Abstract
The ability of very old animals to make new muscle after injury remains controversial. This issue has major implications for the regenerative potential of damaged geriatric human muscle, to age-related loss of muscle mass (sarcopenia) and to the proposed need for muscle stem cell therapy for the aged. To further address issues of inherent myogenic capacity and the role of host systemic factors in new muscle formation, whole muscle grafts were transplanted between geriatric (aged 27–29 months) and young (3 months) C57Bl/6J mice and compared with autografts in geriatric and young mice. Grafts were sampled at 5 and 10 days for histological analysis. Inflammation and formation of new myotubes was strikingly impaired at 5 days in the geriatric muscle autografts. However, there was a strong inflammatory response by the geriatric hosts to young muscle grafts and geriatric muscles provoked an inflammatory response by young hosts at 5 days. At 10 days, extensive myotube formation in geriatric muscle autografts (equivalent to that seen in young autografts and both other groups) confirmed excellent intrinsic capacity of myogenic (stem) cells to proliferate and fuse. The key conclusion is that a weaker chemotactic stimulus by damaged geriatric muscle, combined with a reduced inflammatory response of old hosts, results in delayed inflammation in geriatric muscle autografts. This delay is transient. Once inflammation occurs, myogenesis can proceed. The presence of well developed myotubes in old muscle autografts at 10 days confirms a very good inherent myogenic response of geriatric skeletal muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progressive loss of skeletal muscle mass and function with age (sarcopenia) is a major problem that has attracted much attention. In humans, significant sarcopenia occurs from 50 to 80 years of age, when about 30% of muscle mass and 35% of myofibers are lost (Frontera et al. 2000). Many complex reasons are proposed for sarcopenia including age-related changes in myofiber biochemistry, denervation of myofibers and an altered extra cellular matrix (ECM) environment with increased fibrosis (that also affects blood vessels and innervation) (Shavlakadze and Grounds 2003; Lynch et al. 2005; Edstrom et al. 2007), in addition to the issues of possible decreased satellite cell (muscle precursor or stem cell) numbers, a slightly delayed myogenic response and possibly impaired new muscle formation (reviewed in (Grounds 1998; Brack 2007; Smythe et al. 2008)). This last aspect of proposed impaired myogenic response leading to a failure of new muscle formation in very old animals and humans is the focus of the present study.
It is widely considered that myogenic precursor cells (myoblasts) in adult skeletal muscle are derived from satellite cells, although there is much interest in the notion that they may also arise from other sources (Grounds and Relaix 2009): such precursors are widely referred to as myogenic stem cells, often in the absence of any exclusive in vivo stem cell markers. Problems with extracting myogenic precursor cells from aged skeletal muscle and a delay in their myogenic response in tissue culture, initially led to the conclusion that the number of these (satellite) cells was reduced in aged muscles and they had impaired replicative capacity and myogenesis (Mouly et al. 2005). Classical counting of satellite cells in tissue sections using electron microscopy or immunostaining generally concludes that numbers decrease in aged muscles from human and other species (Renault et al. 2002). However, Pax7 is down-regulated in many apparent satellite cells in aged muscle (Collins et al. 2007) and this may apply to other molecular markers with age thus leading to under-estimation of satellite cell numbers. Another possibility is that satellite cells may emigrate from their classical sub-sarcolemmal position into the interstitial space (where they cannot be readily identified) and these, or another myogenic precursor population (stem cells) resident in the interstitium, may account for very small myofibers reported between large myofibers in post-natal muscles (Desaki 2008). The complexity of the situation in human muscles with age-related differences in the extent of myofibre atrophy, differential loss of type II and type I myofibres and satellite cell numbers between various types of skeletal muscles has been comprehensively reviewed recently (Snijders et al. 2009). In rodents, at least a sub-population of satellite cells in aged muscles retains excellent myogenic capacity (Collins et al. 2007) and some decline in satellite cell function may be more important than actual numbers in aging muscles (Brack and Rando 2007; Gopinath and Rando 2008). Furthermore, the capacity of myotubes to fully differentiate within geriatric muscles may be compromised, akin to the abortive myogenesis due to a block of terminal differentiation seen in denervated muscle in situ (Borisov et al. 2005a), although this inhibition to full differentiation is removed when myogenic cells are isolated from the denervated muscle tissue and grown in culture (Borisov et al. 2005b). Such studies emphasise that the behaviour of myogenic cells in culture can be very different to that within muscle tissues in vivo and care must be taken when comparing results (e.g. related to myogenic cells from aged muscles) in these two very different environments. It is of interest that expression of the skeletal muscle specific transcription factors MyoD and myogenin is elevated to a similar extent in very old muscles and in young denervated muscles in rats (Dedkov et al. 2003), supporting the idea that ‘abortive’ regenerative activity in aged muscle may be a consequence of partial denervation of myofibers. The myogenic capacity of very old muscle remains controversial (reviewed (Collins et al. 2007; Grounds and Relaix 2009)). This issue has major implications for the regenerative potential of geriatric human muscle in response to damage. Furthermore, on the basis that impaired muscle regeneration has been proposed as a contributing factor to the relentless age-related loss of muscle mass, stem cell therapy is even suggested for sarcopenia (to replace a presumed loss of satellite cell capacity), yet such a strategy is clearly not justified if the endogenous satellite cell population in aged muscles is viable and capable of good myogenesis.
