Abstract
The aim of this study was to examine the influence of sex on age-related changes in phenotype and functional capacity of rat macrophages. The potential role of estradiol as a contributing factor to a sex difference in macrophage function with age was also examined. Thioglycollate-elicited peritoneal macrophages derived from the young (2 months old) and the naturally senescent intact middle-aged (16 months old) male and female rats were tested for cytokine secretion and antimicrobial activity (NO and H2O2 production and myeloperoxidase activity). Serum concentration of estradiol and the expression of estrogen receptor (ER)α and ERβ on freshly isolated peritoneal macrophages were also examined. Decreased secretion of IL-1β and IL-6 by macrophages from middle-aged compared to the young females was accompanied with the lesser density of macrophage ERα expression and the lower systemic level of estradiol, whereas the opposite was true for middle-aged male rats. Macrophages in the middle-aged females, even with the diminished circulating estradiol levels, produce increased amount of IL-6, and comparable amounts of IL-1β, TNF-α, and NO to that measured in macrophages from the middle-aged males. Age-related changes in macrophage phenotype and the antimicrobial activity were independent of macrophage ERα/ERβ expression and estradiol level in both male and female rats. Although our study suggests that the sex difference in the level of circulating estradiol may to some extent contribute to sex difference in macrophage function of middle-aged rats, it also points to more complex hormonal regulation of peritoneal macrophage activity in females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sex-related differences in the immune response, observed in both the innate and the adaptive immune responses, likely contribute to differences in susceptibility to infectious diseases, response to vaccines, and the incidence of autoimmune diseases in males and females [1–3]. Females typically develop a more vigorous innate and adaptive immune response to antigen challenges [4, 5] which can accelerate pathogen clearance, but could also lead to an increase in the immune-related pathology, such as autoimmune or inflammatory diseases [6, 7].
The immune system exhibits age-related changes, referred to as immunosenescence, which is characterized by a progressive decline in the immune system function, resulting in an augmented susceptibility to infections and tumors, and a decreased response to vaccines [8, 9]. Although the age-dependent changes in the adaptive immune system are well documented, relatively little research has been carried out on the impact of aging on the innate immune response.
Macrophages have diverse functions within the immune system. These cells play an important role in the innate immunity by eliminating the invading pathogens via phagocytosis and production of reactive oxygen and nitrogen species, and also by releasing a number of inflammatory mediators, including cytokines and chemokines that are central to initiation and propagation of the inflammatory process [10]. Macrophages also have an important role in instructing the adaptive immune response, capable of antigen presentation and the activation of T and B cells [10].
Both sex- and age-related differences in different macrophage function—including toll-like receptor signaling, phagocytosis and microbicidal activity, and cytokine secretion—have been reported in both rodent and humans [11–13]. Sex- and age-associated differences in macrophage function likely contribute to sex differences in the overall immune response and the immune response impairment documented in the elderly, respectively.
To date, sex-specific differences in the aging immune system function and the way that sex steroid hormone levels affect the macrophage function were scarcely documented. Serum concentrations of sex hormones in men and women are affected differently by age. In men, a slow and steady decline in plasma testosterone levels was observed with age, while estradiol levels gradually increase because of the age-related rise in peripheral aromatization of testosterone [14]. Women, on the other hand, exhibit a dramatic, menopause-associated reduction in estrogen levels, whereas testosterone concentration, although in decline after menopause, will gradually return to pre-menopausal levels some 20–30 years after menopause [15]. Besides, women lose their immunological advantages after menopause [16]. In line with that, ovariectomy in female mice, which mimics human ovarian loss, abolishes differences in immunosenescence and oxidative stress between peritoneal leukocytes from aged female and male mice [17]. Nevertheless, it has been shown that in rats post-ovariectomy, the circulating estradiol level after initial decline actually slowly rises [18] reaching the values of intact control rats and remaining stable until the age 20 months [19]. Thus, the present study was designed to explore the influence of sex on age-related alterations in macrophage functions by using naturally senescent intact middle-aged male and female rats. Thioglycollate-elicited peritoneal macrophages from the young (2 months old) and the middle-aged (16 months old) male and female rats were tested for their ability to secrete the reactive oxygen metabolites and cytokines, following in vitro stimulation with lipopolysaccharide (LPS). In addition, to examine the potential contribution of estradiol to sex-related differences in macrophage function with age, serum concentration of estradiol and estrogen receptor expression on freshly isolated peritoneal macrophages were measured.
MATERIALS AND METHODS
Animals
Young (2 months old) and middle-aged (16 months old) male and female Albino Oxford rats were obtained from the breeding colony at Immunology Research Center “Branislav Janković,” Belgrade (Serbia). The animals were housed in standard cages (2–3 rats/cage) in a controlled environment (22 ± 2 °C, humidity ranging 40 to 70%, and under 12:12-h light/dark cycle) with free access to conventional food pellets (Veterinarski zavod Subotica, Serbia) and tap water.
Daily vaginal smears were taken from female rats to determine the phase of estrous cycle. The vaginal epithelial cells were examined via a microscope for 7 consecutive days before the experiment. The swabs were performed in the morning (9:00 A.M.) to maintain consistency. The experiments were performed during the proestrus phase in young females with normal estrous cycle and in middle-aged anestrous females. Only nulliparous females were used, as it was shown that reproductive experience (i.e., pregnancy and lactation) may affect macrophage oxidative burst and cytokine production [20, 21].
Animals were euthanized by using the increasing dose of CO2, and healthiness of rats was confirmed for each animal by gross inspection during autopsy. The experimental protocol and all procedures with animals and their care were approved by Ministry of Agriculture and Environmental Protection (license number 323-07-01577/2016-05/14, issued on 02-25-2016) and were in accordance with principles declared in Directive 2010/63/EU of the European Parliament and of the Council from 22 September 2010 on the protection of animals used for scientific purposes (revising Directive 86/609/EEC).
Thioglycollate-Elicited Peritoneal Macrophage Isolation
Rats were intraperitoneally injected with 7 ml of sterile thioglycollate medium, and peritoneal cells were obtained 7 days later by peritoneal lavage. The purity of peritoneal macrophage population obtained by this method was 60–90%, according to the staining with FITC-conjugated anti-CD68 antibodies (“RESULTS” section).
