Abstract
Age-dependent increased risk of inflammatory bowel diseases such as ulcerative colitis is being increasingly realized, and yet therapies targeting this disorder within the purview of aging are limited. The present study attempted to assess the efficacy of green tea epigallocatechin gallate (EGCG) consumption in preventing the severity and progression of dextran sulphate sodium (DSS)-induced ulcerative colitis in 18 months old middle-aged male mice. Acute colitis was induced in animals using DSS and protective effects of EGCG consumption were examined. Different parameters related to disease progression and molecular markers related to oxi-inflammatory stress, localized and systemic cytokine response, epithelial barrier integrity, and cell cycle progression profile were evaluated. DSS treatment induced rapid and severe symptoms of colitis such as consistently increased DAI score, shortened and inflamed colon accompanied by increased levels of inflammatory proteins (TNFα/IL-6/IL-1β) in both the colon tissue and cultured splenocytes indicating exaggerated Th1 immune response. Markers of oxidative stress increased while antioxidant defences and the expression of tight junction genes in the colonic cells were attenuated. Dysregulation in the expression of cell cycle inhibitory genes (p53/p21WAF1/p16Ink4a) indicated possible induction of colitis-induced dysplasia. On the other hand, EGCG consumption strongly attenuated all the measured ostensible as well as molecular markers of the disease progression as evidenced by improved DAI score, cellular antioxidant capacity, attenuated Th1 cytokine response both in the colon and cultured splenocytes, enhanced expression of tight junction genes, and cell cycle inhibitors thereby suggesting systemic effects of EGCG. Together, these observations suggest that drinking EGCG-rich green tea can be a significant way of managing the severity of colitis during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a relatively common inflammatory bowel disease (IBD) that causes non-specific inflammatory damage and ulcers in the colonic mucosa and submucosa. Incidences of UC are increasing globally and the disease is clinically characterized by bloody diarrhoea, abdominal pain, frailty, and tenesmus. Although the cellular and molecular basis of UC are not yet fully understood; however, intestinal epithelial cell injury, chronic oxi-inflammatory stress mediated by different types of cytokines and reactive oxygen species, and compromised gut mucosal barrier integrity have been associated with the pathogenesis of UC (Kaur and Goggolidou 2020). In addition, extra-intestinal manifestations of UC including unwarranted changes in systemic immunological activity (Miao et al. 2013; Rabe et al. 2019), as well as dermatological and musculoskeletal effects have also been reported (Levine and Burakoff 2011). UC presents with an increased frequency of relapses (Liverani et al. 2016) and if not treated properly, the disease can exacerbate the risk of colon cancer and mortality (Munkholm 2003). As such, there is no specific treatment for UC, and the disease is best managed through therapies aimed at the augmentation of redox and inflammatory balance in the intestinal mucosa (Bourgonje et al. 2020).
It is important to note that until recently, IBDs such as UC and Crohn’s disease were predominantly considered diseases of the younger population. However, due to factors such as lack of a specific cure, intermittent relapses, and comorbidities associated with aging; incidences of IBDs are now increasing at an alarming rate in the elderly population and it is projected that the elderly patients of IBD may account for over one-third of total cases worldwide in the present decade (Coward et al. 2019; Faye and Colombel 2022). More significantly, it is also emerging that the development and progression of UC during aging may be more severe and detrimental as compared to the younger population which could be attributed to known predisposition to inflammatory disorders (inflamm-aging), increased senescent cell burden, and presence of senescence-associated secretory phenotype (SASP) in the elderly (Riegler et al. 2000; Bauer et al. 2010; Risques et al. 2011; Liu et al. 2020). Therefore, the elderly are at a greater risk of developing severe colitis and colitis-induced cancer which is being argued as a novel challenge in the management of IBDs (Faye and Colombel 2022). The matter is further exacerbated by the fact that elderly patients are often underrepresented in clinical drug trials of UC (Hruz et al. 2020), and thus, a necessity for specific therapeutic considerations and the needs of the elderly in treating IBDs have recently been warranted (Butter et al. 2018; Hruz et al. 2020). Given the foregoing discussion, the present study attempted to test the protective effects of green tea epigallocatechin gallate (EGCG) supplementation against dextran sulphate sodium (DSS)-induced acute UC in middle-aged male mice. We have previously observed that EGCG confers multiple anti-aging effects such as the attenuation of immunosenescence (Sharma et al. , 2017, 2019), suppression of several markers related to cellular senescence and gut dysbiosis (Kumar et al. 2019; Sharma et al. 2022), and prevention of premature senescence in aging murine peritoneal macrophages (Kumar et al. 2020). The present study further explores whether the anti-aging effects of EGCG could also be translated into an in vivo disease model of aging. This is also significant since there is a growing interest in developing phytochemicals-based natural therapies for mitigating IBDs due to safety, efficacy, and economical considerations (Somani et al. 2015; Hossen et al. 2020). In particular, popular bioactive dietary polyphenols such as quercetin (Dong et al. 2020), resveratrol (Samsami-Kor et al. 2015), kaempferol (Park et al. 2012), as well as EGCG (Brückner et al 2012; Dryden et al. 2013; Oz et al. 2013; Xu et al. 2015; Bitzer et al. 2016; Du et al. 2019) have been demonstrated to influence multiple aspects of colitis in experimental animals as well as clinical subjects. However, to the best of our knowledge, there are no reports that have tested the therapeutic effects of bioactive phytochemicals, such as EGCG, against colitis progression specifically during aging. As the significance of understanding the pathogenesis and effects of UC during aging is gradually emerging (Faye and Colombel 2022); it is only prudent to assess and identify therapeutic options against IBDs specifically during aging which is addressed in the present study. As such, we hypothesized in the current study that EGCG supplementation in middle-aged animals would protect against the severity and progression of DSS-induced acute colitis by maintenance of oxi-inflammatory homeostasis through the modulation of key cellular defence pathways. Our findings indicate that a severe form of acute colitis is induced by DSS in middle-aged animals which is robustly countered by EGCG consumption as evidenced through the protection of intestinal mucosa from inflammatory aggravation, cellular damage, and probable dysplasia mediated by enhanced antioxidant defences and maintenance of immunological homeostasis.
Materials and methods
Animal care and experimental design
Eighteen months old male Swiss albino mice weighing approximately 40–50 g were procured from the animal house facility of CSIR-IHBT, Palampur. Animals were subsequently housed in the experimental animal facility of Shoolini University, Solan under standard experimental conditions (12:12 h reversed light/dark cycle; relative humidity at 50–60%, temperature of 22 ± 2 °C, and adequate ventilation). This particular species and age of animals were chosen based on our previous experience wherein these animals display characteristic markers of immunosenescence as well as cellular senescence beginning at age 12 months with an average lifespan of ~ 24 months in our facility (Sharma et al. 2014b, 2017, 2019, 2022). Moreover, mice aged 18–24 months are considered representatives of human old age as they correspond to humans aged 56–69 years (Flurkey et al 2007). We specifically chose male mice in the present investigation based on our previous observations (Sharma et al. 2019, 2022) as well as the fact that females are generally more resistant to DSS-induced experimental colitis owing to potent anti-inflammatory action of the estrogen hormones (Bábíčková et al. 2015; Goodman et al. 2017). Animals were divided into three groups of five animals each, i.e., the control group, DSS group, and EGCG treated group. Animals of a particular group were housed together and were carefully selected for experiments ensuring that no weak or bullying animal is present in a particular group that may cause imbalanced feed/water consumption in the group. We chose to specifically test a single dose of EGCG in the present work since our previous in vivo studies have identified a working concentration of EGCG (100 mg/kg/animal/day) which is sufficient to confer anti-aging attributes against multiple aspects of immunosenescence and cellular senescence (Sharma et al. 2017, 2019, 2022). All animal experiments were conducted as per guidelines and approval of the institutional animal ethics committee of Shoolini University, Solan (Approval no. IAEC/SU/21/10 dated 10.09.2021).