Recent experiments in our laboratories tested the effects of aging on the very early events of regeneration (during the first week), specifically on myogenesis (myoblast activation and fusion) and new muscle formation using cross-transplantation of whole muscle grafts between young and old (up to 21 months) mice (Smythe et al. 2008). Overall, these results indicate that, while there are slight age-associated delays in inflammation and neovascularisation in response to muscle injury, there is no detrimental effect on myogenesis in the mouse model used in this study. Since extreme age may be required to reveal age-related changes in rodent muscles (Kaminska et al. 1998) we questioned whether the mice were sufficiently old to test the crucial question of the potential failure of myogenic response in geriatric muscles. Our study (Smythe et al. 2008) used two strains of mice (SJL/J and BALB/c) with the oldest being BALB/c mice aged 21 months which is considered ‘old’ since the mean life span of mice is about 2 years. The longevity of laboratory mice has been extensively documented by the Jackson Laboratories (USA) and excellent information related to the mouse as a research tool is available on their website. Genetics influence aging and there are striking differences in the longevity of mice strains with the mean survival age in days being 576 ± 152 for SJL/J mice (that have a myopathy) and much longer at 793 ± 160 and 767 ± 147 for BALB/c and C57Bl/6J strains, respectively. An age related decline in general function is evident by 18 months, mice aged 18–24 months are considered ‘old’ and roughly correspond to 56–69 years in humans, 50% of mice die after this time with very few surviving beyond 30 months (corresponding to ~80 years in humans) and the maximum life-span is 36 months for C57Bl/6J mice (http://research.jax.org/faculty/harrison/ger1vLifespan1.html): unusually old C57Bl mice aged 34–37 months were used in one study of muscle fatigue and endurance (Pagala et al. 1998). To rigorously test whether the capacity of myogenic cells to form new muscle is compromised in really old (geriatric) muscles, our cross-transplantation whole muscle graft experiments (Smythe et al. 2008) were repeated using very old C57Bl/6J mice aged between 27 and 29 months.
Results
Before describing the histological appearance of the grafts it is pertinent to make some comments about the changes in body composition with age, and the health of the very old mice aged 27 to 29 months. Data from 27 to 29 months old mice were pooled together, because the values were very similar and there was no significant difference between these two groups.
Phenotypic characterisation of aging mice (from 3 to 29 months)
The tibial length increased with age with significantly longer tibial bones in 27/29 compared with 3 month old mice (Table 1), indicating some (albeit slow) growth of these bones during this time.
Body weight, and body weight standardised to tibia length, increased by 57 and 53%, respectively from 3 to 24 months of age and decreased by 8 and 11%, respectively from 24 to 27/29 months (Table 1). Data from 27 to 29 month old mice were combined as there was no difference between these age groups. This loss is probably due in large part to sarcopenia as indicated by the loss of muscle mass that was evident by 24 months of age. The weight of quadriceps muscles increased by 28% from 3 to 15 months and decreased by 30% from 15 to 27/29 months. The scenario was similar when the weight of quadriceps muscles was standardised to tibia length: this value increased by 23% from 3 to 15 months and decreased by 32% from 15 to 27/29 months (Table 1, Fig. 1). The weight of gastrocnemius muscles was compared between 27/29 and 3 month old mice and it was not significantly different between these two groups (Fig. 1). It is noted that comparison of only young (3 month) with geriatric (27/29 month) muscles does not demonstrate a marked age-related loss in body or muscle weights, as was also demonstrated over 20 years ago for male C57Bl/6J mice (Brooks and Faulkner 1988). A sarcopenia index calculated as muscle weight/body weight (Edstrom and Ulfhake 2005) confirmed a significant age-related loss of muscle mass at 24 and 27/29 months (Table 1).
Quadriceps (white bars) and gastrocnemius (grey bars) muscle weights standardized to tibia length in female C57Bl/6J mice at different ages (from 3 to 27/29 months). Data are mean ± SD for 10–17 mice in each group. (a) Significantly different from 3 months; (b) significantly different from 15 months; (c) significantly different from 24 months. Difference considered significant when P < 0.05
Geriatric mice (aged 27/29 months)
There was a slow death rate amongst the aging mice and only about half of the reserved mice reached 27–29 months. The surviving mice used in the experiments were presumably more robust than the general population and inadvertently selected for longevity. Most of the geriatric mice had reasonably glossy coats with some evidence of hair loss and greying on their back but otherwise appeared healthy. To our surprise, the geriatric mice tolerated anaesthesia very well with excellent recovery and no deaths within the first few days after surgery. The skin of all young mice showed excellent healing whereas many of the old mice had poor skin healing often with scabbing and adhesions of the skin to the underlying graft. Two mice were clearly unwell with a hunched appearance, the muscles were very wasted, the abdominal fat pad was insignificant, the bones fragile and one of these mice had grossly abnormal liver with fatty inclusions. One other geriatric mouse which appeared otherwise healthy had a grossly enlarged spleen (2.5 g compared with normal 0.17 g).
Histology of the muscle grafts
A total of 39 grafts was analysed at 5 (22 grafts) and 10 days (17 grafts). The whole muscle graft is an excellent model for studying all aspects of the kinetics of regeneration and new muscle formation. In grafted EDL muscles, the vascular and nerve supply is completely disrupted and thus angiogenesis is required for regeneration to occur. This model causes widespread necrosis within the grafted muscle, followed by a highly reproducible series of events leading to complete new muscle formation. Infiltration of inflammatory cells, revascularisation and the subsequent formation of new myotubes occurs progressively from the periphery to the centre of the graft with myogenesis sometimes (but not always) being more pronounced in the area adjacent to the TA muscle. The pattern of histological changes over time is very similar and has been thoroughly documented for adult mice of many strains (BALB/c, SJL/J, C57Bl, FVB) (Shavlakadze et al. 2004; Grounds et al. 2005). In most of the grafts, a classic zonal pattern of regeneration was seen progressively from the periphery of the graft towards the centre where the necrotic donor muscle was infiltrated by inflammatory cells and usually replaced by myoblasts that fused to form myotubes. At 5 days, there was a striking delay in the timing of regeneration in old auto- and cross-transplants, as measured by the extent of leukocyte infiltration and the appearance of myotubes within the grafts as detailed below. However, no age-related differences were apparent by 10 days.
