Abstract
Immunosenescence involves age-associated restructuring changes of innate and adaptative immune functions. We have suggested that these changes of the immune system participate in the rate of ageing through modulating oxi-inflamm-ageing. Thus, age-related changes in the immune system can be biological age markers and predictors of longevity. Gender differences in oxidation status and immune functions have been observed in rats, with males showing higher oxidation and immunosenescence than females of the same age. Oestrogens are sex hormones that actively participate in modulating the mammalian immune function and, therefore, the age-related impairment of the immune response is drastically accelerated in females during the menopausal transition. Ovariectomy in rodents constitutes a good model for mimicking human oestrogen loss and thus the menopausal situation. Recently, we have shown the deleterious effects of oestrogen loss on several functions of leukocytes from immune organs in rats and mice. In addition, ovariectomised rats show similar levels in these immune functions to those in males. The present work studied several functions as well as inflammatory and oxidative stress parameters in mouse peritoneal macrophages and lymphocytes from old sham and ovariectomised females, as well as in males of the same age. In general, the results show that females, which have a higher immune response and a lower oxidation and inflammation than males, appear similar to males in the parameters studied when they have lost oestrogens by ovariectomy. Thus, these data support the positive role of oestrogens in the immune function through the ageing process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is accompanied by a functional decline of all the physiological systems. Regarding the immune system, the immunosenescence process involves age-associated restructuring changes of almost every component of the immune system, including innate and adaptative functions. Moreover, as immune cells produce free radicals and inflammatory compounds in order to perform their defensive functions, immunosenescence is closely linked to the oxidative-inflammatory condition found in the elderly (De la Fuente and Miquel 2009).
Although such immune changes take place in both genders along the ageing process (De la Fuente et al. 2004a), different studies that have compared the immune function in male versus female subjects have shown that it works more effectively and for longer time in females with respect to their male counterparts (Marriott and Huet-Hudson 2006; Nunn et al. 2009). These gender-related differences in immunity, with females producing more vigorous cellular and humoral immune responses, have been attributed to the immunomodulatory action of oestrogens (Bouman et al. 2005; Ackerman 2006). Thus, these hormones have been described as responsible for the higher immunocompetence of females as they modulate lymphocyte development, antibody and cytokine production, cell survival, etc. (Islander et al. 2003; Ackerman 2006).
There is a specific moment in female’s life in which the age-related impairment on the immune competence results drastically accelerated: the menopausal transition. The subsequent oestrogen loss results in a deficit in the immune function and an impaired antioxidant protection (Miquel et al. 2006; Farage et al. 2009), making women more prone to experience disease and disability (Amir et al. 2003). Despite the fact that there has been a dramatic increase in life expectancy during the last decades of the twentieth century, the age at which women encounter the menopausal transition has remained essentially constant at around 50 years, so post-menopausal years now constitute one third of the lifespan of most women. Therefore, the repercussions of menopause on female health have become a subject of increasing interest.
In rats and mice, ovariectomy constitutes a commonly used model for mimicking human ovarian loss, and it is used for searching the overall effects of oestrogens insufficiency. Previous studies published by our research group have described that ovariectomy results in a premature ageing of functions in rat leukocytes from spleen and axillary nodes (De la Fuente et al. 2004a; Baeza et al. 2009), as well as in a premature ageing of the nervous system assessed through sensorimotor and anxiety-like behavioural tests (Baeza et al. 2010a). Moreover, ovariectomy also causes an accelerated imbalance between oxidant production and antioxidant levels in favour of the former, with resulting oxidative stress in spleen, liver, heart and kidney cells (Baeza et al. 2010b). In this context, and considering the role of oxidation and inflammation in the ageing process, we have suggested ovariectomy as a model of premature ageing (De la Fuente and Miquel 2009; Baeza et al. 2010a). In addition, males in mammals generate more oxidant compounds than females, which contribute to explain why females live longer than males (Borras et al. 2007).
Last but not least, we must consider the concept of “biological age,” which arises as a consequence of the different rates of physiological changes in the members of a population of the same chronological age and suggests that chronological and biological age do not necessarily coincide (De la Fuente and Miquel 2009). The immune system has been proposed as a good marker of biological age and predictor of life span (Wayne et al. 1990). Using peritoneal leukocyte suspension (a relatively non-invasive sample of easy access in mice), experimental research previously conducted by our group has shown that peritoneal cells of aged mice display impairments in several of their functions and an increase in a variety of oxidative-inflammatory stress parameters (which have been proposed as markers of biological age, premature ageing and consequently predictors of longevity) (De la Fuente and Miquel 2009; Arranz et al. 2010a, b).
The aim of the present work has been going deeper in the characterization of ovariectomy as a model of premature ageing through the study of several functions and oxidative and inflammatory stress parameters in peritoneal leukocytes of aged sham-operated and ovariectomised female mice, as well as to compare such performance with that from peritoneal leukocytes of males from the same chronological age.
