M, Bower B, Garcia GG, Miller RA (2017) Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 16(6):1256–1266. https://doi.org/10.1111/acel.12656
Genova ML, Lenaz G (2015) The interplay between respiratory supercomplexes and ROS in aging. Antioxid Redox Signal 23(3):208–238. https://doi.org/10.1089/ars.2014.6214
Gilmore TD, Wolenski FS (2012) NF-κB: where did it come from and why? Immunol Rev 246(1):14–35. https://doi.org/10.1111/j.1600-065x.2012.01096.x
Giorgi C, Marchi S, Simoes IC, Ren Z, Morciano G, Perrone M, Patalas-Krawczyk P, Borchard S, Jędrak P, Pierzynowska K, Szymański J, Wieckowski MR (2018) Mitochondria and reactive oxygen species in aging and age-related diseases. Int Rev Cell Mol Biol 340:209–344. https://doi.org/10.1016/bs.ircmb.2018.05.006
Gomez LA, Hagen TM (2012) Age-related decline in mitochondrial bioenergetics: does supercomplex destabilization determine lower oxidative capacity and higher superoxide production? Semin Cell Dev Biol 23(7):758–767. https://doi.org/10.1016/j.semcdb.2012.04.002
Gouspillou G, Sgarioto N, Norris B, Barbat-Artigas S, Aubertin-Leheudre M, Morais JA, Burelle Y, Taivassalo T, Hepple RT (2014) The relationship between muscle fiber type-specific PGC-1α content and mitochondrial content varies between rodent models and humans. PLoS ONE 9(8):e103044. https://doi.org/10.1371/journal.pone.0103044
Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A (2007) An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. CurrBiol 17(19):1646–1656. https://doi.org/10.1016/j.cub.2007.08.047
Guarente L (2011) Sirtuins, aging, and metabolism. Cold Spring HarbSymp Quant Biol 76:81–90. https://doi.org/10.1101/sqb.2011.76.010629
Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27(19):2072–2085. https://doi.org/10.1101/gad.227439.113
Guarente L (2014) Aging research-where do we stand and where are we going? Cell 159(1):15–19. https://doi.org/10.1016/j.cell.2014.08.041
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11(6):859–871. https://doi.org/10.1016/j.devcel.2006.10.007
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 16(70):3. https://doi.org/10.1126/scitranslmed.3001845
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226. https://doi.org/10.1016/j.molcel.2008.03.003
Hadem IKH, Majaw T, Sharma R (2020) Interplay between nutrient sensing molecules during aging and longevity. In: Rath PC (ed) Models, Molecules and Mechanisms in Biogerontology: Cellular Processes, Metabolism and Diseases. Springer Nature, Singapore, pp 393–417
Hafizi Abu Bakar M, Kian Kai C, Wan Hassan WN, Sarmidi MR, Yaakob H, Zaman Huri H (2015) Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: the roles of long chain fatty acids. Diabetes Metab Res Rev 31(5):453–475. https://doi.org/10.1002/dmrr.2601
Hall JA, Dominy JE, Lee Y, Puigserver P (2013) The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest 123(3):973–979. https://doi.org/10.1172/jci64094
Hall AR, Burke N, Dongworth RK, Hausenloy DJ (2014) Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol 171(8):1890–1906. https://doi.org/10.1111/bph.12516
Han B, Sivaramakrishnan P, Lin C-CJ, Neve IAA, He J, Tay LWR, Sowa JN, Sizovs A, Du G, Wang J, Herman C, Wang MC (2017) Microbial genetic composition tunes host longevity. Cell 169(7):1249–1262e13. https://doi.org/10.1016/j.cell.2017.05.036
Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C (2007) Lifespan extension by conditions that inhibit translation in Caenorhabditiselegans. Aging Cell 6(1):95–110. https://doi.org/10.1111/j.1474-9726.2006.00267.x
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 13(4):251–62. https://doi.org/10.1038/nrm3311
Hardie DG, Schaffer BE, Brunet A (2016) AMPK: an Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol 26(3):190–201. https://doi.org/10.1016/j.tcb.2015.10.013
Harman D (1956) Aging: a theory based on free radicals and radiation chemistry. J Gerontol 11(3):288–300. https://doi.org/10.1093/geronj/11.3.298
Harman D (1972) The biologic clock: the mitochondria? J Am GeriatrSoc 20(4):145–147. https://doi.org/10.1111/j.1532-5415.1972.tb00787.x
Hasan-Olive MM, Lauritzen KH, Ali M, Rasmussen LJ, Storm-Mathisen J, Bergersen LH (2019) A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem Res 44(1):22–37. https://doi.org/10.1007/s11064-018-2588-6