Day 5 grafts
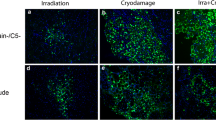
Yg/Yh autografts. The pattern of regeneration of young C57Bl/6J autografts at day 5 has been extensively described in mice (White et al. 2000; White et al. 2002; Shavlakadze et al. 2006) and 4 Yg/Yh autografts (from 2 mice) examined in the present study showed the standard appearance (Fig. 2a, Fig. 3a). Day 5 young autografts consisted of a necrotic core with disintegrating persisting myofibers occupying about half of the total cross-sectional area of the graft (48 ± 16%) and a well-pronounced peripheral regenerating zone (37 ± 12% of the total graft area) where inflammatory cells were abundant and were also infiltrating the necrotic core (Fig. 4a) Newly formed desmin positive myotubes were evenly distributed through the regeneration zone (Fig. 3a) and many desmin positive activated crescent shaped satellite cells were seen ‘cuffing’ necrotic myofibers in the centre of the grafts (Fig. 3a). On average there were 413 ± 25 myotubes (round desmin positive cells with well pronounced cytoplasm) in the regenerating zone of day 5 young (Yg/Yh) autografts (Fig. 4a).
Representative appearance of Yg/Yh (a), Og/Oh (b), Og/Yh (c) and Yg/Oh (d) grafts sampled at 5 days and Yg/Yh (e) and Og/Oh (f) grafts sampled at 10 days after transplantation, stained with H&E. The TA muscle is positioned at the bottom of each image and the EDL muscle graft on top of the TA. An extensive zone of inflammation with disintegrating myofibers and purple colour inflammatory cells (zone indicated by arrows) advances from the periphery to the necrotic core (dotted outline) of day 5 Yg/Yh (a), Og/Yh (c) and Yg/Oh (d) grafts, but is not conspicuous in day 5 Og/Oh (b) grafts. Necrotic myofibers are fully replaced by myotubes in day 10 Yg/Yh (e) and Og/Oh (f) grafts. Scale bar is 200 μm
High power images of Yg/Yh (a), Og/Oh (b), Og/Yh (c) and Yg/Oh (d) grafts sampled at 5 days (stained for desmin) and Yg/Yh (e) and Og/Oh (f) grafts sampled at 10 days (stained with H&E) after transplantation. The TA muscle is positioned at the bottom of each image and the transplanted EDL muscle graft on top of the TA. An extensive regeneration zone with abundant desmin positive young myotubes (arrows) and crescent shaped activated satellite cells (asterisks) that are ‘cuffing’ necrotic myofibers is seen in day 5 Yg/Yh (a) and Yg/Oh (d) grafts. Most of the Og/Oh (b) graft is occupied by the persisting necrotic core, with minimal inflammation and very few desmin positive myotubes (arrows). An advanced inflammatory zone with removal of necrotic muscle (Inf/Reg), but with very few desmin positive myotubes (arrows), is present in the Og/Yh (c) graft at 5 days. At day 10 following transplantation, Yg/Yh (e) and Og/Oh (f) transplants have an identical appearance and young densely packed myotubes are present through the entire area of the grafts. Scale bar is 50 μm
Area of regeneration and necrosis (a) and the number of myotubes (b) in day 5 grafts and the density of myotubes in day 10 grafts (c). At day 5 after transplantation healthy young grafts normally contain a peripheral zone of regeneration (consisting of inflammatory cells, phagocytosed myofibres with myoblasts and myotubes) with some surviving myofibres, and a necrotic central zone. The area of regeneration and necrosis is presented in day 5 grafts as a percent of the total graft area (a). Total numbers of myotubes (round shaped desmin positive cells with well pronounced cytoplasm and central nuclei) were also counted in all grafts (b). Day 10 grafts were mainly composed of young desmin positive myotubes. Only two Yg/Oh grafts from one mouse still displayed a remaining necrotic core. The density of desmin positive myotubes is presented per 0.1 mm2 of the graft area (c). All numbers are mean ± SER. ** Significant (P < 0.005) compared to the Yg/Yg values
Og/Oh autografts. Seven old autografts from 4 mice were examined (Table 2). All old autografts showed very little inflammation or regeneration (11 ± 3.4%), with few if any desmin positive myoblasts and myotubes (7 ± 2); they consisted mainly of persisting necrotic muscle tissue (89 ± 3.4%) (Fig. 2b, Fig. 3b, Fig. 4a, b).
Og/Yh cross transplants. Six old grafts implanted into 3 young mice were examined (Table 2). The Og/Yh cross-transplants had extensive inflammation advancing from the periphery to the central necrotic core (34 ± 7%) (Fig. 2c, Fig. 4a) representing a zone of regenerative activity, although very few desmin positive myoblasts and myotubes (41 ± 20) were seen within this zone of inflammatory activity that is an essential prelude to subsequent formation of new muscle (Fig. 3c, Fig. 4b).
Yg/Oh cross transplants. Five young grafts implanted into 3 old mice were examined (Table 2) and they had a similar appearance to young autografts. All Yg/Oh cross transplants had an extensive zone of regeneration (60 ± 12%) with evenly distributed newly formed desmin positive myotubes (312 ± 77) (Fig. 2d, Fig. 3d, Fig. 4a, b).
Day 10 grafts
One graft out of each group was considered unsuccessful (thin and fibrotic) due to technical reason and was excluded from the analyses. For most of the grafts the necrotic zone was completely removed, with necrotic myofibers present in only a minority of grafts (2 out of 6 Yg/Oh). The overall morphological appearance of young and old 10 day grafts was similar and they were composed of desmin positive myotubes (Fig. 2e, f, Fig. 3e, f). The average density of desmin positive myotubes (calculated per 0.1 mm2 of the graft area) was similar in all day 10 grafts (Fig. 4c). These data were expressed as numbers/area of graft because of the extensive and uniform appearance of the many myotubes: if myotubes had been larger, or there had been more fibrosis between myotubes, this would result in a lower density of myotubes, but this was not the case in any grafts.