Materials and methods
Female and male ICR-CD1 mice were obtained from Harlan Iberica (Barcelona, Spain) at the age of 6 months. They were specific pathogen-free, as tested by Harlan according to Federation of European Laboratory Science Associations recommendations. Female mice were randomly divided and each group was housed in a polyurethane box, at a constant temperature (22 ± 2°C) on an inverted 12-h light/dark cycle. All mice were fed standard laboratory diet (Panlab A04), with free access to food and water. Animals were treated according to the guidelines of the European Community Council Directives 1201/2005 EEC.
Surgical procedure (ovariectomy)
Although female rats and mice become anovular at 10–12 months of age, they maintain plasma oestrogen levels of about 40 pg/ml until 2 years of age, similar to those of young animals in diestrus (Lu et al. 1979), in contrast to the ovaries of women, where the synthesis of gonadal steroid is suppressed at menopause (Nelson 2008). For this reason, female mice were submitted to ovariectomy or sham-operated (ovaries were manipulated but not excised) at 13 months of age according to the method previously described in Baeza et al. (2010a). After surgery, mice were housed individually for some hours to allow recovery and then re-grouped in their home cages. Intact male mice were housed in the same conditions as females.
Collection of leukocyte suspensions
When animals reached 20 months of age, peritoneal cellular suspensions were obtained between 8:00 and 10:00 a.m. without killing the animals.
Each mouse was held by the cervical skin, the abdomen was cleansed with 70% ethanol and 3 ml of sterile Hank’s solution (previously tempered at 37°C) were injected intraperitoneally. After abdominal massage, approximately 80% of the injected volume was recovered. The following studies were performed using unfractioned peritoneal leukocytes in order to better reproduce the in vivo immune response and oxidative status. Macrophages and lymphocytes from the peritoneal suspensions were identified by their morphology and quantified in Neubauer chambers using optical microscopy (40×). Cellular viability, determined in each experiment using the Trypan Blue exclusion test, was in all cases higher than 95%. The peritoneal suspensions were adjusted to a specific number of macrophages, lymphocytes or total leukocytes depending on the parameter analysed, as described in the corresponding section.
Assays of peritoneal macrophages function
Macrophages were counted and then adjusted by dilution with Hank’s solution to a concentration of 5 × 105 macrophages/ml, with the exception of the microbicidal capacity assay, where aliquots were adjusted to 1 × 106 leukocytes/ml.
Chemotaxis capacity
The assay was carried out according to the method previously described by Boyden in 1962 with some modifications (Alvarado et al. 2005a). Chambers with two compartments separated by a filter (Millipore, Ireland) of 3 μm pore diameter were used. Aliquots of peritoneal suspensions were placed in the upper compartment, and the chemoattractant agent fMet-Leu-Phe (Sigma) was placed in the lower compartment at a concentration of 10−8 M. The number of lymphocytes in the lower face of the filter (CI) was calculated by counting, in an optical microscope, the total number of macrophages in one-third of the lower face of the filters. All CI were assayed in duplicate.
Phagocytosis capacity
Phagocytosis of inert particles was assayed following the method described by De la Fuente in 1985. Peritoneal suspensions were incubated in MIF plates (KARTELL) for 30 min at 37°C in a humidified atmosphere of 5% CO2. The adhered monolayer obtained was washed with pre-warmed PBS, and then Hank’s solution and latex beads (1.09 mm diameter, diluted to 1% in phosphate-buffered saline, Sigma) were added. After 30 min of incubation, plates were washed, fixed and stained. The number of particles ingested by 100 macrophages was counted in an optical microscope (100×) and expressed as phagocytic index (PI). The percentage of macrophages with one or more latex beads ingested was also determined and expressed as phagocytic efficiency (PE).
Digestion capacity: intracellular ROS levels
The digestion capacity of the foreign material is carried out principally through production of reactive oxygen species (ROS). Intracellular ROS levels were measured by fluorometry following a method previously described (Alvarado et al. 2005a). 2,7-Dichlorofluorescin diacetate (DCF-DA; MOLECULAR PROBES) was used as a probe because it is oxidized in the cytoplasm by ROS to 2,7-dichlorofluorescin (H2DCF), a highly fluorescent compound. The peritoneal suspension was incubated with DCF-DA (1 mM) and phorbol myristate acetate (PMA; 50 ng/ml) (stimulated samples) or Hank’s solution (nonstimulated samples). Finally, the samples were analyzed by fluorometry. The results are expressed as the stimulation index in response to PMA (phorbol miristate acetate), with the value of the nonstimulated samples being 100%.
Assays of peritoneal lymphocyte functions
Lymphocytes obtained from the peritoneal suspension were counted and then adjusted by dilution with Hank’s solution to a concentration of 1 × 106 lymphocytes/ml, with the exception of the chemotaxis assay, where aliquots were adjusted to 5 × 105 lymphocytes/ml.