Chemicals and Immunoconjugates
Lipopolysaccharide (LPS), phenylmethyl sulfonyl fluoride (PMSF), α-isonitrosopropiophenone (ISPF), phenol red, phorbol 12-myristate 13-acetate (PMA), horseradish peroxidase (HRPO), zymosan, nitrotetrazolium blue chloride (NBT), and phenol red-free RPMI-1640 medium (RPMI) were purchased from Sigma (St. Lewis, MO, USA). Fetal calf serum (FCS) was obtained from Gibco (Grand Island, NY, USA). Thioglycollate medium was acquired from the “Torlak” Institute (Belgrade, Serbia).
Monoclonal PE-conjugated mouse anti-rat CD163 (ED2-like) resident macrophage receptor (clone HIS 36), monoclonal FITC-conjugated mouse anti-rat major histocompatibility complex class II (MHC II-FITC) antibody (clone OX-6), monoclonal FITC-conjugated mouse anti-rat CD40 (CD40-FITC), FITC-conjugated goat anti-rabbit IgG (GAR-FITC), PE-conjugated donkey anti-rabbit IgG (DAR-PE), PCP-conjugated streptavidin (Sav-PCP), and appropriate IgG isotype controls were purchased from BD Biosciences Pharmingen (Mountain View, CA, USA). Monoclonal FITC-conjugated mouse anti-rat CD68 (CD68-FITC, clone ED1), biotin-conjugated mouse anti-rat CD68 (clone ED1), and mouse anti-rat CD169 (clone ED3) antibodies were obtained from Serotec (Oxford, UK). Rabbit anti-rat toll-like receptor (TLR) 4 antibodies, unconjugated polyclonal rabbit anti-estrogen receptor α (ERα), and polyclonal rabbit anti-estrogen receptor β (ERβ) were obtained from Abcam (Cambridge, MA, USA). PCPCy5.5-conjugated goat anti-mouse IgG (GAM-PCPCy5.5) was purchased from Biolegend (San Diego, CA, USA).
Flow Cytometric Analysis (FCA)
Flow cytometric analysis of peritoneal cells was performed immediately following their isolation. Macrophages were selected and gated by light scatter characteristics, and debris was gated out on the basis of low forward scatter and low side angle scatter. Samples were analyzed on a FACSVerse™ flow cytometer using BD FACSuite™ software (Becton Dickinson, Mountain View, CA, USA).
For the single-color staining, cells were immunolabeled with PE-conjugated mouse anti-rat CD163, specific for membrane antigen CD163, or fixed with 0.25% paraformaldehyde and permeabilized by 0.2% Tween 20 prior to the addition of FITC-conjugated mouse anti-rat CD68, which is specific for intracellular antigen CD68. In the first set of two-color staining, cells were first stained with membrane antigen-specific anti-MHC II-FITC or anti-TLR 4 (followed by DAR-PE), than fixed with 0.25% paraformaldehyde and permeabilized by 0.2% Tween 20 prior to the addition of anti-CD68b (followed by Sav-PCP). The second set of two-color staining was commenced by the addition of anti-CD163-PE and completed by the addition of anti-CD40-FITC or anti-CD169 (followed by GAM-PCPCy5.5).
For the analysis of steroid hormone receptor nuclear expression, cells were fixed with 0.25% paraformaldehyde and permeabilized with 0.05% Triton X-100. Cells were incubated overnight with anti-ERα or with anti-ERβ, washed twice with PBS containing 0.05% Triton X-100, and incubated with GAR-FITC antibody.
PCR Analyses of Interferon Regulatory Factor-4 Expression
Total RNA was isolated from freshly isolated peritoneal macrophage cells using an ABI Prism 6100 Nucleic Acid PrepStation (Applied Biosystems, Foster City, CA, USA) and Total RNA Chemistry (Applied Biosystems). Reverse transcription was performed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and 5 μl of complementary DNA (cDNA) was used for real-time PCR. Triplicate 25-μl reactions were run under Applied Biosystems 7500 universal cycling conditions. Gene Expression Master Mix and commercial TaqMan Gene Expression Assays for rat interferon regulatory factor (IRF)4 (Rn01435145_m1) were obtained from Applied Biosystems. All procedures were performed according to the manufacturer’s recommendations. β-Actin was the internal standard to normalize for input cDNA variations, as it displayed an optimal stability among various samples tested. Quantitative differences in gene expression levels were assessed using Applied Biosystems SDS software (v 1.4.0.) and the 2−ΔΔCt method.
Serum Concentration of Estradiol
Serum estradiol levels were determined using the IMMULITE solid-phase competitive chemiluminescent enzyme immunoassay (EIA) on an IMMULITE 1000 analyzer (Euro/DPC, Caernarfon, UK), according to the guidelines provided by the manufacturer.
Macrophage Cultures
For cytokine release determination, cell aliquots adjusted to 1 × 106/ml in RPMI/5%FCS were pooled in such a manner that two pools per group were obtained and plated to 24-well flat-bottomed tissue culture plates in duplicate of each pool. For macrophage activity determination, cell aliquots of each animal were plated in duplicate to 96-well flat-bottomed tissue culture plates (1 × 106/ml in RPMI/5%FCS for nitric oxide production; 2.5 × 106/ml in RPMI/5%FCS for NBT reduction, hydrogen peroxide secretion, and myeloperoxidase activity).
Cells were cultured to adhere for 2 h at 37 °C in a moist atmosphere with 5% CO2 in the air. The non-adherent cells were removed by washing the plates twice with warm (37 °C) PBS. Adherent macrophage was additionally incubated at 37 °C and 5% CO2 for 24 h (cytokine assays, NBT reduction, and myeloperoxidase activity) or for 48 h (nitric oxide production) with 1 μg/ml LPS/RPMI/5%FCS, or with RPMI/5%FCS. The only exception was test for hydrogen peroxide release, in which adherent macrophages were incubated only with RPMI/5%FCS for 24 h. Optical densities (OD) were determined by Multiscan Ascent plate reader (Labsystems, Helsinki, Finland).
Hydrogen Peroxide Release Assay
Hydrogen peroxide (H2O2) release was determined as previously described [22] according to a method based on the HRPO-dependent oxidation of phenol red [23]. After 24 h of resting in RPMI/5%FCS, adherent macrophages were washed twice with 200 μl of warm PBS (37 °C) and primed for peroxide production with 100 μl of 25 nM of PMA in phenol red solution (10 mM potassium phosphate buffer pH 7, 140 mM NaCl, 5.5 mM dextrose, 0.56 mM phenol red, and 19 U/ml of HRPO) for 1 h at 37 °C and 5% CO2. PMA was prepared from a stock solution (10−2 M in DMSO, stored at −70 °C). Incubations were terminated with 10 μl of 0.5 M NaOH, and ODs were determined at 620 nm. The concentration of H2O2 in the samples was calculated using standard concentrations of H2O2 (1–100 μM).