Discussion
Features of geriatric muscles and mice
In the present study, many of the female C57Bl/6J mice died while waiting to become sufficiently old (27/29 months) to be used in the experiments. The cause of death was not investigated: however, it seems likely that there is a similar array of age-related causes of death and pathological changes in a wide range of tissues to that reported for geriatric rats (Carlson et al. 2001). Furthermore, it seems likely that the relatively few surviving geriatric mice used in experiments represent a more robust sub-population, as reported for rats (Edstrom and Ulfhake 2005).
There is general agreement that in really old skeletal muscles, myofiber size is decreased (atrophy), numbers of myofibers remain similar (although some reports dispute this), there are changes in myofiber types, wider seams of interstitial connective tissue (endomysium and perimysium) as well as degeneration of nerves and blood vessels (reviewed in (Desaki 2008)), also reduced movement/mobility indicating impaired muscle function (Pagala et al. 1998; Carlson et al. 2001; Edstrom and Ulfhake 2005). Many of these changes are widely reported for geriatric rats (e.g. >30 months near the end of their life when only about 20% of animals survive) whereas such changes are often not pronounced in slightly younger rats (24 months) (Kaminska et al. 1998; Carlson et al. 2001; Desaki 2008). Pathological changes include wide variation in myofiber sizes (some very large and other very small) in various muscle bundles, grouping of type 1 myofibers, central myonuclei in myofibers or myotubes, activated satellite cells, immature myofibers in the interstitial space and degenerating/necrotic myofibers. However, other studies report relatively minor changes even in >30 month Fischer Brown Norway rats (Baker et al. 2008). These different reports may reflect variation between strains, the specific muscle examined and especially the fact that 30 month old rats are not a homogeneous group and the extent of histological change seems to relate more to the degree of motor impairment rather than chronological age (Edstrom and Ulfhake 2005).
For mice, detailed descriptions of histological and other changes in geriatric muscles are infrequent. Extensive studies of muscle of geriatric C57Bl mice in the present and other studies (unpublished data) identified more irregular myofiber sizes and a small incidence of centrally nucleated myofibers; however, there was no evidence of severe pathology or of gross denervation as has been reported for some (but not all) geriatric rats.
A classic study of contractile properties on aging male C57Bl/6J mice reported that body mass increased by 25% between 3 and 10 months and then decreased by 13% between 10 and 27 months (Brooks and Faulkner 1988). Similar continual growth of female C57Bl/6J mice was seen in our study with body weight increasing up to 24 months and significantly decreasing from 24 to 27/29 months. Brooks and Faulkner (1988) also observed that the weights of soleus and EDL mouse muscles decreased by 20 and 13%, respectively between 10 and 27 months of age. It is useful to note that there was no significant difference when comparing the weights of soleus muscles between these young (3 months) and geriatric (27 month) mice (Brooks and Faulkner 1988), as was also seen for gastrocnemius muscles in our study: only comparison with intermediate ages demonstrated a loss of muscle mass with age (sarcopenia). Our data for whole body and quadriceps muscle weights, that both showed a significant increase from 3 to 15 and 24 months and then a decrease after this time until 27/29 months, agree with these early data (Brooks and Faulkner 1988).
To demonstrate sarcopenia, the muscle weights should be standardised to some parameter of body size. A saropenia index calculated by the ratio of muscle/body weight for our mice showed significant sarcopenia at 24 and 27/29 months compared to both 3 and 15 month of age. While the ratio of muscle/body weight is proposed as a useful sarcopenia index to indicate how well adapted the muscle is in relation to body weight (Edstrom and Ulfhake 2005), excessive abdominal adipose tissue or large tumours can distort this reference body weight: indeed, tumours are often reported in geriatric rats and in our study tumours of liver and spleen were noted in some mice. Instead we propose that the ratio of muscle/tibial length is a more reliable reference. In our study, the absolute quadriceps muscle mass was significantly lower at 24 months compared to 15 months and continued to decline by 27/29 months when it became significantly lower compared to all examined younger ages. When quadriceps muscle weights were standardised to tibia length, there was a trend towards reduced muscle weight from 15 to 27/29 months and in geriatric 27/29 month old mice standardised quadriceps muscle weights were significantly lower compared to all examined younger ages.
Regeneration of young and old whole muscle grafts
Day 5 grafts: early events of inflammation and myogenesis
The lack of inflammation at 5 days in old autografts (Og/Oh) contrasts with the extensive inflammation seen in old muscles implanted into young hosts (Og/Yh). The Og/Yh grafts emphasise the impact of the environment on the early events of regeneration such as inflammation (strong response by young compared with old host to old muscle) and also demonstrate a delayed myogenic response by the old muscle. The importance of systemic host factors in the early events of muscle regeneration was also demonstrated, after damage to muscles in situ by cold injury, by elegant parabiotic studies between young and old (24 month) C57Bl mice (reviewed in (Brack and Rando 2007)). However, our results show that systemic factors in geriatric host mice are capable of a strong inflammatory response when they receive the appropriate signals (e.g. from young muscle) and are sufficient to support early events of myogenesis (in young muscles), as demonstrated by the observation that the extent of inflammation and new myotube formation was similar within young grafts transplanted into young (Yg/Yh) and old (Yg/Oh) hosts. Successful regeneration of the Yg/Oh transplants does not support the notion that the old host systemic environment is inhibitory to myogenesis.
The extensive inflammation throughout young grafts in old hosts (Yg/Oh) at 5 days, compared with old autografts (Og/Oh), indicates that damaged geriatric muscle lacks the appropriate signals to chemoattract the old leukocytes. This accords with studies using the corneal micropocket assays to measure angiogenesis in vivo, where old muscle (21 months) provided a far weaker initial stimulus for angiogenesis compared with young (3 months) muscle (Smythe et al. 2008) and it seems likely that a decrease in such stimulatory factors would be even more pronounced in the geriatric muscles (27–29 months). The nature of these signalling molecules that stimulate angiogenesis and attract neutrophils and macrophages to the site of damage (e.g. chemotactic cytokines, breakdown products of extracellular matrix), that are decreased in damaged geriatric muscle, remains to be defined.