Chemotaxis assay
The lymphocyte chemotaxis capacity was evaluated as described above for macrophages.
Proliferation assay
The proliferation of lymphocytes in response to the mitogens Concanavalin A (ConA) and lipopolisaccharide (LPS) was assayed following a method previously described (Alvarado et al. 2005a). Aliquots of lymphocyte suspension were dispensed in 96-well flat-bottomed microtiter plates (NUNC) and incubated in the presence or absence (controls) of ConA or LPS (1 μg/ml; Sigma) during 48 h at 37°C in a humidified atmosphere of 5% CO2. Then, 3H-thymidine (ICN, Costa Mesa, USA) was added to each well and, after 8 h, cells were harvested and thymidine uptake was measured in a beta counter. The results were expressed as percentage of proliferation in response to ConA or LPS, with 100% being the thymidine uptake counts per minute (cpm) in control wells (without mitogen). Each sample was assayed in triplicate.
Cytotoxicity assay
An enzymatic colorimetric assay was used for cytolysis measurements of target cells (Cytotox 96 TM Promega, Boerinher Ingelheim) based on the determination of the activity of the enzyme lactate dehydrogenase (LDH), using tetrazolium salts as previously described (Alvarado et al. 2005a). YAC-1 cells, from a murine lymphoma, were used as target cells. Each sample was assayed in triplicate. Results were expressed as percentage of tumoral cell lysis.
Cytokine levels
The levels of interleukin-2 (IL-2) and IL-10 were determined on supernatants of lymphocyte cultures in the presence of ConA, and IL-6 levels were determined on supernatants of lymphocyte cultures in the presence of LPS. After 48 h of incubation with mitogens, the supernatants were collected and frozen at −20°C until assay. Cytokines were measured using different enzyme-linked immunosorbent assays (ELISA; R&D System), and the results were expressed as pg/ml.
Apoptosis assay
This assay was carried out following the technique previously described by Wirleitner et al. (1998) with some modifications. The fluorescent probe JC-1 (Molecular Probes) was used. Aliquots of the peritoneal suspension were set in a 96-bottom well opaque plate. In control wells, PBS was added and, in apoptosis-induced wells, H2O2 was added at a concentration of 500 μM (the final concentration in the well was 50 μM). Moreover, some wells carrying only probe (in order to evaluate autofluorescence of the probe) were also set. Afterwards, the plate was incubated for 6 h at 37°C. The probe was then added to control and stimulated wells, and the plate was incubated 30 min more. Finally, fluorescence was measured at 485 nm. Results are expressed as % stimulation in response to H2O2, with 100% being the basal fluorescence (in absence of H2O2). Each sample was assayed in triplicate.
Antioxidant defences and oxidant levels in peritoneal leukocytes
Glutathione levels
Total glutathione and its oxidized form (GSSG) were spectrophotometrically evaluated according to the method of Tietze (1969), with some modifications (Alvarado et al. 2006), by monitoring the change in absorbance at 412 nm. To calculate the GSSG/GSH ratio, a good marker of oxidative stress situation, the GSH (reduced form) was obtained by subtracting the GSSG values from the total glutathione values. The results were expressed as pmol/106 cells.
Glutathione peroxidase assay
Glutathione peroxidase (GPx) enzyme, which plays a fundamental role in the glutathione system allowing its antioxidant function, was measured using the original technique described by Lawrence and Burck (1976) with some modifications (Alvarado et al. 2006). The reaction was followed spectrophotometrically by the absorbance decrease at 340 nm. Results were expressed as mU/106 cells.
Catalase assay
The activity of CAT was determined following the method described by Beers and Sizer (1952), slightly modified (Alvarado et al. 2006). The reaction was initiated by the addition of H2O2 and spectrophotometrically measured at 240 nm for 50 s. Results were expressed as U/106 cells.
Lipid oxidative damage of peritoneal leukocytes
Malondialdehyde levels
The protocol followed was according to the technique previously described by Chirico et al. (1993) with slight modifications (Alvarado et al. 2006). As mobile phase 50 mM KH2PO4 pH 6.8/methanol (90/10 v/v) was used. The flow rate of the mobile phase was adjusted to 0.4 ml/min. The eluted fractions from the HPLC were monitored at a λ = 532 nm in a Waters 486 ultraviolet detector. Standard curves were prepared fresh daily using MDA-bis solution (dimethyl acetal, Sigma). Results were expressed as nmol/106 cells.
Life span
Life span of the female sham group was 103 ± 6 weeks, 105 ± 4 weeks in the female ovariectomised group and 86 ± 4 weeks in intact males.