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15(6):848–60. https://doi.org/10.1016/j.cmet.2012.04.019
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 271(44):27879–87. https://doi.org/10.1074/jbc.271.44.27879
Herbener GH (1976) A morphometric study of age-dependent changes in mitochondrial populations of mouse liver and heart. J Gerontol 31(1):8–12. https://doi.org/10.1093/geronj/31.1.8
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135. https://doi.org/10.1038/nrm.2017.95
Hernandez AR, Hernandez CM, Truckenbrod LM, Campos KT, McQuail JA, Bizon JL, Burke SN (2019) Age and Ketogenic Diet Have Dissociable Effects on Synapse-Related Gene Expression Between Hippocampal Subregions. Front Aging Neurosci. 13(11):239. https://doi.org/10.3389/fnagi.2019.00239
Hirota Y, Kang D, Kanki (2012) The physiological role of mitophagy: new insights into phosphorylation events. Int J Cell Biol 2012:354914. https://doi.org/10.1155/2012/354914
Hofer SJ, Davinelli S, Bergmann M, Scapagnini G, Madeo F (2021) Caloric Restriction Mimetics in Nutrition and Clinical Trials. Front Nutr 8:717343. https://doi.org/10.3389/fnut.2021.717343
Hong YH, Varanasi US, Yang W, Leff T (2003) AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem 278(30):27495–27501. https://doi.org/10.1074/jbc.m304112200
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238. https://doi.org/10.1038/nrm3293
Hunt ND, Li GD, Zhu M, Miller M, Levette A, Chachich ME, Spangler EL, Allard JS, Hyun DH, Ingram DK, de Cabo R (2012) Effect of calorie restriction and refeeding on skin wound healing in the rat. Age (Dordr) 34(6):1453–1458. https://doi.org/10.1007/s11357-011-9321-6
Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280(32):29060–29066. https://doi.org/10.1074/jbc.m503824200
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403(6771):795–800. https://doi.org/10.1038/35001622
Ingram DK, Roth GS (2015) Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev 20:46–62. https://doi.org/10.1016/j.arr.2014.11.005
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. caosmithCell 115(5):577–590. https://doi.org/10.1016/s0092-8674(03)00929-2
Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, Fujimoto S, Terada Y (2013) Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol 305(4):F495–509. https://doi.org/10.1152/ajprenal.00642.2012
Iyer A, Brown L, Whitehead JP, Prins JB, Fairlie DP (2015) Nutrient and immune sensing are obligate pathways in metabolism, immunity, and disease. FASEB J 29(9):3612–3625. https://doi.org/10.1096/fj.15-271155
Jensen NJ, Nilsson M, Ingerslev JS, Olsen DA, Fenger M, Svart M, Møller N, Zander M, Miskowiak KW, Rungby J (2020) Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur J Endocrinol 182(2):233–242. https://doi.org/10.1530/eje-19-0710
Jheng H-F, Tsai P-J, Guo S-M, Kuo L-H, Chang C-S, Su I-J, Chang C-R, Tsai Y-S (2012) Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32(2):309–319. https://doi.org/10.1128/MCB.05603-11
Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS (2015) Modulating mTOR in aging and health. Interdiscip Top Gerontol 40:107–127. https://doi.org/10.1159/000364974
Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ES, Quintana-Ascencio PF, Caswell H, Baudisch A, Vaupel JW (2014) Diversity of ageing across the tree of life. Nature 505(7482):169–173. https://doi.org/10.1038/nature12789
Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LMD, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C (2012) The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11(5):801–809. https://doi.org/10.1111/j.1474-9726.2012.00844.x
Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, Prolla TA, Leeuwenburgh C (2013) Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS ONE 8(7):e69327. https://doi.org/10.1371/journal.pone.0069327
Jung Kim M (2018) Betaine enhances the cellular survival via mitochondrial fusion and fission factors, MFN2 and DRP1. Anim Cells Syst 22(5):289–298. https://doi.org/10.1080/19768354.2018.1512523
Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK (2005) Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310(5751):1193–1196. https://doi.org/10.1126/science.1115535
Kaletsky R, Murphy CT (2010) The role of insulin/IGF-like signaling in C. elegans longevity and aging. Dis Model Mech 3(7–8):415–419. https://doi.org/10.1242/dmm.001040