Induction of DSS-induced colitis
A DSS-based ulcerative colitis model was used for testing the effects of EGCG consumption based on our previous observations (Sharma et al. 2020). In the DSS group, animals were orally administered DSS (36–50 kDa, MP Biomedicals, Ontario, USA) at 3.5% (w/v) in drinking water (~ 6 ml/animal/day/cage) for seven consecutive days followed by a two-day recovery period and subsequent sacrifice (Fig. 1A). DSS was freshly prepared after every two days during the entire course of treatment and animals were daily monitored for signs of disease induction such as weight loss, diarrhea, occult and rectal blood, and general frailty. In the EGCG group, treatment with EGCG (at 100 mg/kg/animal/day) was started 30 days before the administration of DSS and was also continued throughout the DSS administration regimen till animal sacrifice (Fig. 1A). EGCG was prepared daily and orally administered in drinking water to the animals in the morning hours (~ 9:00 AM IST). No further drinking water was provided to animals until next day that enabled them to actively consume EGCG as and when supplied. Feed and water intake of all animals were regulated (5 g feed/day/animal and 6 ml water/day/animal) and monitored daily. Control group animals were also maintained on rationed water and animal feed similar to other groups but without any DSS treatment. To further ensure appropriate sensitization, all animals were maintained on rationed feed and water for two weeks before initiating the EGCG consumption protocol based on our prior experiences and observations in aging studies (Sharma et al. 2022). At the end of the study, mice in different groups were euthanized following which the colon tissue and spleen were collected. Colon tissue length was immediately measured and then processed for downstream analyses of various molecular, biochemical, and immunological parameters as detailed in subsequent sections. Splenocytes were isolated, enumerated, and cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS in a CO2 incubator at 37 °C for 48 h following which the culture supernatant was collected, aliquoted, and stored at − 80 °C till further analyses (Sharma et al. 2014c).
EGCG treatment ameliorates the ostensible effects of DSS-induced colitis in 18 months old Swiss albino mice. A Schematic description of study design. EGCG treatment (@100 mg/kg/animal/day) was initiated 30 days prior to the DSS application and was continued along with DSS as well as during recovery period until the end of the experiment (Day 39). DSS (@3.5% w/v) was orally supplied to animals for seven consecutive days (30–36) followed by a 2 day recovery period. B Daily body weight, C Daily feed intake D Daily water intake E DAI score. Values are mean ± SD (n = 5). *Represents significant difference at p < 0.05 when compared to control; #represents significant difference at p < 0.05 when compared to DSS group
Disease activity index (DAI)
The DAI was calculated by daily recording the parameters of stool consistency, rectal bleeding, and loss of body weight as described previously (Sharma et al. 2020). To assess DAI, scores were assigned as follows: for weight loss: 0, no loss; 1, 1–5%; 2, 5–10%; 3, 10–20%; and 4, > 20%; for stool consistency: 0, normal; 1 and 2, loose stool; 3 and 4, diarrhea; and for rectal bleeding 0, normal; 1 and 2 mild occult blood; 3 and 4, gross bleeding (Sharma et al. 2020).
Estimation of interleukins
A portion of the colon tissue from each animal was homogenized in 1 × PBS (pH 7.4) containing a protease inhibitor cocktail (HiMedia, India, Cat. #ML051). The homogenized samples were centrifuged at 10,000×g for 15 min at 4 °C and their supernatants were collected, aliquoted, and stored at − 80 °C till further analyses. Sandwich ELISA was performed in the colon tissue homogenates as well as in splenocytes culture supernatant for the estimation of IL-6, TNF-α, IL-1β, and IL-10 levels using commercially available ELISA MAX™ Deluxe set kits (BioLegend, San Diego, U.S.A) as per the manufacturer’s protocol. Total proteins in the samples were determined by Bradford assay and results are expressed relative to total protein concentration.
Measurement of total antioxidant activity
Total antioxidant activity was determined in the colon tissue homogenates using Cayman’s antioxidant assay kit (Cat. #709001). The assay relies on the ability of antioxidants present in the sample to inhibit the oxidation of ABTS by metmyoglobin which is then compared with the Trolox standard. Briefly, a portion of the colon tissue from each animal was washed with ice-cold PBS containing protease inhibitor and homogenized followed by centrifugation at 10,000×g for 15 min at 4 °C. The supernatant was collected, aliquoted, and stored at − 80 °C until further analysis as per the manufacturer’s protocol. The total antioxidant capacity in the sample is quantified as millimolar Trolox equivalent.
Measurement of protein carbonyl content
Protein carbonyl content in samples was assessed using Cayman’s protein carbonyl kit (Cat. #10005020). In this assay, protein carbonyls in the tissue homogenate are measured by the amount of protein-hydrozone produced when 2,4-dinitrophenylhydrazine (DNPH) reacts with protein carbonyl which is then quantified by measuring absorbance at 370 nm. Briefly, a small section of colon tissue was homogenized in cold PBS containing EDTA followed by centrifugation at 10,000×g for 15 min at 4 °C. The supernatant was collected, aliquoted, and stored at − 80 °C until further analysis as per the manufacturer’s protocol. The total protein carbonyl present in the sample was measured in nanomoles.
Measurement of lipid peroxidation by TBARS
Thiobarbituric acid reactive substance (TBARS) assay determines the lipid peroxidation status and was quantified using Cayman’s TBARS assay kit (Cat. #10009055) as per the manufacturer’s protocol. In this method, malondialdehyde (MDA), an oxidative stress marker, is measured in the reaction of malondialdehyde-thiobarbituric acid (MDA-TBA) under high temperature which produces a colored complex that is detected spectrophotometrically. Briefly, the colon tissue was homogenized in RIPA lysis buffer (Millipore, CA, USA, Cat. #20-188) containing protease inhibitor cocktail (HiMedia, India, Cat. #ML051) followed by centrifugation at 1600×g for 10 min at 4 °C. The supernatant was collected, aliquoted, and stored at − 80 °C until further analysis as per the manufacturer’s protocol. The total lipid peroxidation in the colon sample is quantified as micromolar MDA equivalent.
RNA isolation and qRT-PCR
Samples of colon tissue were stored in RNAlater™ stabilization solution (Thermo Fisher, USA, Cat. #AM7020) till RNA isolation. Total RNA from tissue samples was isolated using the TRI-reagent (Sigma-Aldrich, USA, Cat. #T9424). Briefly, 70–80 mg of colon tissue from each animal was homogenized in TRI-reagent and total RNA was isolated as per the manufacturer’s protocol. The quality and quantity of the isolated RNA were determined and the RNA was aliquoted and stored at − 80 °C until further analysis. qRT-PCR was performed using CFX96 Touch Real-Time PCR Detection System (BioRad Inc.). In brief, 50 ng of RNA template was used per reaction using the Thermo Scientific Verso SYBR Green 1-Step qRT-PCR Low ROX Kit (Cat. #AB-4106/C) as per the manufacturer’s protocol. The list of primer sequences used is presented in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was utilized as a housekeeping control to quantify relative mRNA expression using the ΔΔCt method as previously described (Sharma et al. 2017).