Statistical analysis
Results were expressed as the mean ± SD of the values. Normality of the samples was checked by the Kolmogorov–Smirnov test, and homogeneity of variances, with the Levene test. The data were statistically evaluated by the Student’s t-test. P < 0.05 was taken as the minimum significance level.
Results
Peritoneal macrophages functions
The chemotaxis index (Fig. 1a) was significantly higher in female sham mice than in males (P < 0.01). However, in ovariectomised animals, the CI got significantly impaired (P < 0.001), resulting in values similar to those found in males.
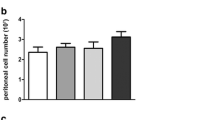
Peritoneal macrophages functions studied: chemotaxis index (CI) (a), phagocytic index (PI) (b) and stimulated intracellular ROS levels (%) (c) in sham and ovariectomised (ovx) female and intact male mice. Each column represents the mean ± SD of the number of animals shown in brackets. ***P < 0.001, **P < 0.01, *P < 0.05 with respect to the female sham group. ## P < 0.01 with respect to the ovx group
In the phagocytic index (PI) (Fig. 1b), sham females showed higher values than their male counterparts (P < 0.05), while such difference was not found between ovariectomised females and males. Although ovariectomy slightly decreased PI, no significant effect had been found. Moreover, no differences due to sex or ovariectomy had been shown regarding the phagocytic efficiency (being the PE indexes 96.5 ± 1.4 for sham females, 97 ± 3 for ovariectomised females and 89.38 ± 6.52 for intact males).
The last step studied in the phagocytic process was the ability of macrophages to digest the particles ingested by the production of intracellular ROS. These ROS levels (Fig. 1c) were slightly decreased in ovariectomised females with respect to control sham mice, and although no significant differences were found between these two groups, ovariectomy resulted in statistically lower ROS levels than those found in control males of the same age (P < 0.01).
Peritoneal lymphocytes functions
Figure 2 shows the lymphocytes chemotaxis index (a), the proliferative response of peritoneal lymphocytes in response to the mitogens Con A and LPS (b and c, respectively) and the NK activity (d). In the four parameters studied, females showed values significantly higher than those found in males (P < 0.001). However, in ovariectomised females these functions suffered a significant impairment, being the values similar to those obtained in males.
Peritoneal lymphocytes functions studied: chemotaxis index (a), proliferative response to ConA (b), proliferative response to LPS (c) and natural killer activity (d) in sham and ovariectomised (ovx) female and intact male mice. Each column represents the mean ± SD of the number of animals included in each experimental group (in brackets). ***P < 0.001, **P < 0.01 with respect to the female sham group. ### P < 0.001 with respect to the ovx group
The levels of three cytokines secreted in response to mitogens are shown in Fig. 3. IL-2 and IL-10 levels (two anti-inflammatory cytokines) were analysed in supernatants of cultured cells stimulated with the mitogen ConA (Fig. 3a and b, respectively). IL-6 (a pro-inflammatory cytokine) was studied in supernatants of cultured cells stimulated with LPS (Fig. 3c). Males displayed lower levels of IL-2 (P < 0.05) and IL-10 (P < 0.001) with respect to sham females. When ovariectomy was performed, the levels of these two cytokines decreased (it must be highlighted the striking decrease of IL-10 in ovariectomised mice with respect to sham, P < 0.001), showing these values no statistical differences with those from their male counterparts. With respect to IL-6, no significant differences due to ovariectomy were found in females, but males showed higher IL-6 levels (P < 0.05) than ovariectomised females of the same age.
Levels of the cytokines IL-2 (a), IL-10 (b) and IL-6 (c) in sham and ovariectomised (ovx) female and intact male mice. Each column represents the mean ± SD of the number of animals included in each experimental group (in brackets). ***P < 0.001, *P < 0.05 with respect to the female sham group. # P < 0.05 with respect to the ovx group
With respect to apoptosis, a key mechanism in differentiation and regulation of the immune cells once the pathogens have been cleared, the results showed that H2O2-induced apoptosis increased in ovariectomised females with respect to sham animals (93 ± 4% for sham females and 99 ± 3% for ovariectomised females, P < 0.01), although no differences were found with respect to males (98 ± 7%).
Antioxidant defences, oxidants compounds and lipid peroxidation damage of peritoneal leukocytes
Catalase (CAT) and glutathione peroxidase (GPx) activities were studied for they represent two of the most important antioxidant enzymes in cells. The results showed that CAT activity was similar between sham females and males (15.5 ± 1.66 and 15.7 ± 3 U/106 cells, respectively), and that ovariectomy did not significantly alter this activity (13.9 ± 1.54 for ovariectomised females). Although the same lack of effect of ovariectomy over GPx activity was found (299 ± 66 mU/106 cells for sham females, 360 ± 11 for ovariectomised females), males showed a GPx activity more than twice higher than females (751 ± 19 for intact males, P < 0.001 with respect to sham females and P < 0.01 with respect to ovariectomised females).