NBT Reduction Assay
Phagocytosis was determined according to modified methods of Choi et al. and Pick et al. [24, 25], based on the fact that phagocytosis of zymosan particles induces NADPH oxidase-dependent superoxide anion generation that further mediates reduction of yellow NBT to blue formazan. After 24 h of LPS stimulation or resting in RPMI/5%FCS, adherent macrophages were rinsed twice with 200 μl of warm PBS (37 °C) and stimulated with zymosan (125 μg/ml) in the presence of 0.5 mg/ml NBT for 1 h at 37 °C and 5% CO2. The cells were then fixed with methanol, the plates were air-dried, and formazan sediment was dissolved by shaking the plates with 2 M KOH and 10% DMSO for 15 min (200 cycles/min). Subsequent solubilization of blue precipitate by KOH/DMSO increases assay sensitivity. Phagocytosis of zymosan was determined by measuring ODs at 620 nm. The amount of engulfed zymosan particles was proportional to the reduction of yellow NBT to blue formazan. The results are expressed as OD (620 nm) × 1000.
Myeloperoxidase Activity Assay
Myeloperoxidase activity (MPO) was determined according to modified methods of Bradley and coworkers [26], by measuring the oxidation of o-phenylene diamine (OPD). After 24 h of LPS stimulation or resting in RPMI/5%FCS, adherent macrophages were washed twice with 200 μl of warm PBS (37 °C), and the reaction was started by adding substrate solution (1 mg/ml OPD in 10 mM citrate buffer, pH 5.0, 0.001% Triton X-100, and 8.8 mM H2O2). After 10 min at 22 °C in the dark, the reaction was stopped by the addition of 2 M H2SO4 and ODs were determined at 492/620 nm. The results are expressed as OD (492/620 nm) × 1000.
Cytokine Assays
Culture supernatants collected following 24 h of macrophage stimulation with LPS were frozen at −70 °C until assayed. Commercially available ELISA kits for tumor necrosis factor (TNF)-α interleukin (IL)-6 (Biolegend Inc., San Diego, CA, USA), IL-1β (Thermo Scientific, Waltham, MA, USA), and transforming growth factor (TGF)-β (R&D Systems Inc., Minneapolis, MN, USA) were used. Prior to performing ELISA, the thawed culture supernatants were centrifuged on 250g, 15 min, +4 °C. All assays were carried out according to the manufacturer’s instructions.
Nitric Oxide Production
Culture supernatants collected after 48 h of LPS stimulation or resting in RPMI/5%FCS were immediately analyzed for nitrite concentration by a method based on the Griess reaction [27]. The concentration of nitrite in the samples was determined according to optical densities measured at 405 nm and calculated using a NaNO2 (1–100 μM) as a standard.
Statistical Analysis
Since the experimental design included variations in two factors, sex (males vs females) and age (young vs middle-aged), data were analyzed by two-way ANOVA with Bonferroni’s multiple comparison post hoc test using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Student’s t test was used for comparisons of RPMI-stimulated with LPS-stimulated macrophages from age- and sex-matched rats. Differences were regarded as statistically significant if p < 0.05.
RESULTS
Body Weight and Peritoneal Cell Yield in Young and Middle-Aged Male and Female Rats
Although young rats of both sexes weighed similarly at the beginning of the experiment, the aging has increased the body weight only in males, thus making the middle-aged male rats heavier than the middle-aged female rats (Fig. 1a). In spite of the observed differences in the weight change, the absolute number of cells recovered following the peritoneal lavage was not significantly different between the young and the middle-aged male and female rats (Fig. 1b). Consequently, the yield of peritoneal cells, expressed as number of cells per gram of body weight, was increased in the middle-aged females when compared to the middle-aged male rats (Fig. 1c).
Body weight (a), total number of recovered peritoneal exudate cells (b), and peritoneal cell yield (c) in young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant interaction of sex × age (p < 0.05) for body weight, whereas significant main effect of sex (p < 0.01) was identified for peritoneal cell yield. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.001 vs young sex-matched rats; a p < 0.05 and b p < 0.01 vs male age-matched rats.
Phenotypic Characteristics of Macrophages from Young and Middle-Aged Male and Female Rats
ED1 and ED2 monoclonal antibodies were used to confirm that the cells analyzed in the present study comprised mainly macrophages. ED1 is a CD68-like antibody that recognizes rat pan-macrophage, while ED2 exclusively recognizes CD163, a resident rat macrophage marker [28].
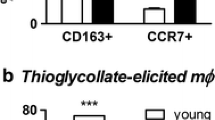
The percentage of CD68+ and CD163+ cells and the mean expression density of CD68 and CD163 (according to CD68 and CD163 MFI on CD68+ and CD163+ cells, respectively) observed in thioglycollate-elicited peritoneal macrophage-like cells (according to FSC and SSC characteristics) were comparable between the young male and female rats (Fig. 2a, b). Aging increased the proportion of CD68+ and CD163+ cells in both male and female rats to a similar extent, so that their percentages remained comparable between the middle-aged rat experimental groups of both sexes (Fig. 2a, b). Also, the mean expression density of CD68 and CD163 increased in both male and female rats with age. However, the increase in mean expression density of CD68 reached statistical significance only in male rats, so that in middle-aged rats, it was higher in male than in female rats (Fig. 2a). In contrast, CD163 mean surface density significantly increased with age only in female rats, but the value of this parameter in middle-aged rats did not significantly differ between sexes (Fig. 2b).
The representative flow cytometry profile (upper panel), the proportion (middle panel), and mean fluorescence intensity (MFI, lower panel) for CD68+ (a) and CD163+ (b) peritoneal macrophages in young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant main effect of age for the proportion of CD68+ macrophages (p < 0.05), and for the proportion (p < 0.0001) and MFI (p < 0.001) of CD163+ macrophages. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.01, **p < 0.001 vs young sex-matched rats; a p < 0.05 vs male age-matched rats.