Inflammation and angiogenesis are essential pre-requisites in vivo for new muscle formation. If these critical events are impaired then removal of the necrotic damaged tissue and progression of myogenesis cannot occur. Instead fibrosis eventually results (Robertson et al. 1992). This point is emphasised by studies in young adult C57Bl mice where inflammation was blocked, by specific antibodies or soluble receptors to the pro-inflammatory cytokine tumour necrosis factor (Grounds et al. 2005), and the regenerative response of whole muscle grafts was essentially paralysed at 5 days with much persisting necrotic muscle tissue (70–90%) similar to the appearance of old autografts in the present study. However, by 7 days compensatory factors allowed regeneration to progress, the necrotic tissue was phagocytosed by inflammatory cells (<20% necrotic core remaining) and excellent new muscle formation then occurred with myotubes occupying the entire graft by 14 days (Grounds et al. 2005; Radley et al. 2008).
Inflammation and neovascularisation of injured skeletal muscle are regulated by many components, including the extracellular matrix and release of growth factors from both muscle and non-muscle cell types and these and many other factors alter with age (reviewed in (Grounds 1998; Smythe et al. 2008)). Activation of the inflammatory response of the elderly would seem to be a particularly critical target, in order to accelerate subsequent cellular events required for effective new muscle formation in response to injury.
In addition to the weaker inflammatory stimulus produced by geriatric muscle, there is also a delayed activation of myogenic cells in geriatric muscle. This is demonstrated by the observation that while inflammation was well advanced in old grafts transplanted into young hosts (Og/Yh) at 5 days, myoblast activation and formation of new myotubes was delayed in these old muscles. This agrees with many reports from tissue culture studies of myogenic precursors (presumed satellite cells) extracted from old muscles, and from in vivo studies, where reduced local production of growth factors and a lack of receptors for key growth factors is associated with old muscle (reviewed in (Grounds 1998)). However, such an initial short delay in activation does not result in impaired myogenesis. The timing of such complex cellular responses required for new muscle formation is probably critical in vivo, with prolonged delays resulting in fibrosis that will further impair myogenesis.
Regulation of the quiescent state and activation of satellite cells in vivo is complex (Grounds and Relaix 2009) and Notch/Wnt signalling has been implicated in the delayed myogenic response in old animals. Activation of Notch enhanced myotube formation at 5 days after cold injury of aged (24 month) mouse muscle and inhibition of Notch correspondingly impaired muscle formation in young muscles (Conboy 2003): it was concluded that the Delta ligand for Notch is deficient in old mice and thus Notch signalling is impaired in very old muscles. Further short-term studies to test the importance of circulating factors used parabiosis, where the vascular supply was conjoined between combinations of young (2–3 months) and old (19–26 months) mice (on a C57Cl background strain) for 4 weeks prior to freeze injury of muscles (Conboy 2005; Brack et al. 2007). This showed that the impaired myotube formation and sustained proliferation of myogenic cells seen at 5 days after injury of old (compared with young) muscles, was reversed by exposure to the young circulation and the young serum upregulated Delta and enhanced Notch signalling in myofiber explants isolated after parabiosis, and in cultured satellite cells (Conboy 2005). Other studies using the parabiotic model concluded that elevated Wnt signalling may generally promote an aging phenotype, possibly resulting from increased amounts of Wnt or Wnt-like molecules in the aged serum that may delay activation of satellite cells (Brack et al. 2007; Brack 2008). It was further proposed that Notch-1 receptor activation is necessary during early activation and proliferation of satellite cells, whereas members of the Wnt family (possibly serum derived) are important for myogenic lineage commitment and myoblast differentiation: see Gopinath and Rando (2008) for a review of these factors and the stem cell niche in aged muscle. It is striking that adverse effects of the old systemic environment on myogenesis were not apparent in our study where the kinetics of activation and fusion of myogenic cells (by 5 days) in regenerating young muscles exposed to the serum of geriatric hosts (Yg/Oh) appeared similar to that seen in young autografts (Yg/Yh). Since the parabiotic studies did not examine muscles beyond 6 days after injury, there is unfortunately no information regarding whether good new muscle was subsequently formed in old muscles after an initial (transient) delay as is reported in our study and older literature: a crucial point.
Day 10 grafts: myotubes and maturation
To test whether the onset of myogenesis was merely delayed (rather than impaired) in old muscles, grafts were also examined at 10 days. The extent of inflammation and extensive new muscle formation in old autografts (Og/Oh) at 10 days strongly supports an excellent inherent myogenic capacity of geriatric muscles and indicates merely a delay in the whole regenerative process within the old grafts. The extent of myotube formation was similar to that seen in all groups at 10 days. This agrees with the previous study, using mice up to 21 months of age where the extent of new muscle formation in regenerating grafts at 7 days was similar in all young and old host combinations (Smythe et al. 2008). These studies show that new muscle formation (myogenesis) in regenerating geriatric muscle within old hosts can be very good. Two decades ago, the long-term regeneration of old EDL autografts and cross-transplanted EDL grafts between young and old rats after 60 days was examined with respect to physiological function and morphology (Carlson and Faulkner 1989). Compared to young autografts, regenerated autografts in 24 month old rats had impaired function and were characterised by smaller muscle fibers and higher amount of interstitial connective tissue. In cross-transplants the success of regeneration was associated with the age of the host and old muscles grafted into young hosts regenerated much better than young muscle grafted into old hosts. This study appears to relate strongly to host factors and an impaired capacity for successful re-innervation of the regenerated muscles in old hosts and makes no comment on their early myogenic response (Carlson and Faulkner 1989). Further studies in rats show excellent new muscle formation and similar muscle mass and contractile properties of grafts at 41 days when EDL muscles from geriatric rats (aged over 32 months, near the end of their lives) were cross-transplanted into young hosts (Og/Yh) and compared with young autografts (Yg/Yh) (Carlson et al. 2001): this study did not include equivalent grafts into old hosts because of problems with survival of geriatric host rats. The same study (Carlson et al. 2001) showed excellent regeneration of geriatric rat muscles (sometimes exceeding that of young rats) at 41 days after damage in situ by the myotoxic anaesthetic bupivacaine (a model where the nerve and blood supply remains relatively intact) again confirming good myogenic capacity of geriatric muscles although the very early cellular events (prior to 41 days) were not investigated.