Statistical analyses
Data are expressed as mean ± S.D. Significant differences among means of various groups were assessed by using one-way ANOVA followed by a Tukey’s post-hoc test. A value of p < 0.05 was considered statistically significant.
Results
EGCG improves the general health of mice during colitis
It was observed that DSS treatment induced severe symptoms of colitis in aged animals. DSS group animals showed a continuous decline in body weight as compared to the control animals starting from the second day of DSS administration and an average weight loss of 33.31% was observed at the end of the experiment (Fig. 1B). Animals also appeared frail and sedentary accompanied by evidence of occult and rectal blood along with decreased feed and water intake although no mortality was observed (Fig. 1C, D). However, once the DSS treatment regimen ended, animals were able to maintain a steady body weight state although no recovery could be established. In addition, the DAI score also registered a significant increase from the third day onwards and continued until the end of the experiment suggesting rapid and severe induction of colitis (Fig. 1E). On the other hand, EGCG-treated animals registered an average decline of 16.01% in body weight at the end of the experiment while DAI score also significantly and consistently decreased as compared to control animals beginning from the third day of DSS treatment (Fig. 1B–E).
EGCG consumption preserves the colon length
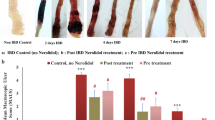
The effects of colitis on the colon tissue were first noticeable by signs of inflammation in the caecum and drastic overall shortening of the colon length. In DSS group animals, the colon length registered a significant decrease of over 51% as compared to the control group while EGCG treated animals recorded a non-significant decline of 11.51% (Fig. 2A). Representative images of colon tissue in various groups are presented in Fig. 2B. The spleen of DSS-treated animals also showed a 24% albeit non-significant increase in size as compared to control group animals suggesting signs of possible systemic inflammation while a reverse effect of EGCG treatment was observed (Fig. 2C).
EGCG treatment attenuates DSS-induced inflammatory damage in the colon
In the DSS group animals, a strong and significant general increase in various pro-inflammatory interleukins in the colon tissue homogenates was recorded as compared to the control group (Fig. 3A–C). While IL-6 levels recorded a 54.5 folds significant increase, TNF-α recorded a significant increase of 1570 folds, and IL-1β registered a significant increase of 37.14 folds as compared to the control group (Fig. 3A–C). Estimation of anti-inflammatory interleukin IL-10 also revealed an increase of 3.29 folds (Fig. 3D), although, the ratio of pro-inflammatory cytokines to IL-10 clearly revealed a strong and significant increase in the pro-inflammatory environment in the colon tissue of DSS group animals as compared to the control (Fig. 3E–G). On the other hand, EGCG treatment appeared to strongly attenuate the pro-inflammatory damage and maintain inflammatory homeostasis as evident by a robust and significant decline in all tested interleukins as compared to the DSS group animals (Fig. 3A–D). Moreover, in the case of TNF-α and IL-10, the decline due to EGCG treatment was at par with the control group (Fig. 3B, D). Similarly, when analysed relative to IL-10, EGCG treatment significantly improved the inflammatory environment which was at par with the control groups in the case of IL-6 and TNF-α (Fig. 3E, F).
EGCG treatment suppresses inflammatory stress in the colon and augments Th1/Th2 immune balance as assessed through the measurement of A IL-6, B TNF-α, C IL-1β, D IL-10, E IL-6/IL-10 ratio, F TNF-α/IL-10 ratio, G IL-1β/IL-10 ratio. Values are mean ± SD (n = 5). Significant difference amongst means presented as ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05
EGCG treatment suppresses DSS-induced systemic inflammatory activation
To assess whether DSS-induced colitis induced systemic activation of immune cells, splenocytes culture supernatants were assessed for markers of inflammatory proteins. It was observed that similar to the colon tissue, a strong and significant increase in the expression of IL-6, TNF-α, IL-1β, and IL-10 proteins was apparent in animals of the DSS group although the rate of change was much smaller than those observed in the colon tissue homogenates (Fig. 4A–D). Analyses of the pro-inflammatory to anti-inflammatory protein (IL-10) ratio also revealed a pattern similar to the colon tissue homogenates except for IL-6 (Fig. 4E–G). However, except for IL-6, EGCG-treated animals showed robust and significant suppression of all tested cytokines which were also at par with control IL-1β and TNF-α levels (Fig. 4A–D). EGCG also maintained inflammatory homeostasis as evidenced by the ratio of pro-inflammatory to anti-inflammatory cytokines which were at par with the control except for IL-1β levels (Fig. 4E–G). Further, an enhanced rate of proliferation of splenocytes was also evident since a 4.94 folds increase in total splenocytes in the DSS group as compared to the control group animals was observed (Fig. 4H). However, no effect of EGCG treatment on the numbers of total splenocytes could be observed (Fig. 4H).
EGCG treatment suppresses inflammatory cytokines in the splenocytes culture supernatant as assessed through the measurement of A IL-6, B TNF-α, C IL-1β, D IL-10, E IL-6/IL-10 ratio, F TNF-α/IL-10 ratio, G IL-1β/IL-10 ratio, H Total number of splenocytes. Values are mean ± SD (n = 5). Significant difference amongst means presented as ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05
EGCG treatment augments cellular antioxidant defences and attenuates oxidative damage
EGCG-treated animals demonstrated a robust and significant 3.29- and 1.97-folds increase in total antioxidant capacity of the colon tissues as compared to the control and DSS group animals respectively (Fig. 5A). Similarly, over 15 folds significant increase in cellular NRF-2 mRNA expression was also observed in EGCG-treated animals as compared to control (Fig. 5B). On the other hand, analyses of cellular oxidative damage through the estimation of lipid peroxidation levels revealed a strong and significant increase in DSS treated animals by a factor of 4.38 folds as compared to the control while lipid peroxidation damage in EGCG treated animals was at par with the control (Fig. 5C). Although protein carbonyl levels also appeared to increase in the DSS-fed group; however, no significant difference amongst any of the tested groups could be observed (Fig. 5D).
Influence of EGCG on colonic antioxidant capacity and oxidative damage. A Total antioxidant capacity, B NRF-2 relative gene expression, C MDA levels, D Protein carbonyl content. Values are mean ± SD (n = 5). Significant difference amongst means presented as ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05
Intestinal epithelial integrity and functions are maintained in EGCG-treated animals
To assess whether DSS-induced structural and functional damage could be ameliorated by EGCG treatment, we analysed the expression of tight junction proteins (ZO-1 and occludin) and mucous secreting proteins (mucin-2). It was observed that the DSS challenge strongly downregulated the expression of ZO-1 and occludin in colon tissue by a factor of 4.34 folds and 1176.47 folds respectively as compared to the control (Fig. 6A, B). Similarly, relative expression of Mucin-2 mRNA in DSS group animals decreased by over 3000 folds as compared to the control (Fig. 6C). On the other hand, a robust and significant increase in ZO-1, occludin, and mucin-2 mRNA expression was recorded in EGCG-treated animals as compared to both the control and DSS-treated animals (Fig. 6A–C).