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S (2004) Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14(10):885–890. https://doi.org/10.1016/j.cub.2004.09.057
Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR (2007) Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell 6(6):827–839. https://doi.org/10.1111/j.1474-9726.2007.00346.x
Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K (2002) Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277(6):3829–3835. https://doi.org/10.1074/jbc.m107895200
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F (2014) Geroscience: linking aging to chronic disease. Cell 159(4):709–713. https://doi.org/10.1016/j.cell.2014.10.039
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366(6454):461–464. https://doi.org/10.1038/366461a0
Kenyon C (2005) The plasticity of aging: insights from long-lived mutants. Cell 120(4):449–60. https://doi.org/10.1016/j.cell.2005.02.002
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. https://doi.org/10.1038/ncb2152
Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277(5328):942–946. https://doi.org/10.1126/science.277.5328.942
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721. https://doi.org/10.1126/science.290.5497.1717
Kojima T, Kamei H, Aizu T, Arai Y, Takayama M, Nakazawa S, Ebihara Y, Inagaki H, Masui Y, Gondo Y, Sakaki Y, Hirose N (2004) Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. ExpGerontol 39(11–12):1595–1598. https://doi.org/10.1016/j.exger.2004.05.007
Koubova J, Guarente L (2003) How does calorie restriction work? Genes Dev 17(3):313–321. https://doi.org/10.1101/gad.1052903
Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17(8):1049–1061. https://doi.org/10.1038/ncb3195
Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD (2007) Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell 6(5):607–618. https://doi.org/10.1111/j.1474-9726.2007.00312.x
Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz Z (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci 97(11):5807–5811. https://doi.org/10.1073/pnas.110148297
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122(Pt 20):3589–3594. https://doi.org/10.1242/jcs.051011
Leduc-Gaudet J-P, Picard M, St-Jean Pelletier F, Sgarioto N, Auger M-J, Vallée J, Robitaille R, St-Pierre DH, Gouspillou G (2015) Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6(20):17923–17937. https://dx.doi.org/10.18632%2Foncotarget.4235
Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130(3):440–455. https://doi.org/10.1016/j.cell.2007.05.058
Lee JH, Budanov AV, Karin M (2013) Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 18(6):792–801. https://doi.org/10.1016/j.cmet.2013.08.018
Lenhare L, Crisol BM, Silva VR, Katashima CK, Cordeiro AV, Pereira KD, Luchessi AD, da Silva AS, Cintra DE, Moura LP, Pauli JR, Ropelle ER (2017) Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp Gerontol 97:17–21. https://doi.org/10.1016/j.exger.2017.07.009
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. https://doi.org/10.1016/j.cell.2007.12.018
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388. https://doi.org/10.1016/j.cmet.2011.03.009
Li B, Liu J, Gu G, Han X, Zhang Q, Zhang W (2020) impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J Neurochem 154(5):502–518. https://doi.org/10.1111/jnc.15001
Li X, Zhang W, Cao Q, Wang Z, Zhao M, Xu L, Zhuang Q (2020) Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov 6(1):1–4. https://doi.org/10.1038/s41420-020-00316-9
Lin K, Dorman JB, Rodan A, Kenyon C (1997) daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278(5341):1319–1322. https://doi.org/10.1126/science.278.5341.1319
Lipsky MS, King M (2015) Biological theories of aging. Dis Mon 61(11):460–466. https://doi.org/10.1016/j.disamonth.2015.09.005
Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, Saito T, Saido T, Zhu J, Wu LJ, Mattson MP (2019) SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun 10(1):1886. https://doi.org/10.1038/s41467-019-09897-1
Lloret A, Badia MC, Mora NJ, Pallardó FV, Alonso MD, Vina J (2009) Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimer’s Dis 17(1):143–149. https://doi.org/10.3233/JAD-2009-1033
Loewith R, Hall MN (2011) Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189(4):1177–1201. https://doi.org/10.1534/genetics.111.133363