CD68+ macrophage populations that express markers of cell activation (CD68+MHCII+, Fig. 3a, and CD68+TLR4+, Fig. 3b) were more prominent in young female than in young male rats (Fig. 3a, b). Aging significantly diminished the proportion of CD68+MHCII+ in females (Fig. 3a) and increased the proportion of CD68+TLR4+ in males (Fig. 3b).
Representative dot plots of two-color flow-cytometric analysis (upper panel) and the proportion (lower panel) of CD68+MHCII+ (a), CD68+TLR4+ (b), CD163+CD169+ (c), and CD163+CD40+ (d) peritoneal macrophages in young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant main effect of age (p < 0.05) for the proportion of CD163+CD169+ macrophages. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.05 vs young sex-matched rats; a p < 0.05 and b p < 0.01 vs male age-matched rats.
The proportion of CD163+CD169+ macrophages, i.e., those that reflect the macrophage’s relative anti-inflammatory capacity [29], was not significantly different between the young male and female rats. The percentage of these cells increased with aging in female rats (Fig. 3c). In contrast, the percentage of resident CD163+ macrophages that express the activation marker CD40 did not differ between sexes in young rats and did not change during aging in either of sexes (Fig. 3d).
The Expression of ERα and ERβ on Peritoneal Cells from Young and Middle-Aged Male and Female Rats
More than 90% of the freshly isolated peritoneal cells from young rats of both sexes expressed ERα (Fig. 4a, left panel) and ERβ (Fig. 4b, left panel). The percentage of ERα+ and ERβ+ cells was similar in young rats of both sexes and was not affected by aging in neither male nor female rats (Fig. 4a, b, left panels). However, the mean expression density of ERα was higher in peritoneal cells derived from young female than from young male rats, whereas that of ERβ was comparable between the peritoneal cells of animals of both sexes, according to ERα and ERβ MFI in ERα+ and ERβ+ cells, respectively (Fig. 4a, b, right panels). Although aging increased the mean expression density of ERα in peritoneal cells from male rats and decreased it in the cells from female rats, the mean expression density of ERα was still higher in cells from the middle-aged female rats than from their male counterparts (Fig. 4a, right panel). Aging diminished the ERβ expression density in both male and female rats’ cells (Fig. 4b, right panel). However, the ERβ expression density was lower in macrophages from the middle-aged females than in those from the corresponding male group (Fig. 4b, right panel).
The representative flow cytometry profiles (left panel) and the mean fluorescence intensity (MFI, right panel) of ERα+ (a) and ERβ+ (b) peritoneal macrophages in young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant interaction of sex × age for macrophage ERα MFI (p < 0.0001) and for macrophage ERβ MFI (p < 0.05). Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.001 vs young sex-matched rats; a p < 0.01 and b p < 0.001 vs male age-matched rats.
Hydrogen Peroxide Release, NBT Reduction, and MPO Activity in Macrophages from Young and Middle-Aged Male and Female Rats
Macrophages from young rats of both sexes produced comparable amount of H2O2. Aging increased H2O2 production only in macrophages derived from male rats (Fig. 5a). Consequently, macrophages from middle-aged male rats produced greater amount of H2O2 compared with middle-aged females (Fig. 5a).
PMA-stimulated release of hydrogen peroxide (a), zymosan-stimulated NBT reduction (b), and MPO activity (c) in RPMI- and LPS-stimulated adherent peritoneal macrophages of young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant main effect of age for macrophage hydrogen peroxide release (p < 0.05) and significant interaction of sex × age for RPMI-stimulated (p < 0.01) and LPS-stimulated (p < 0.001) macrophage MPO activity. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.05, **p < 0.01, and ***p < 0.001 vs young sex-matched rats; a p < 0.05, b p < 0.01, and c p < 0.001 vs male age-matched rats.
Phagocytosis of zymosan, measured indirectly by a degree of NBT reduction following the oxidative burst, was comparable in macrophages from young and middle-aged rats of both sexes, irrespective of the LPS stimulation (Fig. 5b). The LPS stimulation of macrophages did not affect the degree of NBT reduction when compared to macrophages from sex- and age-matched groups treated with RPMI only.
Among the young rats, macrophages derived from males expressed a greater MPO activity compared with those from females, and aging decreased the MPO activity of macrophages in both sexes, irrespective of the LPS stimulation (Fig. 5c). Moreover, for both RPMI- and LPS-treated macrophages, aging more robustly diminished the MPO activity in macrophages from males, resulting in greater MPO activity in cells from middle-aged female than in middle-aged male rats (Fig. 5c). The LPS stimulation did not affect MPO activity when compared to sex- and age-matched groups treated with RPMI only.
Production of NO by Macrophages from Young and Middle-Aged Male and Female Rats
Macrophages from young male rats produced higher levels of NO compared to young females’ macrophages, irrespective of the LPS stimulation (Fig. 6). The production of NO increased with age in RPMI-treated macrophages from both male and female rats, whereas the LPS-stimulated production increased with age only in female rats’ macrophages (Fig. 6). Macrophages from the middle-aged rats of both sexes exhibited comparable RPMI- and LPS-stimulated production of NO. However, stimulation with LPS increased NO release only in macrophages derived from young male (p < 0.01, LPS-treated vs RPMI- treated) and female rats (p < 0.001, LPS-treated vs RPMI-treated), but not in macrophages from the middle-aged animals.
The production of NO by RPMI- and LPS-stimulated adherent peritoneal macrophages of young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant interaction of sex × age for RPMI-stimulated (p < 0.05) and LPS-stimulated (p < 0.01) macrophage NO production. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.05 and **p < 0.001 vs young sex-matched rats; a p < 0.001 vs male age-matched rats.
Cytokine Release by LPS-Stimulated Macrophages from Young and Aged Male and Female Rats
Overall, macrophages from the young females produced higher levels of pro-inflammatory cytokines IL-1β (Fig. 7a) and IL-6 (Fig. 7b) than macrophages from the young males, whereas the levels of TNF-α (Fig. 7c) and TGF-β (Fig. 7d) were similar in macrophages from the young rats of both sexes.
Levels (pg/ml) of IL-1β (a), IL-6 (b), TNF-α (c), and TGF-β (d) in the culture supernatants of LPS-stimulated adherent peritoneal macrophages from young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed the significant interaction of sex × age for macrophage production of IL-1β (p < 0.001), IL-6 (p < 0.0001), TNF-α (p < 0.05), and TGF-β (p < 0.01). Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant differences: *p < 0.05, **p < 0.01, and ***p < 0.001 vs young sex-matched rats; a p < 0.01 and b p < 0.001 vs male age-matched rats.