Glutathione is the most important non-enzymatic antioxidant defence in cells, and reduced (GSH), oxidized (GSSG) levels and the GSSG/GSH ratio, are shown in Fig. 4a, b and c, respectively. The GSH content in peritoneal leukocytes from sham females was similar to that in males, and at the same time, GSSG was significantly higher in males (P < 0.01), which results in males displaying a tendency of higher GSSG/GSH ratio with respect to sham females. Ovariectomy caused no alteration in GSH levels, but it clearly increased GSSG levels (P < 0.01), with the subsequent rise in the GSSG/GSH ratio (P < 0.01), being such ratio a reliable measure of the cellular oxidative stress levels.
Antioxidant defences, oxidants compounds and lipid peroxidation damage: reduced glutathione levels (a), oxidized glutathione levels (b), ratio GSSG/GSH (c) and MDA levels (d) in sham and ovariectomised (ovx) female and intact male mice. Each column represents the mean ± SD of the number of animals included in each experimental group (in brackets). **P < 0.01 with respect to the female sham group. # P < 0.05 with respect to the ovx group
A consequence of the oxidative stress situation is the increase of lipid peroxidation, which can be measured by MDA levels. The results (Fig. 4d) showed higher MDA levels in ovariectomised females than in sham mice (P < 0.01). MDA levels in males were similar to those found in ovariectomised females, and although they were higher than those in sham female mice, no statistical differences had been found.
Discussion
In the present work we have shown that ovariectomy in female mice, which mimics human ovarian loss and has been proposed as a model of premature ageing of the nervous and immune systems (Baeza et al. 2010a), causes, in peritoneal leukocytes from aged mice, similar levels to males of immunosenescence and oxidative stress.
Sex steroids are involved in various physiological processes besides reproduction (Huber and Gruver 2001). Although there is extensive literature on the effect of oestrogen and its potential to modulate various aspects of the immune response, publications on progesterone are not as extensive, and a number of hypotheses for the function of progesterone have been inferred from conclusions on oestrogen research. In general, it is thought that oestrogen has a stimulatory effect on the humoral immune response, whereas the effect of progesterone is inhibitory (Pauklin and Petersen-Mahrtz 2009; Gameiro and Romao 2010).
In mammals, females live longer than males. This seems to be a consequence of the presence of oestrogens and their capacity to up-regulate the expression of antioxidants and decrease oxidant production (Viña et al. 2006). In addition, males, in comparison to females, show premature immunosenescence (De la Fuente et al. 2004a; De la Fuente and Miquel 2009; De la Fuente and Gimenez Llort 2010) as has been found, for example, in leukocytes from spleen and axillary nodes of female rats with respect to males (De la Fuente et al. 2004a). This fact, together with the higher oxidation observed in males with respect to females (Borras et al. 2007), supports the idea of an older biological age in males versus females. Several parameters of leukocyte function have been proposed as markers of biological age and predictors of longevity, and we have also suggested the role of immune cells in oxi-inflamm-ageing and in the rate of ageing (Guayerbas and De la Fuente 2003; De la Fuente and Miquel 2009). The present work is the first in which immune function and oxidative stress parameters are studied in peritoneal leukocytes comparing old males and both sham and ovariectomised females.
A great deal of research shows that the ageing process is linked to an impairment of peritoneal macrophage functions such as the chemotaxis, phagocytosis and microbicidal capacities (Guayerbas and De la Fuente 2003; Guayerbas et al. 2004; De la Fuente et al. 2004b). However, in the present study it should be highlighted that sham females reach old ages with a macrophage function better preserved with respect to males. Thus, although females and males have the same chronological age, their biological age is not the same, as peritoneal macrophages from sham-operated females display a better immune competence than males in this respect. Regarding the chemotaxis capacity, the boosting effect of oestrogens over leukocyte mobility to peripheral tissues has been described (Cid et al. 1994; Cannon and St Pierre 1997), which could explain why the removal of the main source of oestrogens through ovariectomy has a negative repercussion on this function. The present study shows no relevant effect of ovariectomy on either the phagocytic or the digestion capacities.
Since macrophages collaborate with lymphocytes in their antigen response, an altered macrophage function contributes to the senescent decline in immune function (Sebastián et al. 2005; De la Fuente and Miquel 2009). Lymphocytes are important effectors cells whose activation is essential for key immune responses, such as the chemotaxis and proliferative capacities, the production of cytokines and the NK activity. It has been described that oestrogens are able to stimulate, in these cells, chemotaxis (Cid et al. 1994; Cannon and St Pierre 1997), proliferation (Porter et al. 2001; Bilbo and Nelson 2001) and NK activity (Yang et al. 2000; Keller et al. 2001). In the present work, ovariectomy causes the deterioration of the peritoneal lymphocyte functions studied in females, resulting in levels similar to those of males of the same chronological age. This fact is in accordance with the data obtained in leukocytes from lymphoid organs in aged ovariectomised rats (De la Fuente et al. 2004a). Thus, the privileged immune-competence of females versus males due to oestrogens actions is confirmed.