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19(2):181–192. https://doi.org/10.1016/j.cmet.2013.12.008
López-Lluch G (2017) Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech Ageing Dev 162:108–121. https://doi.org/10.1016/j.mad.2016.12.005
López-Lluch G, Navas P (2016) Calorie restriction as an intervention in ageing. J Physiol 594(8):2043–2060. https://doi.org/10.1113/jp270543
López-Lluch G, Hernández-Camacho JD, Fernández-Ayala DJM, Navas P (2018) Mitochondrial dysfunction in metabolism and ageing: shared mechanisms and outcomes? Biogerontology 19(6):461–480. https://doi.org/10.1007/s10522-018-9768-2
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G (2016) Metabolic Control of Longevity. Cell 166(4):802–821. https://doi.org/10.1016/j.cell.2016.07.031
Lorenzini A, Salmon AB, Lerner C, Torres C, Ikeno Y, Motch S, McCarter R, Sell C (2014) Mice producing reduced levels of insulin-like growth factor type 1 display an increase in maximum, but not mean, life span. J Gerontol A Biol Sci Med Sci 69(4):410–419. https://doi.org/10.1093/gerona/glt108
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science. 307(5708):384–7. https://doi.org/10.1126/science.1104343
Luo H, Chiang HH, Louw M, Susanto A, Chen D (2017) Nutrient Sensing and the Oxidative Stress Response. Trends EndocrinolMetab 28(6):449–460. https://doi.org/10.1016/j.tem.2017.02.008
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318. https://doi.org/10.1038/nrm2672
Madeo F, Zimmermann A, Maiuri MC, Kroemer G (2015) Essential role for autophagy in life span extension. J Clin Invest 125(1):85–93. https://doi.org/10.1172/jci73946
Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M (2010) Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci 123(6):917–926. https://doi.org/10.1242/jcs.059246
Makino N, Oyama J, Maeda T, Koyanagi M, Higuchi Y, Shimokawa I, Mori N, Furuyama T (2016) FoxO1 signaling plays a pivotal role in the cardiac telomere biology responses to calorie restriction. Mol Cell Biochem 412(1–2):119–130. https://doi.org/10.1007/s11010-015-2615-8
Malena A, Loro E, Di Re M, Holt IJ, Vergani L (2009) Inhibition of mitochondrial fission favours mutant over wild-type mitochondrial DNA. Hum Mol Genet 18(18):3407–3416. https://doi.org/10.1093/hmg/ddp281
Mancuso C, Pani G, Calabrese V (2006) Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep 11(5):207–213. https://doi.org/10.1179/135100006X154978
Manczak M, Kandimalla R, Fry D, Sesaki H, Reddy PH (2016) Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum Mol Genet 25(23):5148–5166. https://doi.org/10.1093/hmg/ddw330
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB (2014) mTOR inhibition improves immune function in the elderly. Sci Transl Med 6(268):268ra179. https://doi.org/10.1126/scitranslmed.3009892
Mao K, Wang K, Liu X, Klionsky DJ (2013) The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Dev Cell 26(1):9–18. https://doi.org/10.1016/j.devcel.2013.05.024
Marinho HS, Real C, Cyrne L, Soares H, Antunes F (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox biol 2:535–562. https://doi.org/10.1016/j.redox.2014.02.006
Martel J, Ojcius DM, Ko YF, Ke PY, Wu CY, Peng HH, Young JD (2019) Hormetic effects of phytochemicals on health and longevity. Trends Endocrin Metab 30(6):335–346. https://doi.org/10.1016/j.tem.2019.04.001
Martin-Montalvo A, de Cabo R (2013) Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal 19(3):310–320. https://doi.org/10.1089/ars.2012.4866
Mattson MP, Arumugam TV (2018) Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab 27(6):1176–1199. https://doi.org/10.1016/j.cmet.2018.05.011
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13(9):1016–1023. https://doi.org/10.1038/ncb2329
Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N (2014) Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 13(4):769–771. https://doi.org/10.1111/acel.12213
Miquel J, Economos AC, Fleming J, Johnson JE Jr (1980) Mitochondrial role in cell aging. Exp Gerontol 15(6):575–591. https://doi.org/10.1016/0531-5565(80)90010-8
Miquel S, Champ C, Day J, Aarts E, Bahr BA, Bakker M, Bánáti D, Calabrese V, Cederholm T, Cryan J, Dye L (2018) Poor cognitive ageing: vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res Rev 42:40–55. https://doi.org/10.1016/j.arr.2017.12.004