Aging increased the production of IL-1β (Fig. 7a) and TNF-α (Fig. 7c) in macrophages from male rats. In contrast, aging diminished the production of IL-1β (Fig. 7a) and IL-6 (Fig. 7b) in female rats’ macrophages. However, both male and female rats’ macrophages belonging to the middle-aged group displayed higher production of TGF-β than macrophages from the corresponding young animals (Fig. 7d).
These opposite changes induced by aging observed in macrophages from male and female rats resulted in similar levels of production of IL-1β (Fig. 7a) and TNF-α (Fig. 7c) in middle-aged rats of both sexes. However, macrophages from the middle-aged female rats produced higher levels of IL-6 (Fig. 7b) and TGF-β (Fig. 7d) when compared to macrophages from the middle-aged male rats.
The Expression of Interferon Regulatory Factor-4 in Peritoneal Cells from Young and Middle-Aged Male and Female Rats
Relative expression of messenger RNA (mRNA) IRF4 was higher in freshly isolated peritoneal cells derived from the young male, than from the young female rats (Fig. 8). With age, mRNA IRF4 decreased in the male rats, whereas in the female rats, it increased (Fig. 8). Consequently, mRNA IRF4 expression was higher in peritoneal cells from the middle-aged female compared with the middle-aged male rats (Fig. 8).
Fold change in IRF4 mRNA expression in freshly isolated peritoneal cells from young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed the significant interaction of sex × age (p < 0.0001) for macrophage IRF4 mRNA expression. Data (mean ± SEM) are representative of two experiments (n = 5–6). Values represent arbitrary units (young males are set as 1). Statistically significant difference: *p < 0.05 vs young sex-matched rats; a p < 0.05 vs male age-matched rats.
Serum Concentration of Estradiol in Young and Middle-Aged Male and Female Rats
Serum estradiol level was significantly higher in young females than in young male rats (Fig. 9). The aging has slightly, but significantly, increased the level of estradiol in male rats, but has robustly diminished it in female rats, resulting in lower estradiol level in the middle-aged female than male rats (Fig. 9).
Serum levels of estradiol in young (2 months old) and middle-aged (16 months old) male and female rats. Two-way ANOVA revealed significant interaction of sex × age (p < 0.0001) for serum levels of estradiol. Data (mean ± SEM) are representative of two experiments (n = 5–6). Statistically significant difference: *p < 0.05, **p < 0.001 vs young sex-matched rats; a p < 0.001 vs male age-matched rats.
DISCUSSION
The present study supports the earlier findings of superior innate immune response observed in young female compared to the young male rats [6], which was manifested in the greater ability of macrophages from females to secrete the pro-inflammatory cytokines IL-1β and IL-6 in response to LPS. Furthermore, our study discerns sex differences in substantial age-related changes in the innate immune compartment in middle-aged rats. The most noticeable observations were the opposite changes with age in the LPS-stimulated production of IL-1β and the expression of IRF4 mRNA in macrophages from rats of two sexes. IRF4 has been shown to downregulate the TLR-dependent induction of pro-inflammatory cytokines [30]. In the middle-aged female rats, the decreased macrophage secretion of IL-1β and IL-6 and the increased IRF4 mRNA expression were accompanied with the lesser density of macrophage ERα expression and the lower systemic level of estradiol compared to the young ones. This is in line with the previously reported finding that estradiol positively regulates cytokine production in macrophages via ERα expression [31]. Besides, the ERα signaling has been related to transcriptional regulation of IRF5 [4]. IRF4 competes with IRF5 for MyD88 interaction, to inhibit the IRF5-mediated induction of pro-inflammatory cytokines [30]. Since MHCII molecules co-localize and synergize with TLR4 in inducing an innate immune response [32], a diminished proportion of MHCII-expressing macrophages in the middle-aged females probably contributed to their diminished LPS-induced IL-6 and IL-1β production in comparison with the young female rats. We have previously reported the increase in both the frequency of TLR4-expressing cells among peritoneal macrophages and the density of TLR4 expression on these cells from the aged (2 years old) female rats [33]. Thus, it may be assumed that in female rats, the rise in the frequency of TLR4-expressing cells with age appeared later in life when compared to male rats.
The rise in serum estradiol level in middle-aged male rats probably reflected its synthesis at extragonadal sites. Males maintain circulating active testosterone throughout life at the levels sufficient for conversion to estradiol in extragonadal sites, including adipose tissue and bone [34]. In agreement with this, the present study showed that male, but not female, rats gained weight as a result of aging. Macrophages from the middle-aged male rats exhibited greater capacity to secrete TNF-α and IL-1β following the LPS stimulation, relative to sex-matched young rats. In addition to the lower IRF4 expression, this was accompanied with the greater frequency of TLR4+ cells within macrophages.
The present study indicated a decreased expression density of ERβ in macrophages derived from the middle-aged rats of both sexes that was unrelated to the level of circulating estradiol which increased and decreased in the male and the female rats, respectively. On the contrary, higher expression density of ERα on macrophages from young females compared to young males [35] and changes in ERα expression in middle-aged rats’ macrophages of both sexes were positively correlated with blood levels of estradiol. These results are in accordance with the notion that estradiol has a slightly higher affinity for ERα over ERβ receptor [36] and that it induces ERα, but not ERβ, expression in macrophages [37]. Furthermore, ERβ has a generally restraining effect on the ERα-mediated gene expression, if expressed on the same cell [38]. It may be assumed that the downturn in expression density of ERβ in macrophages from the middle-aged male rats could have unmasked the ERα-mediated stimulatory effect of 17β estradiol on TGF-β secretion [39].
The frequency of CD163+ cells within macrophages from the middle-aged rats of both sexes was increased compared to young rats. Besides, in the middle-aged female rats, this rise was paralleled by the increase in proportion of resident tissue CD163+CD169+ cells and the increased CD163 expression density in these cells, relative to young females. Substantial capacity of CD163-expressing macrophages to secrete the anti-inflammatory cytokines [40 and the high level of TGF-β expression in CD169+ macrophages [29] may also account for the increased TGF-β secretion in macrophages from the middle-aged female rats. Although some previous findings show that cell CD40 expression may be affected by estradiol [41, 42] and age [43], our results did not corroborate this notion.