The idea of an oxidation-inflammation state linked to the ageing process has been recently described (Franceschi et al. 2000; De la Fuente and Miquel 2009), with males showing, in general, a higher level of oxidation than females (Borras et al. 2007). It has been postulated that the lack of female sex hormones associated with menopause/ovariectomy contributes directly to the acceleration of the ageing process, with a characteristic unbalanced inflammatory state (with higher levels of proinflammatory cytokines and lower levels of anti-inflammatory cytokines) (Pfeilschifter et al. 2002; Kovacs 2005). The decreased levels of the anti-inflammatory cytokine IL-10 found after ovariectomy in the present study strongly corroborate this idea. Moreover, males show lower IL-2 and IL-10 levels and higher IL-6 levels than sham females, so it becomes clear that males exhibit a higher inflammatory state, and that oestrogens play a key role in preserving a moderate inflammatory state in older animals.
Regarding the H2O2–induced apoptosis study, data showing higher percentages of stimulation in ovariectomised females indicate a modulatory role of oestrogens on this parameter in peritoneal leukocytes. It has been described that oestrogens increase the expression of the antiapoptotic protein Bcl-2 and decrease caspases 3 and 8, which could be responsible for their antiapoptotic role (Dubal et al. 1999; Ackerman 2006; Tresguerres et al. 2008). Nevertheless, further research should be conducted in this respect to elucidate the molecular mechanisms involved.
Oxidative stress has been implicated in the pathogenesis of several alterations such as the ageing process and the menopausal transition. In women, the menopausal transition is the moment in life in which this oxidative imbalance is drastically accelerated (Massafra et al. 2000). The results of the present study show that ovariectomy in females accelerates the age-related oxidative state resembling the situation found in males, with higher GSSG levels and, subsequently, a higher GSSG/GSH ratio, which represents a reliable measure of the cellular oxidative stress level (Sies 1999).
Regarding the effect of oestrogens on the antioxidant enzymes, it has been described that these hormones bind to their receptors and activate the MAP kinase and the NFkB signalling pathways, thus up-regulating the expression of antioxidant genes and genes encoding the antioxidant enzymes (Viña et al. 2005, 2008). Although in the present work we have not studied the gene expression of antioxidant enzymes, the activities of catalase and glutathione peroxidase studied are not affected by ovariectomy. Results published in this respect are contradictory, with some researchers having found decreased activity of these enzymes after ovariectomy (Ha et al. 2006; Yalin et al. 2006; Kireev et al. 2007) while others have found no effect or even increased activity (Azevedo et al. 2001; Kankofer et al. 2007), which could be due to the different experimental designs followed.
Finally, lipid peroxidation (through MDA levels) was studied as being one of the determinants of ROS-induced oxidative damage. It is known that MDA levels in peritoneal leukocytes increase with ageing (Alvarado et al. 2005b), and our data in aged mice show that MDA levels after ovariectomy are higher than those found in sham mice and similar to those of males. These findings are in accordance with the vast majority of scientific studies published, which have described an increase in lipid peroxidation in different locations (such as liver, kidney or brain) due to ovariectomy (Yalin et al. 2006; Kiray et al. 2004, 2007; Kankofer et al. 2007).
In recent years, our research group has described the negative effects of oestrogen loss on the function and the oxi-inflammatory state of leukocytes in aged female rats and mice (De la Fuente et al. 2004a; Baeza et al. 2009, 2010a, b). The present work performed in peritoneal leukocytes constitutes another step forward in the characterization of ovariectomy as a good model for assessing premature ageing. Furthermore, gender-related differences in the biological age of mice of the same chronological age have been revealed, with females displaying a much better immune competence and oxi-inflammatory state than males. According to the results obtained in the present study, the effect of the oestrogen loss caused by ovariectomy strongly supports the positive role of these hormones on the immune functions through the ageing process.