An important detailed time course of very early and later events of regeneration in old muscle was described over 20 years ago in rats aged 3 months, 1 and 2 years (Sadeh 1988) in response to intramuscular injection of bupivacaine (marcaine). Inflammation was rapid, myotubes were present at 3 days and myogenesis was essentially completed by 7 days in the young rats, this time-course was similar although slightly slower (e.g. myotubes first seen at day 4) in 1 year old rats. The delay in the early events of inflammation and myotube formation was more pronounced in 2 year old rats, with myotubes not formed until day 7. Mature myotubes were conspicuous in 2 year old rats by 14 days, demonstrating successful new muscle formation. Sadeh (1988) also reported that subsequent maturation of these new myofibers in old rats was slightly impaired and split myofibers became evident over time (at 1 and 2 months), in agreement with many other longer-term studies of regenerating muscles in geriatric rats.
These observations by Sadeh (1988) of delayed inflammation but subsequent excellent myogenesis in aging rats agree closely with the initial delay of inflammation and early myogenic events seen in geriatric muscle autografts in mice in our study. The extent of new muscle formation (at 4 weeks) was also comparable when isolated myofibers from young (1–2 months) or old (22–30 months) mice were implanted into irradiated muscles of young hosts (Collins et al. 2007) and the myogenic potential of satellite cells from old and young muscles was generally similar. Whether the impaired myotube formation and sustained proliferation of myogenic cells seen at 5 days after muscle injury in old (24 months) mice (Conboy 2005) merely reflects such a delay in the onset of myogenesis is not clear, since muscles were not examined at later times.
Differences in regeneration between models of muscle injury
It is noted that regenerative events (e.g. onset of myoblast replication and myotube formation) in traumatized muscle occur more rapidly, about 1 day earlier, than the equivalent events in mouse muscle grafts (due to issues with the speed of inflammation and revascularisation) (Roberts et al. 1989). What is intriguing, is that myoblast proliferation (measured by labelling replicating DNA with tritiated thymidine and subsequent autoradiography) in injured young muscles ceases by about 5 days even where new muscle formation is incomplete whereas, in transplanted grafts myogenesis is sustained for much longer, up to about 10 days (Roberts et al. 1989). This suggests some inhibition of ongoing myogenesis by the adjacent uninjured adult host muscle tissue that is in intimate association with the damaged muscle: indeed such cessation of myoblast replication may coincide with full differentiation and maturation of the myotubes into myofibers. It appears that such factors that shut down myoblast replication and enhance differentiation may be reduced in denervated muscle, where much ‘abortive myogenesis’ is observed (Borisov et al. 2005a). This may also be the case in aged muscles, where partial denervation of geriatric muscles may initiate intrinsic ‘abortive myogenesis’ and problems with re-innervation can limit the maturation of new myofibers formed in response to experimental damage (Carlson 1995; Carlson et al. 2001; Edstrom and Ulfhake 2005). A lack of factors in geriatric muscles that suppress myoblast replication is supported by observations in EDL muscle of geriatric rats (aged 32–34 months) injured by intramuscular injection of Marcaine where, unlike old control muscles, continuing myogenesis at 41 days and ‘the presence of numerous activated satellite cells was a prominent feature of the very old Marcaine-treated muscles’ (Carlson et al. 2001). Studies with isolated myofibers derived from very old (22–30 months) mouse muscles (Collins et al. 2007) also concluded that aging perturbs the myogenic progression of activated satellite cells and this is a widely held view (reviewed (Brack and Rando 2007)). Many factors are known to help switch myoblast replication into differentiation, but the precise nature of these factors that appear to be reduced in denervated, and probably aging (partially-denervated) muscles, is of much interest to define. Such factors may be derived from intact myofibers, from segments of ‘relatively undamaged’ myofibers contiguous with the damaged region, from myoblasts and myotubes or could be associated with cells in the interstitial connective tissue or extracellular matrix components.
Concluding remarks
The inflammatory and neovascular responses to skeletal muscle injury (by whole muscle grafting) are strikingly delayed at 5 days for geriatric muscle in an old environment, but this effect is transient and overcome by 10 days, with very good new muscle formation then being observed. The initial delay in infiltration of inflammatory cells into geriatric muscle autografts seems to be a combined effect of (i) a less vigorous inflammatory response by geriatric hosts and (ii) a less potent chemotactic stimulus produced by the damaged geriatric muscle. Neither of these is a limiting problem per se when combined instead with young hosts or young muscles. Identification of the precise chemotactic signals that differ between the young and geriatric muscle is an interesting challenge. In old animals and humans, the use of drugs to accelerate the initiation of inflammation (an essential pre-requisite for myogenesis) in response to situations of experimental, accidental or surgical damage, would seem a good strategy to speed up the early events of aged muscle regeneration. Even when inflammation is present (iii) there is a delayed onset of myogenic cell activation in geriatric muscles. The delay in regenerative events merely postpones, but does not prevent subsequent myogenesis in the old muscles. This confirms (iv) that myogenic (stem) cells retain a good capacity for myogenesis in geriatric skeletal muscles. This graft model differs to other models of muscle damage where old/geriatric muscles are injured in situ. Muscle grafts are initially isolated from the vascular supply (and require revascularisation) and what may be especially important is that the graft is disconnected from the immediate influence of extant old myofibers that may exert an adverse effect on muscle differentiation and maturation (due to denervation). There appears no justification for proposed stem cell therapy to treat sarcopenia, since the intrinsic myogenic (stem) cells seem quite adequate and excellent new muscle can be formed even in geriatric muscle. Instead, the environment of the muscle cells is critical with respect to both local and systemic factors.