DSS-induced suppression of cell cycle inhibitors is reversed by EGCG treatment
Downregulation of cell cycle inhibitors is associated with UC-associated colon carcinogenesis. In the present study, we observed a general decline in the mRNA expression of all tested cell cycle inhibitors in the DSS group as compared to the control group (Fig. 7A–C). Specifically, a 33.3 folds decrease in p53 expression, 47.6 folds downregulation in p16Ink4a expression, and a 2.27 folds decline in p21WAF1 mRNA expression in the DSS group as compared to the control group were recorded (Fig. 7A–C). Conversely, EGCG-treated animals demonstrated a general increase in the expression of cell cycle inhibitors as compared to the DSS group which was also significantly higher than p53 and p21WAF1expression in the control group (Fig. 7A–C).
Discussion
The present study attempted to assess the protective effects of green tea EGCG consumption on the progression and severity of DSS-induced colitis in aged Swiss albino male mice. DSS is a widely used agent for studying UC due to its rapid induction and clinically relevant parameters (Eichele and Kharbanda 2017). However, molecular understanding of its pathogenesis is still incomplete and it appears that aging augments the detrimental effects of UC (Liu et al. 2019). This is not surprising since aging is accompanied by a systemic increase in inflammation (inflamm-aging) and predisposition to inflammatory disorders, remodelling of the intestinal epithelial barrier as well as ‘leaky gut’ that can aggravate the development and progression of UC (Tran and Greenwood-Van Meerveld 2013; Ren et al. 2014). In this regard, we have previously profiled age-dependent immunological changes in Swiss albino mice used in the present study that confirmed prevalent systemic inflammation and Th1/Th2 immune imbalance (Sharma et al. 2014b). In the present study, we observed a rapid onset of colitis symptoms in aged animals, most evidently weight loss, which appeared to steadily increase with progressive DSS treatment. Previous studies in younger animals have reported 20–30% weight loss on account of DSS treatment which is dependent on the animal strain used, as well as the duration and dosage of DSS administered (Laroui et al. 2012; Chassaing et al. 2014; Kwon et al. 2021). The rapid loss of weight during DSS treatment could be attributed to a combination of DSS-induced cytotoxicity and inflammation (Hwang et al. 2016) as well as the development of UC symptoms such as diarrhoea, bloody stool, and reduced feed intake (Kaur and Goggolidou 2020). Specific data on acute DSS-induced colitis in aged animals are limited, and our results are contrary to a recent study in aged C57BL/6 mice which only showed a near 15% decrease in body weight (Liu et al. 2020). However, this disparity could easily be attributed to the lower dose of DSS [2% (w/v)] as well as the different animal strain used compared to the present study. Although we did not directly compare colitis development relative to age in this work; however, based on our observations as well as previous reports on DSS-induced colitis in young mice (Glauben et al. 2006; Verma et al. 2014; Sharma et al. 2020; Bouameur et al. 2022); the unusually massive change in inflammatory markers, expression of intestinal epithelial integrity as well as cell cycle suppressor genes in the present study suggests that DSS may have caused more severe cellular and molecular damage in aging male animals. This is also supported by recent studies which demonstrated that UC is more detrimental during aging both in humans and experimental animals as validated through parameters such as weight loss, maintenance of epithelial barrier integrity, and inflammation (Liu et al. 2019, 2020).
Several dietary agents and phytochemicals have demonstrated the potential to attenuate the effects of UC both in preclinical as well as clinical studies, and as such, there is a growing interest in the development of natural products-based therapies for the management of IBDs (Holleran et al. 2020; Picardo et al. 2020; Gupta et al. 2022). EGCG is the prominent catechin in green tea and has been associated with several health beneficial effects including anti-inflammatory, anti-oxidant, and anti-cancer activities (Musial et al. 2020; Kim and Heo 2022). We and others have previously reported that EGCG can also beneficially modulate several deleterious aspects of aging including immunosenescence, gut dysbiosis, cellular senescence, as well as inflamm-aging resulting in the extension of organismal healthspan and lifespan (Shin et al. 2016; Sharma et al. 2017, 2019, 2022; Holczer et al. 2018; Kumar et al. 2019, 2020; Sharma and Diwan 2022). In the present study, we observed that EGCG pre-treatment for 30 days remarkably reversed the physical markers of UC in aged animals such as a robust decrease in weight loss, DAI index, and colon length. Previous reports using young experimental animals have also shown that EGCG can attenuate experimental colitis by improving anti-inflammatory responses and maintenance of the epithelial barrier integrity (Bing et al. 2017; Du et al. 2019). DSS-induced colitis is accompanied by acute oxi-inflammatory stress in the colon and indeed we observed a massive upregulation of several markers of inflammation and oxidative damage in the colon tissue of DSS-treated animals. A similar, albeit less aggravating trend was observed in the splenocytes of DSS-treated animals that was further associated with a robust increase in the total splenocytes population. Although we did not specifically measure oxi-inflammatory stress markers in peripheral blood, our observations in the spleen are indicative of systemic effects of DSS-induced colitis. On the other hand, EGCG group animals showed very effective downregulation of all measured inflammatory markers, but curiously, no significant effect on the total splenocytes population could be observed. This indicated that the apparent suppressive effects of EGCG on interleukins may not be due to reduced proliferation of immune cells but could be related to subdued activation of splenic immune cells. In this regard, previous studies have demonstrated that EGCG can suppress the secretion of proinflammatory cytokines in immune cells activated due to various inflammatory insults that may be implicated in the observed effects of EGCG in the present study (Peairs et al. 2010; Huang et al. 2021; Ma et al. 2021). The ratio of pro-inflammatory (IL-6/IL-1β/TNF-α) to anti-inflammatory cytokine (IL-10) is an indicator of the Th1/Th2 cytokine balance and is associated with disease progression as well as the level of immunological homeostasis (Sharma et al. 2014a; Joshi et al. 2015). In the present study, a strong imbalance in Th1/Th2 ratio, as evidenced by upregulation of Th1 immune response, was observed in the colon of DSS-treated animals that were robustly countered by EGCG treatment. Our observations are similar to previous reports which demonstrated that improving the Th1/Th2 cytokine profile is a therapeutic target of various UC management strategies (Bing et al. 2017; Saba et al. 2020). However, no specific trend in Th1/Th2 cytokine profile for splenocytes could be observed which could at least partially be related to the considerable heterogeneity in the investigated splenic immune cell population. In addition to the management of inflammatory stress, a strong upregulation of cellular antioxidant defences in terms of total antioxidant capacity, expression of transcription factor Nrf2, as well as concomitantly decreased oxidative stress was also apparent in EGCG-treated animals. Together, this indicates that EGCG supplementation robustly maintained the oxi-inflammatory homeostasis in the colon even in the wake of an enduring inflammatory threat and thus protected intestinal epithelial cells from damage and loss of function. Our results are supported by several previous studies which have demonstrated that EGCG application can offer protection against inflammatory aggravation by enhancing Nrf2-dependent and independent activation of cellular antioxidant potential (Han et al. 2012; Kanlaya et al. 2016; Li et al. 2016; Sun et al. 2017).