References
Ackerman LS (2006) Sex hormones and the genesis of autoimmunity. Arch Dermatol 142:371–376
Alvarado C, Álvarez P, Guayerbas N, Puerto M, Jiménez L, De la Fuente M (2005a) El daño peroxidativo en leucocitos peritoneales de ratones viejos disminuye si se suplementa la dieta con galletas enriquecidas con antioxidantes. Relación con la supervivencia. Rev Esp Geriatr Gerontol 40:351–356
Alvarado C, Álvarez P, Jiménez L, De la Fuente M (2005b) Improvement of leukocyte functions in prematurely ageing mice after a 5-week ingestion of a diet supplemented with biscuits enriched in antioxidants. Antiox Redox Signal 7:1203–1210
Alvarado C, Alvarez P, Jimenez L, De la Fuente M (2006) Oxidative stress in leukocytes from young prematurely ageing mice is reversed by supplementation with biscuits rich in antioxidants. Dev Comp Immunol 30:1168–1180
Amir SH, Kuhle CL, Fitzpatrick LA (2003) Comprehensive evaluation of the older woman. Mayo Clin Proc 78:1157–1185
Arranz L, Caamano JH, Lord JM, De la Fuente M (2010a) Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: possible role of nuclear factor kappa B. J Gerontol A Biol Sci Med Sci 65:941–950
Arranz L, De Castro NM, Baeza I, Maté I, Viveros MP, De la Fuente M (2010b) Environmental enrichment improves age-related immune system impairment: long-term exposure since adulthood increases life span in mice. Rej Res 13:415–428
Azevedo RB, Lacava ZGM, Miyasaka CK, Chaves SB, Curi R (2001) Regulation of antioxidant enzyme activities in male and female rat macrophages by sex steroids. Braz J Med Biol Res 34:683–687
Baeza I, Alvarado C, Alvarez P, Salazar V, Castillo C, Ariznavarreta C, Fdez-Tresguerres J, De la Fuente M (2009) Improvement of leucocyte functions in ovariectomised aged rats after treatment with growth hormone, melatonin, oestrogens or phytoestrogens. J Reprod Immunol 80:70–79
Baeza I, De Castro NM, Gimenez-Llort L, De la Fuente M (2010a) Ovariectomy, a model of menopause in rodents, causes a premature ageing of the nervous and immune system. J Neuroimmunol 219:90–99
Baeza I, Fdez-Tresguerres J, Ariznavarreta C, De la Fuente M (2010b) Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in aged ovariectomized rats. Biogerontology 11:687–701
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bilbo SD, Nelson RJ (2001) Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 280:R207–R213
Borras C, Gambini J, Viña J (2007) Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci 12:1008–1013
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11:411–423
Boyden SV (1962) The chemotaxis effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med 115:453–466
Cannon JG, St Pierre BA (1997) Gender differences in host defense mechanisms. J Psychiatr Res 31:99–113
Chirico S, Smith C, Marchant C, Mitchinson MJ, Halliwell B (1993) Lipid peroxidation in hyperlipemic patients. A study of plasma using an HPLC-based thiobarbituric acid test. Free Radic Res Commun 19:51–57
Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS (1994) Estradiol enhances leukocyte binding to tumor necrosis factor-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type I and vascular cell adhesion molecule type I. J Clin Invest 93:17–25
De la Fuente M (1985) Changes in the macrophage function with ageing. Comp Biochem Physiol A 81:935–938
De la Fuente M, Gimenez Llort L (2010) Models of ageing of neuroimmunomodulation: strategies for its improvement. Neuroimmunomodulation 17:213–216
De la Fuente M, Miquel J (2009) An update of the oxidation-inflammation theory of ageing: the involvement of the immune system in the oxi-inflamm-ageing. Curr Pharm Des 15:3003–3026
De la Fuente M, Baeza I, Guayerbas N, Puerto M, Castillo C, Salazar V, Ariznavarreta C, Fdez-Tresguerres J (2004a) Changes with ageing in several leukocyte functions of male and female rats. Biogerontology 5:389–400
De la Fuente M, Hernanz A, Guayerbas N, Alvarez P, Alvarado C (2004b) Changes with age in peritoneal macrophage functions. Implication of leukocytes in the oxidative stress of senescence. Cell Mol Biol 50(Suppl):OL683–OL690
Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM (1999) Estradiol modulates Bcl-2 in cerebral isquemia: a potential role for oestrogen receptors. J Neurosci 19:6385–6393
Farage MA, Neill S, MacLean AB (2009) Physiological changes associated with the menstrual cycle: a review. Obstet Gynecol Surv 64:58–72
Franceschi C, Bonafé M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-ageing. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908:244–254
Gameiro C, Romao F (2010) Changes in the immune system during menopause and ageing. Front Biosci (Elite Ed) 2:1299–1303
Guayerbas N, De la Fuente M (2003) An impairment of phagocytic function is linked to a shorter life span in two strains of prematurely ageing mice. Dev Comp Immunol 27:339–350
Guayerbas N, Puerto M, Alvarez P, De la Fuente M (2004) Improvement of the macrophage functions in prematurely ageing mice by a diet supplemented with thiolic antioxidants. Cell Mol Biol 50:OL677–OL681