Experimental procedures
Phenotypic characterisation of mice aged, 3, 15, 24 and 27/29 months
In order to clearly document sarcopenia over time, a detailed phenotypic characterisation was carried on 4 groups of inbred female C57Bl/6J mice aged 3 to 29 months of age (Table 1). Mice aged 15 and 24 months were only sampled for this characterisation analysis; whereas the mice aged 3 and 27/29 months were used for the muscle transplantation experiments (Table 2). The older mice aged 15, 24 and 27/29 months were obtained from a colony of old mice at the Queensland breeding facility (Royal Brisbane hospital). After transportation, mice were allowed to acclimatise for at least 1 week before sampling or surgery. Female C57Bl/6J mice aged 3 months were obtained from the Animal Resource Centre (Murdoch, Western Australia). All animal procedures were carried out in strict accordance with the guidelines of the National Health and Medical Research Council of Australia.
Transplantation of whole muscle grafts in mice
Muscles were transplanted between female C57Bl/6J mice aged 27 to 29 months (geriatric) and younger mice aged 10–12 weeks with the different groups summarised in Table 2. Mice were anesthetized with a gaseous mixture of 1.5% isoflurane (Merial), N2O, and O2 and whole muscle autograft transplantation was performed as described previously (Roberts and McGeachie 1992; Roberts et al. 1997; Shavlakadze et al. 2004; Smythe et al. 2008). In brief, all mice received 2 grafts of whole intact extensor digitorum longus (EDL) muscles, either from the same mouse (autograft), or a cross-transplant from a different aged mouse of the same strain. The EDL muscles were removed from both hindlegs of donor mice and relocated over the tibialis anterior (TA) muscles of the host. Each EDL muscle was sutured proximally to the distal tendon of the quadriceps femoris muscle and distally to the distal tendon of the TA, and the skin closed with sutures. Numbers of animals used in whole muscle graft transplantation experiments are summarized in Table 2. (Note: while each mouse received 2 grafts, all of these grafts were not used for the present study).
Muscle sampling
For sampling, mice were anesthetized and euthanased by cervical dislocation. Mice were weighed, quadriceps and gastrocnemius muscles from both legs removed and weighed as a general assessment of any loss of muscle mass and the abdominal fat pads were also removed and weighed in many but not all cases. When sampling whole muscle grafts, surgery was taken as day 0 and muscles were sampled at 5 and 10 days after grafting. Whole EDL muscle grafts attached to the underlying TA muscles were removed, immersed overnight in full-strength fixative (10% buffered formalin, pH 7.0) at room temperature and processed in a Lynx automatic tissue processor. Muscle grafts were dissected in the mid-region, embedded with both cut surfaces at the top of the paraffin block, and 5 μm transverse sections were collected onto silanated glass slides and stained with Haematoxylin and Eosin (H&E) for histological analysis.
Immunocytochemistry for Desmin
Polyclonal rabbit anti-desmin (DAKO; Carpinteria, CA) primary antibody was used to identify muscle cells (myoblast and myotubes) in regenerating grafts. The primary antibody was detected with donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories; West Chester, PA) in conjunction with avidin d-peroxidase (Vector Laboratories). Paraffin sections were de-waxed, rehydrated, and antigen retrieval was performed before immunostaining for desmin as described previously, with color development using peroxidase substrate DAB (Roche Diagnostics; Sydney, Australia) and counterstaining with hematoxylin (Shavlakadze et al. 2004).
Histological and morphometric analyses
All samples were coded, then analysed “blind” using a light microscope. Each section was examined at low power m through to high power (under 100× oil immersion) and analysed specifically for the infiltration of the inflammatory cells and the presence of myogenic cells, in addition to persisting necrotic muscle tissue. Digital images of the entire grafts stained with H&E and immunostained for desmin were taken and tiled using ImagePro Plus 4.5.1 (Microsoft) software and an automated microscope stage movement mechanism.
Images of the H&E stained grafts were used to measure the areas of regeneration/inflammation and necrosis. These areas were expresses as the percentage of the total graft area. Myotubes (round shaped desmin-positive cells with well-pronounced cytoplasm and centralized nuclei (Fig. 3)) were counted on digital images of the grafts immunostained for desmin. Since in day 5 grafts myotube distribution is uneven throughout the graft (they are only in the regeneration zone), the total numbers of myotubes were presented per graft. However, in day 10 grafts where more mature myotubes occupied the entire area (in most cases) their number was standardised per 0.1 mm2 of the graft to assess myotube density.
