Abstract
Risky sexual behaviour is a major health issue in society, and it is therefore important to understand factors that may predispose individuals to such behaviour. Research suggests a link between risky sexual behaviour and personality, but the basis of this link remains unknown. Hans Eysenck proposed that personality is related to sexual behaviour via biological underpinnings of both. Here we test the viability of this perspective by analysing data from identical and non-identical twins (N = 4,904) who completed a questionnaire assessing sexual attitudes and behaviour as well as personality. Using genetic modelling of the twin data, we found that risky sexual behaviour was significantly positively correlated with Impulsivity (r = .27), Extraversion (r = .24), Psychoticism (r = .20), and Neuroticism (r = .09), and that in each case the correlation was due primarily to overlapping genetic influences. These findings suggest that the genetic influences that shape our personality may also predispose us to risky sexual behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Risky sexual behaviour is a major health issue in society. High-risk sexual behaviours include failure to use condoms and birth control, having a large number of lifetime sex partners, non-discriminating sex-partner recruiting patterns, participating in concurrent sex partnerships, and having sex after heavy alcohol consumption (Aral 2001; Cook and Clark 2005; Hoyle et al. 2000). Though these behaviours do not necessarily lead to negative outcomes, they tend to correlate together forming a pattern of behaviour that is a primary risk factor for sexually transmitted disease (STD) and unplanned pregnancy. The short-term and long-term consequences of STD include cervical cancer, pelvic inflammatory disease, infertility, and complications in pregnancy (Aral 2001). Consequences of unplanned pregnancy can include medical, social, financial, and psychological difficulties (Delgado-Rodriguez et al. 1997; Fullerton et al. 1997; Geller 2004).
Identifying and understanding factors that may influence or predispose to risky sexual behaviour is important in order to design appropriate interventions and prevention campaigns, and to determine their target groups. Research identifying environmental (social and cultural) conditions that increase risky sexual behaviour (Aral 2001; Marston and King 2006) has been helpful, but it is also important to determine whether dispositional differences affect individuals’ susceptibility to sexual risk behaviours. A review by Hoyle et al. (2000) suggested that individuals high on personality traits such as impulsivity and sensation seeking are more likely to engage in risky sexual behaviour. Results were more equivocal for the Big Five personality traits, perhaps partly due to the sparsity of studies, most of which have small sample sizes and varying methodologies or conceptions of sexual risk behaviours. More recent studies have found low agreeableness, low conscientiousness, high neuroticism (Trobst et al. 2002), high extraversion (Schmitt 2004), and low openness (Miller et al. 2004) to be associated with riskier sexual behaviour; it appears the latter is associated with all of the major personality traits (albeit in different directions), rather than just exhibiting a specific relationship with one dimension of personality. Hoyle et al. (2000) suggested that future research needed not only to further establish the basic link between personality and risky sexual behaviour, but to empirically investigate the basis of the link; there has been no progress on this as yet.
Eysenck (1976) theoretically proposed that personality is related to sexual behaviour via biological processes underlaid by genetic variation. From twin studies, we know that individual differences in all dimensions of personality are known to be partly (≈30–60%) due to genetic differences (Jang et al. 1996; Loehlin 1992). However, it is only very recently that research has found specific evidence that genes also influence individual differences in risky sexual behaviour. Bricker et al. (2006) estimated a heritability of 28% for age of first intercourse, which is associated with lack of condom use and higher number of lifetime sexual partners (Abma and Sonenstein 2001; Darroch et al. 2001), and Mustanski et al. (2007) found heritabilities for age of first intercourse and lifetime number of sexual partners ranging from 42 to 61% for males and females. In our own data, we found 34% heritability for a broad measure of risky sexual behaviour (Verweij et al. 2009). According to Eysenck’s (1976) perspective, the genetic variation that underlies differences in personality would be expected to overlap with the genetic variation underlying differences in risky sexual behaviour. Here we aim to test that prediction.
We analysed questionnaire data from a large twin sample, including a risky sexual behaviour checklist, the revised Eysenck Personality Questionnaire short version (Eysenck et al. 1985) scales Extraversion, Neuroticism, and Psychoticism, as well as an impulsivity scale (Eysenck and Eysenck 1977). Using genetic modelling of the twin data, we tested the hypotheses that (1) variation in risky sexual behaviour, Impulsivity, Extraversion, Neuroticism, and Psychoticism is influenced by genetic factors; (2) risky sexual behaviour correlates with Impulsivity, Extraversion, Psychoticism, and Neuroticism; and (3) these correlations are due to genetic correlation between the traits.
Methods
Participants
Participants were drawn from the Australian National Health and Medical Research Council Twin Registry (ATR), and were recruited in two phases from a large twin-family study of alcohol use and abuse.
In phase one all twin pairs (N = 4,269, aged between 18 and 25) participating in an extensive Health and Lifestyle Questionnaire (HLQ) were asked whether they were willing to participate in an anonymous study about sexual behaviour and attitudes. All those who agreed were mailed the sex questionnaire between July 1991 and October 1992. To ensure anonymity, informed consent was obtained separate from the questionnaires, and twins were not asked for their name or date of birth, but to make up a ten digit identification number. Both twins of a pair used the same number so that the responses of twin pairs could be linked.