Mocchegiani E (2016) Nutrition in the Elderly: General Aspects. In: Malavolta M, Mocchegiani E (eds) Molecular basis of Nutrition and Ageing. Academic Press Elsevier, London, United Kingdom, pp 41–56
Moosmann B, Behl C (2008) Mitochondrially encoded cysteine predicts animal lifespan. Aging Cell 7(1):32–46. https://doi.org/10.1111/j.1474-9726.2007.00349.x
Moreno-Loshuertos R, Enríquez JA (2016) Respiratory supercomplexes and the functional segmentation of the CoQ pool. Free Radic Biol Med 100:5–13. https://doi.org/10.1016/j.freeradbiomed.2016.04.018
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280(16):16456–16460. https://doi.org/10.1074/jbc.m501485200
Orr WC, Sohal RS (1994) Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263(5150):1128–1130. https://doi.org/10.1126/science.8108730
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL 3rd, Goodyear LJ, Tong Q (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging 1(9):771–783. https://doi.org/10.18632/aging.100075
Pamplona R, Barja G, Portero-Otin M (2002) Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann N Y Acad Sci 959(1):475–490. https://doi.org/10.1111/j.1749-6632.2002.tb02118.x
Parkinson Study Group (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 328(3):176–183. https://doi.org/10.1056/NEJM199301213280305
Patti ME, Corvera S (2010) The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev 31(3):364–95. https://doi.org/10.1210/er.2009-0027
Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A (2009) Is the oxidative stress theory of aging dead? Biochim Biophys Acta 1790(10):1005–1014. https://doi.org/10.1016/j.bbagen.2009.06.003
Perrone CE, Malloy VL, Orentreich DS, Orentreich N (2013) Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp Gerontol 48(7):654–660. https://doi.org/10.1016/j.exger.2012.07.005
Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G (2008) Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med 46(1):88–94. https://doi.org/10.1016/j.freeradbiomed.2008.09.031
Polito L, Kehoe PG, Forloni G, Albani D (2010) The molecular genetics of sirtuins: association with human longevity and age-related diseases. Int J Mol Epidemiol Genet 1(3):214–225
Rambold AS, Kostelecky B, Lippincott-Schwartz J (2011) Fuse or die: Shaping mitochondrial fate during starvation. Commun Integr Biol 4(6):752–754. https://doi.org/10.4161/cib.17667
Rana A, Oliveira MP, Khamoui AV, Aparicio R, Rera M, Rossiter HB, Walker DW (2017) Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat Commun 8:448. https://doi.org/10.1038/s41467-017-00525-4
Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H (2014) Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1$25 million people. Lancet 383(9932):1899–1911. https://doi.org/10.1016/S0140-6736(14)60685-1
Rebrin I, Sohal RS (2004) Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol 39(10):1513–1519. https://doi.org/10.1016/j.exger.2004.08.014
Reddy PH, Manczak M, Yin X (2017) Mitochondria-division inhibitor 1 protects against amyloid-β induced mitochondrial fragmentation and synaptic damage in Alzheimer’s disease. J Alzheimer’s Dis 58(1):147–162. https://doi.org/10.3233/JAD-170051
Reeve AK, Grady JP, Cosgrave EM, Bennison E, Chen C, Hepplewhite PD, Morris CM (2018) Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson’s disease. NPJ Parkinson’s Dis 4:9. https://doi.org/10.1038/s41531-018-0044-6
Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45(6):410–418. https://doi.org/10.1016/j.exger.2010.03.014
Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA (2017) A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab 26(3):539–546e5. https://doi.org/10.1016/j.cmet.2018.04.005
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434(7029):113–118. https://doi.org/10.1038/nature03354
Romanello V, Sandri M (2016) Mitochondria quality control and muscle mass maintenance. Front Physiol 6:422. https://doi.org/10.3389/fphys.2015.00422
Rossetti L (2000) Perspective: Hexosamines and nutrient sensing. Endocrinology 141(6):1922–1925. https://doi.org/10.1210/endo.141.6.7566
Rottenberg H, Hoek JB (2017) The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 16(5):943–955. https://doi.org/10.1111/acel.12650