The robust presence of oxi-inflammatory stress in the colon tissue environment is likely to affect epithelial cells’ functional homeostasis and integrity. Indeed, a strong decrease in the expression of tight junction genes in the DSS treated group was apparent while EGCG treated animals had significantly higher levels of these genes even when compared to the control animals. These observations suggest that EGCG application could maintain and even enhance the architecture and functional homeostasis of the intestinal epithelial cells and thus potentially prevent the leaky gut which could explain the lack of systemic effects of DSS-induced inflammatory aggravation in EGCG-treated animals. Our observations are also consistent with previous studies wherein the application of dietary bioactive phytochemicals improved the intestinal integrity and functions through the maintenance of tight junction proteins during progressive UC (Cao et al. 2018; Dong et al. 2020; Li et al. 2020; Qu et al. 2021). In addition to inflammatory damage, a more serious implication of UC is the development of colitis-associated neoplasia leading to colonic carcinoma (Foersch and Neurath 2014). Persistent oxi-inflammatory stress can suppress the cell cycle regulatory pathways (such as p53/p21WAF1 and p16Ink4a/Rb) resulting in increased and unwarranted cellular proliferation (Poehlmann et al. 2013; Gudkov and Komarova 2016). The acute inflammatory stress in DSS-induced colitis can enhance the aberrant proliferation of colon cells and thus increase the risk of carcinogenesis (Inoue et al. 2007). It has been observed that the loss of p53 functions due to mutations in the p53 gene is one of the earliest and essential neoplastic events in UC-associated tumorigenesis that can both initiate and augment the progression of tumorigenesis and inflammation (Fujii et al. 2004; Chang et al. 2007; Cooks et al. 2013; Schulz-Heddergott et al. 2018; Su et al. 2019). Similarly, a decrease in the expression of p21WAF1 (the downstream target of p53), as well as p53-independent p16Ink4a expression, has also been associated with increased incidences of neoplasia and carcinoma in IBDs (Seril et al. 2003; Wong et al. 2003). In fact, the relative expression profile of cell cycle inhibitors is considered a marker for assessing the severity and progression of UC, and suppression in cyclins expression is associated with impending neoplasia (Ioachim et al. 2004). Previous studies suggest that multiple cycles of DSS can ultimately induce tumorigenesis in experimental animals that can be further accelerated by using a combination of carcinogen azoxymethane and DSS (Clapper et al. 2007; Arnesen et al 2021). In the present study, we observed a strong inhibition of p53/p21WAF1/p16Ink4a in the colon of DSS-treated animals suggesting the possible onset of dysplasia. In the context of aging, since senescence acts as a barrier to tumorigenesis, loss of cell cycle suppressor genes in aged animals in the wake of acute inflammatory stress can ablate the senescence program in colon cells, and it is reasonable to speculate that this loss of cellular senescence could be involved in promoting UC-associated carcinogenesis during aging. Moreover, the molecular cross-talk between p53 expression and persistent inflammatory aggravation is well known wherein increased inflammatory cytokines such as IL-6, as observed in the present study, can directly suppress p53 activity and its downstream effects (Yonish-Rouach et al. 1991; Gudkov et al. 2011; Brighenti et al. 2014) thereby promoting tumorigenesis which can be correlated in the present study. It thus can be speculated that the remarkable increase in antioxidant potency and attenuated oxi-inflammatory damage on account of EGCG treatment may have maintained cellular redox and functional homeostasis in the colon despite the looming DSS threat ultimately resulting in the apparent suppression of colitis and possible colitis-induced dysplasia in aged animals (Fig. 8). However, it is also interesting to note that EGCG is considered a gerosuppressor as it inhibits several features of cellular senescence including the increased expression of cell cycle inhibitory genes such as p53 and p21WAF1 in cellular models of senescence as well as in naturally aged animals (Kumar et al. 2019; Lilja et al. 2020; Sharma et al. 2022). Considering this and based on the observations in the present study, it seems that the effects of EGCG on cell cycle regulation are contrary in the wake of DSS-induced inflammation in aged animals. Although the exact underlying mechanisms regarding this disparity are yet unclear, however, it needs to be considered that DSS treatment in the present study induced severe inflammation in the colon and splenocytes of aged animals as evident by unusually high levels of inflammatory cytokines. This robust inflammatory environment can overwhelm the p53-mediated protective response to counter oxidative and inflammatory stress which can ultimately suppress p53 activity and expression (Brighenti et al. 2014; Gudkov and Komarova 2016). It is plausible to suggest that EGCG being an antioxidant, countered and neutralized the DSS-induced cellular inflammatory stressors to a relatively lower level such that p53 could now express and respond efficiently to these stressors ultimately augmenting the cellular antioxidant responses (Sablina et al. 2005) as observed in the EGCG treated group.
Schematic representation of the observed effects and anti-colitis mechanism of EGCG. Persistent DSS exposure induced oxidative and inflammatory aggravation to intestinal epithelial cells of the colonic mucosa that ultimately manifested as loss of integrity and colon length. Continued inflammation may have suppressed p53 and related cell cycle inhibitory genes resulting in loss of stress response capacity, uncontrolled proliferation, and probable augmentation of neoplasia. The attribute of EGCG in improving antioxidant capacity, attenuating oxidative and inflammatory damage may be the chief contributors to apparent delayed onset and severity of colitis including improvement in cell cycle inhibitory gene expression
The present study is the first attempt to integrate aging, DSS-induced inflammatory damage, and tumorigenesis vis-à-vis a phytochemical (EGCG) treatment. Our data suggests that aging can strongly predispose elderly to UC, and that EGCG treatment is a potential tool in managing the progression of UC during aging. However, despite its strengths, there are certain limitations to consider. Firstly, although very high levels of inflammatory cytokines were observed in the colon and spleen tissue in the present study; analyses of the systemic presence of inflammatory markers could provide further insights into the extent and severity of prevalent colitis in aged animals. Secondly, the present study evaluated cellular senescence marker genes within the context of developing colitis, but since these are aged animals, it would be prudent to further assess how cellular senescence in the colon tissue per se is affected by DSS as well as EGCG treatment thereof by using other characteristic markers of senescence such as SA-β-activity, cell proliferation, telomere length, and SASP. Thirdly, a chronic model of DSS-induced colitis should also be assessed for comprehending how chronic inflammation and EGCG treatment affect UC, particularly tumorigenesis, in aged animals.
Conclusion
There is increasing evidence to consider that aging presents a more severe form of colitis which requires specific therapeutic considerations. Indeed, our results suggest that aged male animals develop rapid and robust colitis due to DSS treatment, and that consumption of green tea EGCG can beneficially impact the development of UC even in aged animals by ameliorating oxi-inflammatory stressors and attenuating potential colitis-induced progression of tumorigenesis. Although male animals are considered more prone to chemical-induced colitis, however, females can also develop symptoms of DSS-induced colitis, albeit less severe, depending upon the dose and duration of DSS treatment (Zhao et al. 2013; Bábíčková et al. 2015; Nyuyki et al. 2018; Zhang et al. 2019). Further, a recent report has observed that application of DSS (@ 3.5% w/v) for seven days, as also performed in the present study, could successfully induce colitis in young female mice (Ren et al. 2021) thereby indicating that our results in aged male mice could also be valid in female aged mice. Moreover, dietary application of bioactive phytochemicals is potent in managing inflammatory disorders, such as experimental colitis, regardless of the animal gender (Varilek et al. 2001; Dou et al. 2013; Zhao et al. 2013; Ren et al. 2021) and thus, we expect that our observations on protective effects of EGCG in the current study should also be applicable in aged female mice. After allometric scaling for humans, our standardized bioactive dosage of EGCG (100 mg/kg/animal) corresponds to 486 mg of EGCG consumption in humans which is considered a safe and recommended daily dosage for potential health beneficial effects of green tea drinking (Kuriyama et al. 2006; Pang et al. 2016; Sharma et al. 2017). Further studies assessing EGCG consumption in impacting colitis-induced colon cancer, especially during aging are desirable to truly comprehend the anti-colitis effects of green tea.