The present study revealed that the increase in macrophage capacity to produce NO, the unaltered superoxide anion release, as well as the decrease in MPO activity in the middle-aged rats of both sexes were independent of the changes in macrophage ERα/ERβ expression and the blood estradiol levels. This was in contrast with the data showing both in vivo and in vitro effects of estradiol on macrophage generation of reactive oxygen and nitrogen species [44]. Having in mind that some bacterial components such as LPS are able to induce the recruitment of mitochondria to macrophage phagosomes and augment phagosomal NADPH oxidase oxygen production [45], the activities related to oxidative stress in macrophages pre-treated with LPS were also tested. Nevertheless, there were no differences between the unstimulated and the LPS-stimulated macrophages in the NADPH-dependent superoxide anion release (subsequent to zymosan phagocytosis via TLR2/TLR6 and dectin 1) [46, 47] and the MPO activity (indicating macrophage activation upon priming) [48]. Irrespective of the LPS pretreatment, the macrophage superoxide anion release was comparable among different sex and age groups, whereas the MPO activity was significantly higher in the young male than in the young female rats, and abruptly diminished in the middle-aged rats of both sexes. In the middle-aged rats of both sexes, macrophages cultured under basal conditions exhibited the increased NO production and the decreased MPO activity when compared to the young rats. Considering that macrophage MPO may scavenge NO via its catalytic consumption [49], it could be assumed that it is the diminished MPO activity of the unstimulated macrophages from the middle-aged rats that contributed to the rise in NO level in culture supernatants of these cells, when compared to the young sex-matched rats. However, this was not a case when cells were cultured in the presence of LPS, probably due to the more efficient stimulation of iNOS expression by LPS in peritoneal macrophages from the young [50].
The increased amount of H2O2 released from macrophages of the middle-aged, when compared to young male rats, advocates for the increased intensity of respiratory burst following direct stimulation of membrane protein kinase C by PMA, without phagocytosis [51] in these cells. Considering findings showing the preserved superoxide dismutase activity and the impaired catalase and glutathione peroxidase activity in hematopoietic stem cells in middle-aged mice [52], the impaired function of H2O2-degrading enzymes in macrophages from the middle-aged may be anticipated.
In spite of the attenuated macrophage inflammatory response in the middle-aged when compared to the young female rats, macrophages in the middle-aged females produce increased amount of IL-6, and comparable amount of IL-1β, TNF-α, and NO to that measured in the middle-aged males. These findings suggest that even with the diminished circulating estradiol levels, middle-aged females preserve the peritoneal macrophages’ inflammatory features comparable to those of their male counterparts.
Long-term changes in circulating estradiol levels in female rats, such as those observed over the course of estrus cycle, followed by permanently diminished estradiol level in menopause, undoubtedly affect peritoneal environment of female rats and could not be reproduced by short-term in vitro estrogen treatment [53]. Recent data reinforced the central role of microenvironmental factors in governing macrophage function within the peritoneum [54]. It must be taken into consideration that peritoneal macrophages from females, in contrast to males, may be also directly exposed to the ovarian hormones via periovarial space [55]. Opening of the ovarian bursa, consisting of a tunnel-like passage located at the junction of the ovarian bursa and the cephalic tip of the uterine cornu, provides communication between the periovarial space and the peritoneal cavity and allows for a direct contact with the ovarian and the uterine-derived secretions [55]. Estradiol levels in the ovarian vein, uterine endometrium, and vagina are 10–100-folds higher than the levels measured in peripheral blood [56, 57]; so, peritoneal macrophages during pre-menopause may be routinely exposed to this concentration of estradiol. Access to gonadal estradiol in young age may shape macrophage phenotype and function during aging in a different manner than exposure to sole circulating estradiol, such as in males. Therefore, both circulating and tissue levels of estradiol may contribute to changes in phenotype and function of peritoneal macrophages of middle-aged female rats.
CONCLUSION
Middle age is associated with the profound changes in phenotype and function of peritoneal macrophages in rats of both sexes. Whereas the level of circulating estradiol and the expression level of macrophage ERα are allied with the age-related changes in macrophage inflammatory cytokine production (i.e., an increase in males and a decrease in females), the modifications in phenotype and the oxidative metabolism of macrophages in middle-aged rats appear to be independent of estradiol and ERα/ERβ expression. Although our study suggests that the sex difference in the level of circulating estradiol may to some extent contribute to sex difference in macrophage function of middle-aged rats, it also points to more complex hormonal regulation of peritoneal macrophage activity in females.
References
Fischer, J., N. Jung, N. Robinson, and C. Lehmann. 2015. Sex differences in immune responses to infectious diseases. Infection. doi:10.1007/s15010-015-0791-9.
Voskuhl, R.R., and S.M. Gold. 2012. Sex-related factors in multiple sclerosis susceptibility and progression. Nature Reviews Neurology. doi:10.1038/nrneurol.2012.43.
Živković, I., B. Bufan, V. Petrušić, R. Minić, N. Arsenović-Ranin, R. Petrović, and G. Leposavić. 2015. Sexual diergism in antibody response to whole virus trivalent inactivated influenza vaccine in outbred mice. Vaccine. doi:10.1016/j.vaccine.2015.09.006.
Griesbeck, M., S. Ziegler, S. Laffont, N. Smith, L. Chauveau, P. Tomezsko, A. Sharei, G. Kourjian, F. Porichis, et al. 2015. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. The Journal of Immunology. doi:10.4049/jimmunol.1501684.
Hewagama, A., D. Patel, S. Yarlagadda, F.M. Strickland, and B.C. Richardson. 2009. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes & Immunity. doi:10.1038/gene.2009.
Klein, S.L., and K.L. Flanagan. 2016. Sex differences in immune responses. Nature Reviews Immunology. doi:10.1038/nri.2016.90.
Ruggieri, A., S. Anticoli, A. D'Ambrosio, L. Giordani, and M. Viora. 2016. The influence of sex and gender on immunity, infection and vaccination. Annali dell’Istituto Superiore di Sanità. doi:10.4415/ANN_16_02_11.
Mahbub, S., A.L. Brubaker, and E.J. Kovacs. 2011. Aging of the innate immune system: An update. Current Opinion in Immunology. doi:10.2174/157339511794474181.