Ruiz R, Pérez-Villegas EM, Manuel Carrión Á (2016) AMPK Function in Aging Process. Curr Drug Targets 17(8):932–941. https://doi.org/10.2174/1389450116666151102095825
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 59(1):138–43. https://doi.org/10.1016/j.jhep.2013.02.012
Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH (1994) RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78(1):35–43. https://doi.org/10.1016/0092-8674(94)90570-3
Salminen A, Hyttinen JM, Kaarniranta K (2011) AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 89(7):667–676. https://doi.org/10.1007/s00109-011-0748-0
Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med 336(17):1216–1222. https://doi.org/10.1056/NEJM199704243361704
Santos RX, Correia SC, Zhu X, Smith MA, Moreira PI, Castellani RJ, Nunomura A, Perry G (2013) Mitochondrial DNA oxidative damage and repair in aging and Alzheimer’s disease. Antioxid Redox Signal 18(18):2444–2457. https://doi.org/10.1089/ars.2012.5039
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101. https://doi.org/10.1126/science.1106148
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168. https://doi.org/10.1016/j.molcel.2006.03.029
Saxton RA, Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 168(6):960–976. https://doi.org/10.1016/j.cell.2017.02.004
Scheckhuber CQ, Erjavec N, Tinazli A, Hamann A, Nyström T, Osiewacz HD (2007) Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat Cell Biol 9:99–105. https://doi.org/10.1038/ncb1524
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308(5730):1909–1911. https://doi.org/10.1126/science.1106653
Sharma R (2004) Dietary restriction and its multifaceted effects. CurrSci 87:1203–1210
Sharma R, Dkhar P (2014) Biological Basis of Ageing: theories and explanations. In: Sanchetee P (ed) Textbook of Geriatric Medicine. Indian Academy of Geriatrics, Paras Medical Publisher, Hyderabad. pp 24–31, pp 24–31
Sharma A, Smith HJ, Yao P, Mair WB (2019) Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep 20(12):e48395. https://doi.org/10.15252/embr.201948395
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci 101(10):3329–3335. https://doi.org/10.1073/pnas.0308061100
Shi D, Zhao D, Niu P, Zhu Y, Zhou J, Chen H (2018) Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells. BMC Complement Altern Med 18(1):317. https://doi.org/10.1186/s12906-018-2380-9
Shimobayashi M, Hall MN (2014) Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15(3):155–162. https://doi.org/10.1038/nrm3757
Siddle K (2011) Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 47(1):R1–10. https://doi.org/10.1530/jme-11-0022
Sies H (2017) Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox biol 11:613–619. https://doi.org/10.1016/j.redox.2016.12.035
Silva JP, Wahlestedt C (2010) Role of Sirtuin 1 in metabolic regulation. Drug Discov Today 15(17–18):781–791. https://doi.org/10.1016/j.drudis.2010.07.001
Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L (2011) Long-term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging 3(4):374–379. https://doi.org/10.18632/aging.100280
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273(5271):59–63. https://doi.org/10.1126/science.273.5271.59
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143(5):802–812. https://doi.org/10.1016/j.cell.2010.10.002
Soultoukis GA, Partridge L (2016) Dietary Protein, Metabolism, and Aging. Annu Rev Biochem 85:5–34. https://doi.org/10.1146/annurev-biochem-060815-014422
Speakman JR, Mitchell SE (2011) Caloric restriction. Mol Aspects Med 32(3):159–221. https://doi.org/10.1016/j.mam.2011.07.001
Spinelli JB, Yoon H, Ringel AE, Jeanfavre S, Clish CB, Haigis MC (2017) Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358(6365):941–946. https://doi.org/10.1126/science.aam9305
Steinberg GR, Kemp BE (2009) AMPK in Health and Disease. Physiol Rev 89(3):1025–1078. https://doi.org/10.1152/physrev.00011.2008
Steinberg GR, O’Neill HM, Dzamko NL, Galic S, Naim T, Koopman R, Jørgensen SB, Honeyman J, Hewitt K, Chen ZP, Schertzer JD, Scott JW, Koentgen F, Lynch GS, Watt MJ, van Denderen BJ, Campbell DJ, Kemp BE (2010) Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J Biol Chem 285(48):37198–37209. https://doi.org/10.1074/jbc.m110.102434
Sterky FH, Lee S, Wibom R, Olson L, Larsson NG (2011) Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci 108(31):12937–12942. https://doi.org/10.1073/pnas.1103295108