Data availability
All material used and data generated or analysed during this study are included in this published article.
References
Arnesen H, Müller MH, Aleksandersen M, Østby GC, Carlsen H, Paulsen JE, Boysen P (2021) Induction of colorectal carcinogenesis in the C57BL/6J and A/J mouse strains with a reduced DSS dose in the AOM/DSS model. Lab Anim Res 37(1):1
Bábíčková J, Tóthová Ľ, Lengyelová E, Bartoňová A, Hodosy J, Gardlík R, Celec P (2015) Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation 38(5):1996–2006
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the nlrp3 inflammasome. Gut 59(9):1192–1199. https://doi.org/10.1136/gut.2009.197822
Bing X, Xuelei L, Wanwei D, Linlang L, Keyan C (2017) Egcg maintains th1/th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through tlr4/myd88/nf-κb signaling pathway in rats. Can J Gastroenterol Hepatol 2017:3057268. https://doi.org/10.1155/2017/3057268
Bitzer ZT, Elias RJ, Vijay-Kumar M, Lambert JD (2016) (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol Nutr Food Res 60(10):2267–2274
Bouameur S, Menad A, Hamadou T, Amp O, Rgensen B, Ameddah S (2022) Gelidium spinosum red algae ameliorates oxidative /nitrosative stress and inflammation in dss -induced ulcerative colitis in mice. Egypt J Chem 65(3):341–352. https://doi.org/10.21608/ejchem.2021.88910.4293
Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H (2020) Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med 26(11):1034–1046. https://doi.org/10.1016/j.molmed.2020.06.006
Brighenti E, Calabrese C, Liguori G, Giannone FA, Trere D, Montanaro L, Derenzini M (2014) Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: a new pathway connecting inflammation to cancer. Oncogene 33(35):4396–4406
Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A (2012) Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis 6(2):226–235
Butter M, Weiler S, Biedermann L, Scharl M, Rogler G, Bischoff-Ferrari HA, Misselwitz B (2018) Clinical manifestations, pathophysiology, treatment and outcome of inflammatory bowel diseases in older people. Maturitas 110:71–78. https://doi.org/10.1016/j.maturitas.2018.01.015
Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y (2018) Protective effect of Naringin on DSS-induced ulcerative colitis in mice. J Agric Food Chem 66(50):13133–13140. https://doi.org/10.1021/acs.jafc.8b03942
Chang W-CL, Coudry RA, Clapper ML, Zhang X, Williams K-L, Spittle CS, Li T, Cooper HS (2007) Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis 28(11):2375–2381. https://doi.org/10.1093/carcin/bgm134
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M (2014) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104(1):15–25
Clapper ML, Cooper HS, Chang WC (2007) Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions 1. Acta Pharmacol Sin 28(9):1450–1459
Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rozenfeld N, Harpaz N (2013) Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23(5):634–646
Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S et al (2019) Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156(5):1345-1353.e1344. https://doi.org/10.1053/j.gastro.2019.01.002
Dong Y, Lei J, Zhang B (2020) Dietary quercetin alleviated DSS-induced colitis in mice through several possible pathways by transcriptome analysis. Curr Pharm Biotechnol 21(15):1666–1673. https://doi.org/10.2174/1389201021666200711152726
Dou W, Zhang J, Zhang E, Sun A, Ding L, Chou G, Wang Z, Mani S (2013) Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther 345(3):473–482
Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ (2013) A pilot study to evaluate the safety and efficacy of an oral dose of (−)-epigallocatechin-3-gallate–rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis 19(9):1904–1912
Du Y, Ding H, Vanarsa K, Soomro S, Baig S, Hicks J, Mohan C (2019) Low dose epigallocatechin gallate alleviates experimental colitis by subduing inflammatory cells and cytokines, and improving intestinal permeability. Nutrients 11(8):1743. https://doi.org/10.3390/nu11081743
Eichele DD, Kharbanda KK (2017) Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 23(33):6016–6029. https://doi.org/10.3748/wjg.v23.i33.6016
Faye AS, Colombel JF (2022) Aging and IBD: a new challenge for clinicians and researchers. Inflamm Bowel Dis 28(1):126–132. https://doi.org/10.1093/ibd/izab039
Flurkey K, Currer JM, Harrison DE (2007) Mouse models in aging research. The mouse in biomedical research. Academic Press, Cambridge, pp 637–672
Foersch S, Neurath MF (2014) Colitis-associated neoplasia: molecular basis and clinical translation. Cell Mol Life Sci 71(18):3523–3535. https://doi.org/10.1007/s00018-014-1636-x
Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y et al (2004) Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut 53(5):710. https://doi.org/10.1136/gut.2003.028779
Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, Mascagni P, Fantuzzi G, Dinarello CA, Siegmund B (2006) Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol 176(8):5015–5022. https://doi.org/10.4049/jimmunol.176.8.5015
Goodman WA, Havran HL, Quereshy HA, Kuang S, De Salvo C, Pizarro TT (2018) Estrogen receptor α loss-of-function protects female mice from DSS-induced experimental colitis. Cell Mol Gastroenterol Hepatol 5(4):630
Gudkov AV, Komarova EA (2016) P53 and the carcinogenicity of chronic inflammation. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a026161
Gudkov AV, Gurova KV, Komarova EA (2011) Inflammation and p53: a tale of two stresses. Genes Cancer 2(4):503–516. https://doi.org/10.1177/1947601911409747
Gupta M, Mishra V, Gulati M, Kapoor B, Kaur A, Gupta R, Tambuwala MM (2022) Natural compounds as safe therapeutic options for ulcerative colitis. Inflammopharmacology 30(2):397–434. https://doi.org/10.1007/s10787-022-00931-1
Han SG, Han S-S, Toborek M, Hennig B (2012) EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol 261(2):181–188. https://doi.org/10.1016/j.taap.2012.03.024
Holczer M, Besze B, Zámbó V, Csala M, Bánhegyi G, Kapuy O (2018) Epigallocatechin-3-gallate (EGCG) promotes autophagy-dependent survival via influencing the balance of MTOR-AMPK pathways upon endoplasmic reticulum stress. Oxid Med Cell Longev 2018:6721530. https://doi.org/10.1155/2018/6721530
Holleran G, Scaldaferri F, Gasbarrini A, Currò D (2020) Herbal medicinal products for inflammatory bowel disease: a focus on those assessed in double-blind randomised controlled trials. Phytother Res 34(1):77–93. https://doi.org/10.1002/ptr.6517
Hossen I, Hua W, Ting L, Mehmood A, Jingyi S, Duoxia X, Yanping C, Hongqing W, Zhipeng G, Kaiqi Z et al (2020) Phytochemicals and inflammatory bowel disease: a review. Crit Rev Food Sci Nutr 60(8):1321–1345. https://doi.org/10.1080/10408398.2019.1570913
Hruz P, Juillerat P, Kullak-Ublick GA, Schoepfer AM, Mantzaris GJ, Rogler G (2020) Management of the elderly inflammatory bowel disease patient. Digestion 101(suppl 1):105–119. https://doi.org/10.1159/000503099