Weiskopf, D., B. Weinberger, and B. Grubeck-Loebenstein. 2009. The aging of the immune system. Transplant International. doi:10.1111/j.1432-2277.2009.00927.x.
Linehan, E., and D.C. Fitzgerald. 2015. Ageing and the immune system: Focus on macrophages. European Journal of Microbiology and Immunology. doi:10.1556/EUJMI-D-14-00035.
Marriott, I., K.L. Bost, and Y.M. Huet-Hudson. 2006. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. Journal of Reproductive Immunology. doi:10.1016/j.jri.2006.01.004.
Spitzer, J.A. 1999. Gender differences in some host defense mechanisms. Lupus. doi:10.1177/096120339900800510.
Stanojević, S., I. Ćuruvija, V. Blagojević, R. Petrović, V. Vujić, and M. Dimitrijević. 2016. Strain-dependent response to stimulation in middle-aged rat macrophages: A quest after a useful indicator of healthy aging. Experimental Gerontology. doi:10.1016/j.exger.2016.10.005.
Vermeulen, A., J.M. Kaufman, S. Goemaere, and I. van Pottelberg. 2002. Estradiol in elderly men. The Aging Male. doi:10.1080/tam.5.2.98.102.
Laughlin, G.A., E. Barrett-Connor, D. Kritz-Silverstein, and D. von Mühlen. 2000. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. The Journal of Clinical Endocrinology & Metabolism. doi:10.1210/jcem.85.2.6405.
Giefing-Kroll, C., P. Berger, G. Lepperdinger, and B. Grubeck-Loebenstein. 2015. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. doi:10.1111/acel.12326.
Baeza, I., N.M. De Castro, L. Arranz, J. Fdez-Tresguerres, and M. De la Fuente. 2011. Ovariectomy causes immunosenescence and oxi-inflamm-ageing in peritoneal leukocytes of aged female mice similar to that in aged males. Biogerontology. doi:10.1007/s10522-010-9317-0.
Zhao, H., Z. Tian, J. Hao, and B. Chen. 2005. Extragonadal aromatization increases with time after ovariectomy in rats. Reproductive Biology and Endocrinology. doi:10.1186/1477-7827-3-6.
Dimitrijević, M., S. Stanojević, N. Kuštrimović, K. Mitić, V. Vujić, I. Aleksić, K. Radojević, and G. Leposavić. 2013. The influence of aging and estradiol to progesterone ratio on rat macrophage phenotypic profile and NO and TNF-a production. Experimental Gerontology. doi:10.1016/j.exger.2013.07.001.
Barrat, F., B. Lesourd, H.J. Boulouis, D. Thibault, S. Vincent-Naulleau, B. Gjata, A. Louise, T. Neway, and C. Pilet. 1997. Sex and parity modulate cytokine production during murine ageing. Clinical & Experimental Immunology. doi:10.1046/j.1365-2249.1997.4851387.x.
Carvalho-Freitas, M.I., J.A. Anselmo-Franci, E. Teodorov, A.G. Nasello, J. Palermo-Neto, and L.F. Felicio. 2007. Reproductive experience modifies dopaminergic function, serum levels of prolactin, and macrophage activity in female rats. Life Science. doi:10.1016/j.lfs.2007.04.032.
Pick, E., and D. Mizel. 1981. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. Journal of Immunological Methods. doi:10.1016/0022-1759(81)90138-1.
Jr Johnston, R.B., and S. Kitagawa. 1985. Molecular basis for the enhanced respiratory burst of activated macrophages. Federation Proceedings 14: 2927–2932.
Choi, H.S., J.W. Kim, Y.N. Cha, and C. Kim. 2006. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. Journal of Immunoassay and Immunochemistry. doi:10.1080/15321810500403722.
Pick, E., J. Charon, and D. Mizel. 1981. A rapid densitometric microassay for nitroblue tetrazolium reduction and application of the microassay to macrophages. Journal of the Reticuloendothelial Society. 6: 581–593.
Bradley, P., D.A. Priebat, R.D. Christensen, and G. Rothstein. 1982. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology. doi:10.1111/1523-1747.ep12506462.
Green, L.C., D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, and S.R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. doi:10.1016/0003-2697(82)90118-X.
Dijkstra, C.D., E.A. Döpp, P. Joling, and G. Kraal. 1985. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54: 589–599.
Thornley, T.B., Z. Fang, S. Balasubramanian, R.A. Larocca, W. Gong, S. Gupta, E. Csizmadia, N. Degauque, B.S. Kim, et al. 2014. Fragile TIM-4–expressing tissue resident macrophages are migratory and immunoregulatory. Journal of Clinical Investigation. doi:10.1172/JCI73527.
Negishi, H., Y. Ohba, H. Yanai, A. Takaoka, K. Honma, K. Yui, T. Matsuyama, T. Taniguchi, and K. Honda. 2005. Negative regulation of toll-like-receptor signaling by IRF-4. Proceedings of the National Academy of Sciences USA. doi:10.1073/pnas.0508327102.
Calippe, B., V. Douin-Echinard, L. Delpy, M. Laffargue, K. Lélu, A. Krust, B. Pipy, F. Bayard, J.F. Arnal, et al. 2010. 17Beta-estradiol promotes TLR4 triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. The Journal of Immunology. doi:10.4049/jimmunol.0902383.
Frei, R., J. Steinle, T. Birchler, S. Loeliger, C. Roduit, D. Steinhoff, R. Seibl, K. Büchner, R. Seger, et al. 2010. MHC class II molecules enhance toll-like receptor mediated innate immune responses. PloS One. doi:10.1371/journal.pone.0008808.
Dimitrijević, M., S. Stanojević, V. Vujić, I. Aleksić, I. Pilipović, and G. Leposavić. 2014. Aging oppositely affects TNF-α and IL-10 production by macrophages from different rat strains. Biogerontology. doi:10.1007/s10522-014-9513-4.
Simpson, E., G. Rubin, C. Clyne, K. Robertson, L. O’Donnell, S. Davis, and M. Jones. 1999. Local estrogen biosynthesis in males and females. Endocrine-Related Cancer. doi:10.1677/erc.0.0060131.