References
Baker BA, Hollander MS, Mercer RR, Kashon ML, Cutlip RG (2008) Adaptive stretch-shortening contractions: diminished regenerative capacity with aging. Appl Physiol Nutr Metab 33:1181–1191
Borisov AB, Dedkov EI, Carlson BM (2005a) Abortive myogenesis in denervated skeletal muscle: differentiative properties of satellite cells, their migration, and block of terminal differentiation. Anat Embryol (Berl) 209:269–279
Borisov AB, Dedkov EI, Carlson BM (2005b) Differentiation of activated satellite cells in denervated muscle following single fusions in situ and in cell culture. Histochem Cell Biol 124:13–23
Brack A, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3(12):226–237
Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807–810
Brack A, Conboy IM, Conboy MJ, Shen J, Rando TA (2008) A temporal switch from Notch to Wnt signalling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2:50–59
Brooks SV, Faulkner JA (1988) Contractile properties of skeletal muscle from young, adult and aged mice. J Physiol 404:71–82
Carlson BM (1995) Factors influencing the repair and adaption of muscles in aged individuals: satellite cells and innervation. J Gerontol 50A:96–100
Carlson BM, Faulkner JA (1989) Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 256:1262–1266
Carlson BM, Dedkov EI, Borisov AB, Faulkner JA (2001) Skeletal muscle regeneration in very old rats. J Gerontol 56A:B1–B10
Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA (2007) A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25:885–894
Conboy IM (2003) Notch mediated restoration of regenerative potential to aged muscle. Science 302:1577–1757
Conboy IM (2005) Rejuvination of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2003) MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res 311:401–416
Desaki J (2008) Regeneration of muscle fibers in the extensor digitorum longus muscle of the aged rat. J Electron Microsc (Tokyo) 57:59–66
Edstrom E, Ulfhake B (2005) Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 4:65–77
Edstrom E, Altun M, Bergman E, Johnson H, Kullberg S, Ramirez-Leon V, Ulfhake B (2007) Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav 92:129–135
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000) Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88:1321–1326
Gopinath SD, Rando TA (2008) Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7:590–598
Grounds MD (1998) Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci 854:78–91
Grounds M, Relaix F (2009) Myogenic Precursor Cells. Chapter 2 in Section I—Scientific basis of muscle disease. In: Hilton-Jones D, Griggs R, Bushby K, Karpati G (eds) Disorders of Voluntary Muscles. Cambridge University Press, Cambridge
Grounds MD, Davies M, Torrisi J, Shavlakadze T, White J, Hodgetts SI (2005) Silencing TNFα activity using Remicade® or Enbrel® blocks inflammation in whole muscle grafts: an in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell Tissue Res 320:509–515
Kaminska A, Fidzianska A, Schulze G, Coper H, Ossowska K, Wolfarth S, Hausmanowa-Petrusewicz I (1998) Ultrastructural changes in the skeletal muscle of senile rats with significant age-dependent motor deficits. Basic Appl Myol 8:185–190
Lynch GS, Shavlakadze T, Grounds MD (2005) Strategies to reduce age-related skeletal muscle wasting. In: Rattan S (ed) Ageing Interventions and Therapies. World Scientific Publishers, Singapore, pp 63–84
Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, Gidaro T, Jacquemin V, Mamchaoui K, Negroni E, Perie S, Renault V, Silva-Barbosa SD, Butler-Browne GS (2005) The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand 184:3–15
Pagala MK, Ravindran K, Namba T, Grob D (1998) Skeletal muscle fatigue and physical endurance of young and old mice. Muscle Nerve 21:1729–1739
Radley HG, Davies MJ, Grounds MD (2008) Reduced muscle necrosis and long-term benefits in dystrophic mdx mice after cV1q (blockade of TNF) treatment. Neuromuscul Disord 18:227–238
Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V (2002) Regenerative potential of human skeletal muscle during aging. Aging Cell 1:132–139
Roberts P, McGeachie JK (1992) The influence of revascularisation, vasoactive drugs and exercise on the regeneration of skeletal muscle, with particular reference to muscle transplantation. BAM 2:5–16
Roberts P, McGeachie JK, Grounds MD, Smith ER (1989) Initiation and duration of myogenesis in transplants of intact skeletal muscles: an autoradiographic study in mice. Anat Rec 224:1–6
Roberts P, McGeachie JK, Grounds MD (1997) The host environment determines strain-specific differences in the timing of skeletal muscle regeneration: cross-transplantation studies between SJL/J and BALB/c mice. J Anat 191:585–594
Robertson TA, Grounds MD, Papadimitriou JM (1992) Elucidation of aspects of murine skeletal muscle regeneration using local and whole body irradiation. J Anat 181:265–276
Sadeh M (1988) Effects of aging on skeletal muscle regeneration. J Neurol Sci 87:67–74
Shavlakadze T, Grounds MD (2003) Therapeutic interventions for age-related muscle wasting: importance of innervation and exercise for preventing sarcopenia. In: Rattan S (ed) Modulating aging and longevity, Chap 9. Kluwer Academic publisher, The Netherlands, pp 139–166
Shavlakadze T, Davies M, White JD, Grounds MD (2004) Early regeneration of whole skeletal muscle grafts is unaffected by overexpression of IGF-1 in MLC/mIGF-1 transgenic mice. J Histochem Cytochem 52:873–883
Shavlakadze T, Boswell JM, Burt DW, Asante EA, Tomas FM, Davies MJ, White JD, Grounds MD, Goddard C (2006) Rskalpha-actin/hIGF-1 transgenic mice with increased IGF-I in skeletal muscle and blood: impact on regeneration, denervation and muscular dystrophy. Growth Horm IGF Res 16:157–173
Smythe GM, Shavlakadze T, Roberts P, Davies MJ, McGeachie JK, Grounds MD (2008) Age influences the early events of skeletal muscle regeneration: studies of whole muscle grafts transplanted between young (8 weeks) and old (13–21 months) mice. Exp Gerontol 43:550–562
Snijders T, Verdijk LB, van Loon LJ (2009) The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev 8:328–338
White JD, Scaffidi A, Davies M, McGeachie J, Rudnicki MA, Grounds MD (2000) Myotube formation is delayed but not prevented in MyoD-deficient skeletal muscle: studies in regenerating whole muscle grafts of adult mice. J Histochem Cytochem 48:1531–1544
White JD, Collins R, Vermeulen R, Davies M, Grounds MD (2002) The role of p53 in vivo during skeletal muscle post-natal development and regeneration: studies in p53 knockout mice. Int J Dev Biol 46:577–582
Acknowledgments
This study was supported by funding from the National Health and Medical Research Council (Australia). We thank Kirsten Maley for carrying out immunostaining.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shavlakadze, T., McGeachie, J. & Grounds, M.D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 11, 363–376 (2010). https://doi.org/10.1007/s10522-009-9260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-009-9260-0