Huang SC, Kao YH, Shih SF, Tsai MC, Lin CS, Chen LW, Chuang YP, Tsui PF, Ho LJ, Lai JH et al (2021) Epigallocatechin-3-gallate exhibits immunomodulatory effects in human primary t cells. Biochem Biophys Res Commun 550:70–76. https://doi.org/10.1016/j.bbrc.2021.02.132
Hwang DY, Park JH, Yim SV, Son Y, Hong HS (2016) Substance-P protects intestinal epithelium against dextran sulfate sodium-induced toxicity in vitro. Mol Cell Toxicol 12(4):391–398
Inoue T, Murano M, Kuramoto T, Ishida K, Kawakami K, Abe Y, Morita E, Murano N, Toshina K, Nishikawa T et al (2007) Increased proliferation of middle to distal colonic cells during colorectal carcinogenesis in experimental murine ulcerative colitis. Oncol Rep 18(6):1457–1462
Ioachim EE, Katsanos KH, Michael MC, Tsianos EV, Agnantis NJ (2004) Immunohistochemical expression of cyclin D1, cyclin E, p21/waf1 and p27/kip1 in inflammatory bowel disease: correlation with other cell-cycle-related proteins (Rb, p53, ki-67 and PCNA) and clinicopathological features. Int J Colorectal Dis 19(4):325–333. https://doi.org/10.1007/s00384-003-0571-3
Joshi L, Ponnana M, Sivangala R, Chelluri LK, Nallari P, Penmetsa S, Valluri V, Gaddam S (2015) Evaluation of TNF-α, IL-10 and IL-6 cytokine production and their correlation with genotype variants amongst tuberculosis patients and their household contacts. PLoS ONE 10(9):e0137727. https://doi.org/10.1371/journal.pone.0137727
Kanlaya R, Khamchun S, Kapincharanon C, Thongboonkerd V (2016) Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci Rep 6(1):30233. https://doi.org/10.1038/srep30233
Kaur A, Goggolidou P (2020) Ulcerative colitis: understanding its cellular pathology could provide insights into novel therapies. J Inflamm 17(1):15. https://doi.org/10.1186/s12950-020-00246-4
Kim JM, Heo HJ (2022) The roles of catechins in regulation of systemic inflammation. Food Sci Biotechnol. https://doi.org/10.1007/s10068-022-01069-0
Kumar R, Sharma A, Kumari A, Gulati A, Padwad Y, Sharma R (2019) Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 20(2):171–189. https://doi.org/10.1007/s10522-018-9785-1
Kumar R, Sharma A, Padwad Y, Sharma R (2020) Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology 21(3):325–343. https://doi.org/10.1007/s10522-020-09861-3
Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 296(10):1255–1265. https://doi.org/10.1001/jama.296.10.1255
Kwon J, Lee C, Heo S, Kim B, Hyun CK (2021) DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci Rep 11(1):1–6
Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D (2012) Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 7(3):e32084
Levine JS, Burakoff R (2011) Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 7(4):235–241
Li J, Sapper TN, Mah E, Rudraiah S, Schill KE, Chitchumroonchokchai C, Moller MV, McDonald JD, Rohrer PR, Manautou JE et al (2016) Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro-inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol Nutr Food Res 60(4):858–870. https://doi.org/10.1002/mnfr.201500814
Li H, Fan C, Lu H, Feng C, He P, Yang X, Xiang C, Zuo J, Tang W (2020) Protective role of berberine on ulcerative colitis through modulating enteric glial cells–intestinal epithelial cells–immune cells interactions. Acta Pharm Sin B 10(3):447–461. https://doi.org/10.1016/j.apsb.2019.08.006
Lilja S, Oldenburg J, Pointner A, Dewald L, Lerch M, Hippe B, Switzeny O, Haslberger A (2020) Epigallocatechin gallate effectively affects senescence and anti-SASP via SIRT3 in 3T3-L1 preadipocytes in comparison with other bioactive substances. Oxid Med Cell Longev. https://doi.org/10.1155/2020/4793125
Liu AL, Wang HY, Lü H, Qian JM (2019) Effect of aging on experimental colitis in mice and its associated mechanism]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 41(1):28–36. https://doi.org/10.3881/j.issn.1000-503X.10840
Liu A, Lv H, Wang H, Yang H, Li Y, Qian J (2020) Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J Gerontol A 75(7):1284–1292. https://doi.org/10.1093/gerona/glz263
Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A (2016) How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol 22(3):1017–1033. https://doi.org/10.3748/wjg.v22.i3.1017
Ma Y, Liu G, Tang M, Fang J, Jiang H (2021) Epigallocatechin gallate can protect mice from acute stress induced by LPS while stabilizing gut microbes and serum metabolites levels. Front Immunol. https://doi.org/10.3389/fimmu.2021.640305
Miao YL, Xiao YL, Du Y, Duan LP (2013) Gene expression profiles in peripheral blood mononuclear cells of ulcerative colitis patients. World J Gastroenterol 19(21):3339–3346. https://doi.org/10.3748/wjg.v19.i21.3339
Munkholm P (2003) Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 18(Suppl 2):1–5. https://doi.org/10.1046/j.1365-2036.18.s2.2.x
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M (2020) Beneficial properties of green tea catechins. Int J Mol Sci 21(5):1744
Nyuyki KD, Cluny NL, Swain MG, Sharkey KA, Pittman QJ (2018) Altered brain excitability and increased anxiety in mice with experimental colitis: consideration of hyperalgesia and sex differences. Front Behav Neurosci 12:58
Oz HS, Chen T, de Villiers WJ (2013) Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front Immunol 4:132
Pang J, Zhang Z, Zheng TZ, Bassig BA, Mao C, Liu X, Zhu Y, Shi K, Ge J, Yang YJ et al (2016) Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta-analysis. Int J Cardiol 202:967–974. https://doi.org/10.1016/j.ijcard.2014.12.176
Park MY, Ji GE, Sung MK (2012) Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 57(2):355–363. https://doi.org/10.1007/s10620-011-1883-8
Peairs A, Dai R, Gan L, Shimp S, Rylander MN, Li L, Reilly CM (2010) Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol Immunol 7(2):123–132. https://doi.org/10.1038/cmi.2010.1
Picardo S, Altuwaijri M, Devlin SM, Seow CH (2020) Complementary and alternative medications in the management of inflammatory bowel disease. Ther Adv Gastroenterol 13:1756284820927550. https://doi.org/10.1177/1756284820927550
Poehlmann A, Reissig K, Schönfeld P, Walluscheck D, Schinlauer A, Hartig R, Lessel W, Guenther T, Silver A, Roessner A (2013) Repeated H2O2 exposure drives cell cycle progression in an in vitro model of ulcerative colitis. J Cell Mol Med 17(12):1619–1631. https://doi.org/10.1111/jcmm.12150
Qu Y, Li X, Xu F, Zhao S, Wu X, Wang Y, Xie J (2021) Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-Nf-κB axis. Front Immunol 12:679897–679897. https://doi.org/10.3389/fimmu.2021.679897
Rabe H, Malmquist M, Barkman C, Östman S, Gjertsson I, Saalman R, Wold AE (2019) Distinct patterns of naive, activated and memory T and B cells in blood of patients with ulcerative colitis or Crohn’s disease. Clin Exp Immunol 197(1):111–129. https://doi.org/10.1111/cei.13294
Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang SC, Hu Y (2014) Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res 26(2):183–191. https://doi.org/10.1007/s40520-013-0148-0