Campesi, I., M. Marino, A. Montella, S. Pais, F. Franconi. 2017. Sex differences in estrogen receptor α and β levels and activation status in LPS-stimulated human macrophages Journal of Cellular Physiology. 232: 340–345. doi: 10.1002/jcp.25425
Jiang, Y., P. Gong, Z. Madak-Erdogan, T. Martin, M. Jeyakumar, K. Carlson, I. Khan, T.J. Smillie, A.G. Chittiboyina, et al. 2013. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. The FASEB Journal. doi:10.1096/fj.13-234617.
Murphy, A.J., P.M. Guyre, C.R. Wira, and P.A. Pioli. 2009. Estradiol regulates expression of estrogen receptor ERa46 in human macrophages. PloS One. doi:10.1371/journal.pone.0005539.
Matthews, J., and J.A. Gustafsson. 2003. Estrogen signaling: A subtle balance between ER alpha and ER beta. Molecular Interventions. doi:10.1124/mi.3.5.281.
Ashcroft, G.S., J. Dodsworth, E. Van Boxtel, R.W. Tarnuzzer, M.A. Horan, G.S. Schultz, and M. Ferguson. 1997. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-1 levels. Nature Medicine. doi:10.1038/nm1197-1209.
Moestrup, S.K., and H.J. Møller. 2004. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Annals of Medicine 5: 347–354.
Geraldes, P., S. Gagnon, S. Hadjadj, Y. Merhi, M.G. Sirois, I. Cloutier, and J.F. Tanguay. 2006. Estradiol blocks the induction of CD40 and CD40L expression on endothelial cells and prevents neutrophil adhesion: An ER α -mediated pathway. Cardiovascular Research. doi:10.1016/j.cardiores.2006.05.015.
Xie, H., C. Hua, L. Sun, X. Zhao, H. Fan, H. Dou, L. Sun, and Y. Hou. 2011. 17β-estradiol induces CD40 expression in dendritic cells via MAPK signaling pathways in a minichromosome maintenance protein 6-dependent manner. Arthritis & Rheumatology. doi:10.1002/art.30420.
Jackaman, C., H.G. Radley-Crabb, Z. Soffe, T. Shavlakadze, M.D. Grounds, and D.J. Nelson. 2013. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell. doi:10.1111/acel.12062.
Straub, R.H. 2007. The complex role of estrogens in inflammation. Endocrine Reviews. doi:10.1210/er.2007-0001.
West, P., I.E. Brodsky, C. Rahner, D.K. Woo, H. Erdjument-Bromage, O. Tempst, M.C. Walsh, Y. Choi, G.S. Shadel, and S. Ghosh. 2011. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. doi:10.1038/nature09973.
Gantner, B.N., R.M. Simmons, S.J. Canavera, S. Akira, and D.M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. The Journal of Experimental Medicine. doi:10.1084/jem.20021787.
Ozinsky, A., D.M. Underhill, J.D. Fontenot, A.M. Hajjar, K.D. Smith, C.B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proceedings of the National Academy of Sciences. doi:10.1073/pnas.250476497.
Rodrigues, M.R., D. Rodriguez, M. Russo, and A. Campa. 2002. Macrophage activation includes high intracellular myeloperoxidase activity. Biochemical and Biophysical Research Communications. doi:10.1006/bbrc.2002.6724.
Abu-Soud, H.M., and S.L. Hazen. 2000. Nitric oxide is a physiological substrate for mammalian peroxidases. The Journal of Biological Chemistry. doi:10.1074/jbc.M002579200.
Shirato, K., and K. Imaizumi. 2015. Mechanisms underlying the suppression of inflammatory responses in peritoneal macrophages of middle-aged mice. In Physical activity, exercise, sedentary behavior and health, eds. Kazuyuki Kanosue, Satomi Oshima, Zhen-Bo Cao, and Koichiro Oka, 193–202. Tokyo: Springer. doi:10.1007/978-4-431-55333-5_16.
Dieter, P. 1992. Relationship between intracellular pH changes, activation of protein kinase C and NADPHoxidase in macrophages. FEBS Letters. doi:10.1016/0014-5793(92)80012-6.
Porto, M.L., B.P. Rodrigues, T.N. Menezes, S.L. Ceschim, D.E. Casarini, A.L. Gava, T.M.C. Pereira, E.C. Vasquez, B.P. Campagnaro, and S.S. Meyrelles. 2015. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. Journal of Biomedical Science. doi:10.1186/s12929-015-0201-8.
Kovats, S. 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cellular Immunology. doi:10.1016/j.cellimm.2015.01.018.
Linehan, E., Y. Dombrowski, R. Snoddy, P.G. Fallon, A. Kissenpfennig, and D.C. Fitzgerald. 2014. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. doi:10.1111/acel.12223.
Gaytan, F., J. Aceitero, C. Bellido, J.E. Sanchez-Criado, and E. Aguilar. 1991. Estrous cycle-related changes in mast cell numbers in several ovarian compartments in the rat. Biology of Reproduction. doi:10.1095/biolreprod45.1.27.
Baird, D.T., P.E. Burger, G.D. Heavon-Jones, and R.J. Scaramuzzi. 1974. The site of secretion of androstenedione in non-pregnant women. Journal of Endocrinology. 63: 201–212.
Thijssen, J.H., M.A. Wiegerninck, G.H. Donker, and J. Poortman. 1984. Uptake and metabolism of oestriol in human target tissues. Journal of Steroid Biochemistry. 20: 955–958.
Acknowledgements
This study is supported by the Ministry of Education, Science and Technological Development Republic of Serbia, Grant No 175050. The Ministry of Education, Science and Technological Development had no role in the study design, collection, analysis and interpretation of data, writing of the report, and decision to submit the article for publication. Authors express their gratitude to Tatjana Miletić, PhD (Health & Environment Department, Molecular Diagnostics, AIT Austrian Institute of Technology GmbH), for critical reading and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol and all procedures with animals and their care were approved by Ministry of Agriculture and Environmental Protection (license number 323-07-01577/2016-05/14, issued on 02-25-2016) and were in accordance with principles declared in Directive 2010/63/EU of the European Parliament and of the Council from 22 September 2010 on the protection of animals used for scientific purposes (revising Directive 86/609/EEC).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ćuruvija, I., Stanojević, S., Arsenović-Ranin, N. et al. Sex Differences in Macrophage Functions in Middle-Aged Rats: Relevance of Estradiol Level and Macrophage Estrogen Receptor Expression. Inflammation 40, 1087–1101 (2017). https://doi.org/10.1007/s10753-017-0551-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0551-3