Ha BJ, Lee SH, Kim HJ, Lee JY (2006) The role of Salicornia herbacea in ovariectomy-induced oxidative stress. Biol Pharm Bull 29:1305–1309
Huber J, Gruver C (2001) Immunological and dermatological impact of progesterone. Gynecol Endocrinol 6:18–21
Islander U, Erlandsson MC, Hasseus B, Jonsson CA, Ohlsson C, Gustafsson JA (2003) Influence of oestrogen receptor α and β on the immune system in aged female mice. Immunology 110:149–157
Kankofer M, Radzki RP, Bienko M, Albera E (2007) Anti-oxidative/oxidative status of rat liver after ovariectomy. J Vet Med 54:225–229
Keller ET, Zhang J, Yao Z, Qi Y (2001) The impact of chronic oestrogen deprivation on immunologic parameters in the ovariectomised rhesus monkey (Macaca mulatta) model of menopause. J Reprod Immunol 50:41–55
Kiray M, Uysal N, Sönmez A, Acikgöt O, Gönenc S (2004) Positive effects of deprenyl and estradiol on spatial memory and oxidant stress in aged female rat brains. Neurosci Lett 354:225–228
Kiray M, Ergur BU, Bagriyanik A, Pekcetin C, Aksu I, Buldan Z (2007) Suppression of apoptosis and oxidative stress by deprenyl and estradiol in aged rat liver. Acta Histochem 109:480–485
Kireev R, Tresguerres AF, Vara E, Ariznavarreta C, Tresguerres JA (2007) Effect of chronic treatments with GH, melatonin, oestrogens, and phyto-oestrogens on oxidative stress parameters in liver from aged female rats. Biogerontology 8:469–482
Kovacs EJ (2005) Ageing, traumatic injury and oestrogen treatment. Exp Gerontol 40:549–555
Lawrence RA, Burck RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lu KH, Hopper BR, Vargo TM, Yen SS (1979) Chronological changes in sex steroids gonadotropin and prolactin secretion in ageing female rats displaying different reproductive states. Biol Reprod 21:193–203
Marriott I, Huet-Hudson YM (2006) Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34:177–192
Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, Bernabei A (2000) Effects of oestrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol 167:447–452
Miquel J, Ramírez-Boscá A, Ramírez-Boscá JV, Alperi JD (2006) Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch Gerontol Geriatr 42:289–306
Nelson HD (2008) Menopause. Lancet 371:760–770
Nunn CL, Lindenfors P, Pursall ER, Rolff J (2009) On sexual dimorphism in immune function. Philos Trans R Soc B 364:61–69
Pauklin S, Petersen-Mahrtz SK (2009) Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol 183:1238–1244
Pfeilschifter J, Köditz R, Pfohl M, Schatz H (2002) Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23:90–119
Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB (2001) Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol 36:311–326
Sebastián C, Espia M, Serra M, Celada A, Lloberas J (2005) MacrophAgeing: a cellular and molecular review. Immunobiology 210:121–126
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal Biochem 17:502–522
Tresguerres JAF, Kireev R, Tresguerres AF, Borrás C, Vara E, Ariznavarreta C (2008) Molecular mechanisms involved in the hormonal prevention of ageing in the rat. J Ster Biochem Mol Biol 108:318–326
Viña J, Borrás C, Gambini J, Sastre J, Pallardó FV (2005) Why females live longer than males: control of longevity by sex hormones. Sci Ageing Knowledge Environ 23:pe17
Viña J, Sastre J, Pallardó FV, Gambini J, Borrás C (2006) Role of mitochondrial oxidative stress to explain the different longevity between genders: protective effect of oestrogens. Free Radic Res 40:1359–1365
Viña J, Sastre J, Pallardó FV, Gambini J, Borrás C (2008) Modulation of longevity-associated genes by oestrogens or phyto-oestrogens. Biol Chem 389:273–277
Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS (1990) Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol 45:45–98
Wirleitner B, Baier-Bitterlich G, Bock G, Widner B, Fuchs D (1998) 7,8-Dihydroneopterin-induced apoptosis in Jurkat T lymphocytes: a comparison with anti-Fas- and hydrogen peroxide-mediated cell death. Biochem Pharmacol 56:1181–1187
Yalin S, Comelekoglu U, Bagis S, Sahin NO, Ogenler O, Hatungil R (2006) Acute effect of single-dose cadmium treatment on lipid peroxidation and antioxidant enzymes in ovariectomised rats. Ecotoxicol Environ Saf 65:140–144
Yang JH, Chen CD, Wu MY, Chao KH, Yang YS, Ho HN (2000) Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T-cell subpopulation of interferon-gamma production in postmenopausal women. Feril Steril 74:261–267
Acknowledgments
This work was supported by MICINN (BFU2008-04336); Complutense University Research Group (910379) and RETICEF (RD06/0013/0002 and RD06/0013/0003) from ISCIII.
Conflict of interest
The authors state that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baeza, I., De Castro, N.M., Arranz, L. et al. Ovariectomy causes immunosenescence and oxi-inflamm-ageing in peritoneal leukocytes of aged female mice similar to that in aged males. Biogerontology 12, 227–238 (2011). https://doi.org/10.1007/s10522-010-9317-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-010-9317-0