Suchiang K, Sharma R (2011) Dietary restriction regulates brain acetylcholinesterase in female mice as a function of age. Biogerontology 12(6):581–589. https://doi.org/10.1007/s10522-011-9356-1
Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P (2008) Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci 105(9):3438–3442. https://doi.org/10.1073/pnas.0705467105
Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A (2013) Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2:e01098. https://doi.org/10.7554/eLife.01098.001
Sundaram S, Yan L (2016) Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res 36(6):603–611. https://doi.org/10.1016/j.nutres.2016.02.005
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7(2):85–96. https://doi.org/10.1038/nrm1837
Templeman NM, Murphy CT (2018) Regulation of reproduction and longevity by nutrient-sensing pathways. J Cell Biol 217(1):93–106. https://doi.org/10.1083/jcb.201707168
Testa G, Biasi F, Poli G, Chiarpotto E (2014) Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Curr Pharm Des 20(18):2950–2977. https://doi.org/10.2174/13816128113196660699
Thomenius M, Freel CD, Horn S, Krieser R, Abdelwahid E, Cannon R, Balasundaram S, White K, Kornbluth S (2011) Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death. Cell Death Differ 18:1640–1650. https://doi.org/10.1038/cdd.2011.26
Thompson LJ, Strittmatter L, Tardif J, Sharma R, Tremblay-Vaillancourt V, Aubut C, Boucher G, Clish CB, Cyr D, Daneault C, Waters PJ (2015) A metabolic signature of mitochondrial dysfunction revealed through a monogenic form of leigh syndrome. Cell Rep 13(5):981–989. https://doi.org/10.1016/j.celrep.2015.09.054
Tilokani L, Nagashima S, Paupe V, Prudent J (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62(3):341–360. https://doi.org/10.1042/EBC20170104
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410(6825):227–230. https://doi.org/10.1038/35065638
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429(6990):417–423. https://doi.org/10.1038/nature02517
Trovato Salinaro A, Pennisi M, Di Paola R, Scuto M, Crupi R, Cambria MT, Ontario ML, Tomasello M, Uva M, Maiolino L, Calabrese EJ (2018) Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms. Immun Ageing 15(1):1–8. https://doi.org/10.1186/s12979-017-0108-1
Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, Academia EC, O’Leary MN, Ashe TD, La Spada AR, Kennedy BK (2015) Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J Clin Invest 125(8):2952–2964. https://doi.org/10.1172/jci77361
Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK (2008) Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132(6):1025–1038. https://doi.org/10.1016/j.cell.2008.01.030
van der Spoel E, Rozing MP, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJ, Westendorp RG, van Heemst D (2015) Association analysis of insulin-like growth factor-1 axis parameters with survival and functional status in nonagenarians of the Leiden Longevity Study. Aging 7(11):956–963. https://doi.org/10.18632/aging.100841
Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426(6967):620. https://doi.org/10.1038/426620a
Vincent AE, Ng YS, White K, Davey T, Mannella C, Falkous G, Feeney C, Schaefer AM, McFarland R, Gorman GS, Taylor RW, Turnbull DM, Picard M (2016) The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci Rep 6(1):30610. https://doi.org/10.1038/srep30610
Wai T, García-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Rupérez FJ, Barbas C, Iba ~ nez B, Langer T (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265):aad0116. https://doi.org/10.1126/science.aad0116
Wang M, Xu Y, Liu J, Ye J, Yuan W, Jiang H, Wang Z, Jiang H, Wan J (2017) Recent insights into the biological functions of sestrins in health and disease. Cell Physiol Biochem 43:1731–1741. https://doi.org/10.1159/000484060
Weber TA, Reichert AS (2010) Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol 45(7–8):503–511. https://doi.org/10.1016/j.exger.2010.03.018
Weindruch R, Sohal RS (1997) Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med 337(14):986–994. https://doi.org/10.1056/nejm199710023371407
Wellen KE, Thompson CB (2010) Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell 40(2):323–332. https://doi.org/10.1016/j.molcel.2010.10.004