Ren J, Yue B, Wang H, Zhang B, Luo X, Yu Z, Zhang J, Ren Y, Mani S, Wang Z, Dou W (2021) Acacetin ameliorates experimental colitis in mice via inhibiting macrophage inflammatory response and regulating the composition of gut microbiota. Front Physiol 11:577237
Riegler G, Tartaglione MT, Carratú R, D’Incá R, Valpiani D, Russo MI, Papi C, Fiorentini MT, Ingrosso M, Andreoli A et al (2000) Age-related clinical severity at diagnosis in 1705 patients with ulcerative colitis. Dig Dis Sci 45(3):462–465. https://doi.org/10.1023/A:1005424603085
Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, Bronner MP, Al-Lahham B, Kowdley KV, Lindor KD et al (2011) Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res 71(5):1669–1679. https://doi.org/10.1158/0008-5472.Can-10-1966
Saba E, Lee YY, Rhee MH, Kim SD (2020) Alleviation of ulcerative colitis potentially through th1/th2 cytokine balance by a mixture of Rg3-enriched korean red ginseng extract and Persicaria tinctoria. Molecules 25(22):5230. https://doi.org/10.3390/molecules25225230
Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM (2005) The antioxidant function of the p53 tumor suppressor. Nat Med 11(12):1306–1313
Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A (2015) Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res 46(4):280–285. https://doi.org/10.1016/j.arcmed.2015.05.005
Schulz-Heddergott R, Stark N, Edmunds SJ, Li J, Conradi LC, Bohnenberger H, Ceteci F, Greten FR, Dobbelstein M, Moll UM (2018) Therapeutic ablation of gain-of-function mutant p53 in colorectal cancer inhibits Stat3-mediated tumor growth and invasion. Cancer Cell 34(2):298–314
Seril DN, Liao J, Yang GY, Yang CS (2003) Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis 24(3):353–362. https://doi.org/10.1093/carcin/24.3.353
Sharma R, Diwan B (2022) An update on health span and lifespan enhancing attributes of tea amidst the emerging understanding of aging biology. Hum Nutr Metab. https://doi.org/10.1016/j.hnm.2022.200149
Sharma R, Kapila R, Dass G, Kapila S (2014a) Improvement in th1/th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. Age 36(4):9686. https://doi.org/10.1007/s11357-014-9686-4
Sharma R, Kapila R, Haq MRU, Salingati V, Kapasiya M, Kapila S (2014b) Age-associated aberrations in mouse cellular and humoral immune responses. Aging Clin Exp Res 26(4):353–362. https://doi.org/10.1007/s40520-013-0190-y
Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S (2014c) Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res 34(11):968–981. https://doi.org/10.1016/j.nutres.2014.09.006
Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS (2017) Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male Swiss albino mice. Biogerontology 18(3):367–382. https://doi.org/10.1007/s10522-017-9696-6
Sharma R, Kumari M, Kumari A, Sharma A, Gulati A, Gupta M, Padwad Y (2019) Diet supplemented with phytochemical epigallocatechin gallate and probiotic Lactobacillus fermentum confers second generation synbiotic effects by modulating cellular immune responses and antioxidant capacity in aging mice. Eur J Nutr 58(7):2943–2957. https://doi.org/10.1007/s00394-018-01890-6
Sharma A, Tirpude NV, Kulurkar PM, Sharma R, Padwad Y (2020) Berberis lycium fruit extract attenuates oxi-inflammatory stress and promotes mucosal healing by mitigating NF-κB/c-jun/mapks signalling and augmenting splenic treg proliferation in a murine model of dextran sulphate sodium-induced ulcerative colitis. Eur J Nutr 59(6):2663–2681. https://doi.org/10.1007/s00394-019-02114-1
Sharma R, Kumar R, Sharma A, Goel A, Padwad Y (2022) Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J Nutr Biochem 107:109068. https://doi.org/10.1016/j.jnutbio.2022.109068
Shin JH, Jeon HJ, Park J, Chang MS (2016) Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via nrf2. Int J Mol Med 38(4):1075–1082. https://doi.org/10.3892/ijmm.2016.2694
Somani SJ, Modi KP, Majumdar AS, Sadarani BN (2015) Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother Res 29(3):339–350. https://doi.org/10.1002/ptr.5271
Su H, Kang Q, Wang H, Yin H, Duan L, Liu Y, Fan R (2019) Changes in expression of p53 and inflammatory factors in patients with ulcerative colitis erratum in 10.3892/etm.2019.7994. Exp Ther Med 17(4):2451–2456. https://doi.org/10.3892/etm.2019.7253
Sun W, Liu X, Zhang H, Song Y, Li T, Liu X, Liu Y, Guo L, Wang F, Yang T et al (2017) Epigallocatechin gallate upregulates nrf2 to prevent diabetic nephropathy via disabling keap1. Free Radic Biol Med 108:840–857. https://doi.org/10.1016/j.freeradbiomed.2017.04.365
Tran L, Greenwood-Van Meerveld B (2013) Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A 68(9):1045–1056. https://doi.org/10.1093/gerona/glt106
Varilek GW, Yang F, Lee EY, DeVilliers WJ, Zhong J, Oz HS, Westberry KF, McClain CJ (2001) Green tea polyphenol extract attenuates inflammation in interleukin-2–deficient mice, a model of autoimmunity. J Nutr 131(7):2034–2039
Verma N, Verma R, Kumari R, Ranjha R, Paul J (2014) Effect of salicin on gut inflammation and on selected groups of gut microbiota in dextran sodium sulfate induced mouse model of colitis. Inflamm Res 63(2):161–169. https://doi.org/10.1007/s00011-013-0685-1
Wong NA, Mayer NJ, Anderson CE, McKenzie HC, Morris RG, Diebold J, Mayr D, Brock IW, Royds JA, Gilmour HM et al (2003) Cyclin d1 and p21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol 34(6):580–588. https://doi.org/10.1016/s0046-8177(03)00125-4
Xu Z, Wei C, Zhang RU, Yao J, Zhang D, Wang L (2015) Epigallocatechin-3-gallate-induced inhibition of interleukin-6 release and adjustment of the regulatory T/T helper 17 cell balance in the treatment of colitis in mice. Exp Ther Med 10(6):2231–2238
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M (1991) Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352(6333):345–347. https://doi.org/10.1038/352345a0
Zhang LJ, Huang XJ, Shi XD, Chen HH, Cui SW, Nie SP (2019) Protective effect of three glucomannans from different plants against DSS induced colitis in female BALB/c mice. Food Funct 10(4):1928–1939
Zhao J, Hong T, Dong M, Meng Y, Mu J (2013) Protective effect of myricetin in dextran sulphate sodium-induced murine ulcerative colitis. Mol Med Rep 7(2):565–570
Acknowledgements
This study was supported by a grant from the Department of Science and Technology, Government of India under the INSPIRE Faculty scheme (IFA17-LSPA79). The authors express their gratitude to Dr. Anamika Sharma for her guidance in colitis experiments.
Author information
Authors and Affiliations
Contributions
BD investigation; RS funding acquisition, conceptualization, methodology, analyses, wrote/edited manuscript; supervision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diwan, B., Sharma, R. Green tea EGCG effectively alleviates experimental colitis in middle-aged male mice by attenuating multiple aspects of oxi-inflammatory stress and cell cycle deregulation. Biogerontology 23, 789–807 (2022). https://doi.org/10.1007/s10522-022-09976-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09976-9