Whitehall JC, Greaves LC (2020) Aberrant mitochondrial function in ageing and cancer. Biogerontology 21(4):445–459. https://doi.org/10.1007/s10522-019-09853-y
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13(22):2004–2008. https://doi.org/10.1016/j.cub.2003.10.031
Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F (2011) Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 60(3):746–756. https://doi.org/10.2337/db10-1246
Yang W, Hekimi S (2010) A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8(12):e1000556. https://doi.org/10.1371/journal.pbio.1000556
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5590. https://doi.org/10.1074/jbc.m404751200
Yang CC, Chen D, Lee SS, Walter L (2011) The dynamin-related protein DRP-1 and the insulin signaling pathway cooperate to modulate Caernorhabditis elegans longevity. Aging Cell 10(4):724–728. https://doi.org/10.1111/j.1474-9726.2011.00711.x
Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY (2012) Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 75(3):425–436. https://doi.org/10.1016/j.neuron.2012.03.043
Yang W, Cao M, Mao X, Wei X, Li X, Chen G, Zhang J, Wang Z, Shi J, Huang H, Yao X, Liu C (2016) Alternate-day fasting protects the livers of mice against high-fat diet-induced inflammation associated with the suppression of Toll-like receptor 4/nuclear factor κB signaling. Nutr Res. 36(6):586–93. https://doi.org/10.1016/j.nutres.2016.02.001
Yen TC, Chen YS, King KL, Yeh SH, Wei YH (1989) Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun 165(3):994–1003. https://doi.org/10.1016/0006-291X(89)92701-0
Yu D, Tomasiewicz JL, Yang SE, Miller BR, Wakai MH, Sherman DS, Cummings NE, Baar EL, Brinkman JA, Syed FA, Lamming DW (2019) Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension. Cell Rep 29(1):236–248e3. https://doi.org/10.1016/j.celrep.2019.08.084
Yu Y, Wang F, Wang J, Zhang D, Zhao X (2020) Ketogenic diet attenuates aging-associated myocardial remodeling and dysfunction in mice. Exp Gerontol 140:111058. https://doi.org/10.1016/j.exger.2020.111058
Yuan M, Pino E, Wu L, Kacergis M, Soukas AA (2012) Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J Biol Chem 287(35):29579–29588. https://doi.org/10.1074/jbc.m112.386854
Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H (2009) Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci 64(12):1212–1220. https://doi.org/10.1093/gerona/glp132
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, MisraSen J, Gorospe M, Mattson MP (2019) Senolytic therapy alleviates Ab-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22:719–728. https://doi.org/10.1038/s41593-019-0372-9
Zhang G, Deighan A, Raj A, Robinson L, Donato HJ, Garland G, Leland M, Martin-McNulty B, Kolumam GA, Riegler J, Freund A, Wright KM, Churchill GA (2022) Intermittent fasting and caloric restriction interact with genetics to shape physiological health in mice. Genetics 220(1):iyab157. https://doi.org/10.1101/2021.04.02.438251
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6(6):472–483. https://doi.org/10.1016/j.cmet.2007.11.004
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327(5968):1000–1004. https://doi.org/10.1126/science.1179689
Zhao G, Cao K, Xu C, Sun A, Lu W, Zheng Y, Li H, Hong G, Wu B, Qiu Q, Lu Z (2017) Crosstalk between mitochondrial fission and oxidative stress in paraquat-induced apoptosis in mouse alveolar type II cells. Int J Biol Sci 13(7):888–900. https://dx.doi.org/10.7150%2Fijbs.18468
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–658. https://doi.org/10.1111/acel.12344
Zhu Y, Liu X, Ding X, Wang F, Geng X (2019) Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 20(1):1–16. https://doi.org/10.1007/s10522-018-9769-1
Acknowledgements
The authors would like to thank the Department of Biochemistry, North-Eastern Hill University, Shillong, for providing infrastructure and research facilities under UGC-SAP, DBT and DST-FIST, New Delhi. ET and PS thank NEHU for the UGC-Non-NET fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomtheelnganbee, E., Sah, P. & Sharma, R. Mitochondrial function and nutrient sensing pathways in ageing: enhancing longevity through dietary interventions. Biogerontology 23, 657–680 (2022). https://doi.org/10.1007/s10522-022-09978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09978-7