In phase two an additional group of twin pairs in an older cohort (aged between 27 and 52 years old) was asked to participate in the sexual behaviour and attitudes study following the same procedures as above. Twins who expressed willingness to participate were mailed the questionnaire between April and August 1992.
In total, 4,904 twins completed and returned the questionnaire (1,824 males and 3,080 females), a 54% response rate. Of those, 107 single twins were excluded from further analysis due to ambiguous zygosity. The number of participants used for genetic analyses was 4,797, including 667 female MZ, 312 male MZ, 377 female DZ, 185 male DZ, 366 opposite-sex DZ pairs, along with 983 single twins. The mean age for males was 30.5 (SD = 8.3) and for females 31.1 (SD = 8.5).
Zygosity determination
The zygosity of the same-sex twins was determined during completion of the HLQ, based on their response to standard items about physical similarity and being mistaken for each other. Ambiguous responses were clarified by telephone call. According to Ooki et al. (1990) and Martin and Martin (1975), concordance on zygosity between discriminant analyses of questionnaire scores and DNA typing is at least 95%, and telephone clarification will have increased this accuracy.
Measures
Risky sexual behaviour
Our broad measure of risky sexual behaviour is the same as that used in Verweij et al. (2009). It includes a checklist of high risk behaviours such as failure to use condoms or other birth control methods, ever having had a venereal disease, participating in concurrent sex partnerships, non-discriminating sex-partner recruitment, and having sex after heavy alcohol consumption (see Table 1 for all scale items). These behaviours have been identified as increasing risk of STD and unwanted pregnancy (Aral 2001; Cook and Clark 2005; Hoyle et al. 2000), and are known to correlate with each other to form a pattern of risk behaviour. It is this broad pattern of risky sexual behaviour (RSB) that we aim to investigate here. Table 1 shows the eight items and the percentage of people who checked each one. Each checked risk behaviour scored a point, and the points were summed. Additionally, a categorical item assessing respondents’ number of lifetime number of sex partners was included to reflect the importance of this trait to overall sexual risk. Those with three to ten sex partners (39.4% of males and 45.1% females) had one extra point added to their score, and those with more than ten (34.6% of males and 15.8% of females) had two extra points added to their score. These cut-offs were largely dictated by the category cut-offs in the questionnaire item, which had eight response options (0, 1, 2, 3–5, 6–10, 11–20, 20–50, >50). In cases of a missing response to this item (4.5% of males and 5.4% of females), the respondent’s score on the composite scale was treated as missing. Cronbach’s alpha—a measure of internal consistency—for the RSB scale was 0.80.
As the distribution of the risky sexual behaviour scale showed significant skewness, scores were converted (see “Appendix 1”) into six ordinal categories with roughly similar frequencies (Neale et al. 1994; Verweij et al. 2009) for subsequent analyses, which is optimal for threshold modelling in the statistical package Mx (Neale et al. 2006). Since some items referred to heterosexual sex, we tested our concern that participants self-identifying (on another item in the questionnaire) as homosexual (1.6% of the sample) may have artificially lower RSB scores than heterosexuals. However, homosexuals actually had higher scores than heterosexuals, so we decided against excluding those participants from the analysis.
Personality
We measured personality using the 48-item revised Eysenck Personality Questionnaire (EPQ-R) shortened version (Eysenck et al. 1985), plus an Impulsivity scale consisting of seven items from the EPQ-R full version plus seven extra impulsivity items (Eysenck and Eysenck 1977). Here we used the scales Extraversion, Neuroticism, and Psychoticism (12 items each), and the 14-item Impulsivity scale. Cronbach’s alpha for these scales was .88, .84, .54, and .75, respectively, in accordance with findings in other studies (e.g., Eysenck et al. 1985; Sato 2005). Where an item or items were missing, the respondent’s scale score was treated as missing. Table 1 shows descriptive statistics for each personality scale. To be analysed in threshold models along with the RSB scale, personality scales had to be converted to ordinal variables as well, so scores were similarly converted (see “Appendix 1”) into six ordinal categories with roughly similar frequencies (Neale et al. 1994).
Analyses
Maximum-likelihood modelling procedures were employed using the statistical package Mx (Neale et al. 2006), which accounts for twin relatedness. The measures described above were analysed in Mx as raw ordinal data, where it is assumed that thresholds delimiting the different categories overlay a normally distributed continuum of liability. In maximum-likelihood modelling, the goodness-of-fit of a model to the observed data is distributed as chi-square (χ²), and the number of unknown parameters (those to be estimated) is reflected by the degrees of freedom (df). By testing the change in chi-square (Δχ²) against the change in degrees of freedom (Δdf), we can test whether dropping model parameters, or constraining them to be equal, significantly worsens the model fit. In this way we can test hypotheses regarding those parameters.
Genetic modelling
The present study uses the classical twin design, where variance in traits, and covariance between them, is partitioned into genetic (additive, A, and non-additive, D) and environmental (shared within twin pairs, C, and unshared, E) sources. Additive genetic variance results from the sum of allelic effects within and across genes. Non-additive genetic effects include dominance and epistasis (i.e., allelic interactions within and across genes, respectively). Shared environmental variance is that shared between twin pairs, and may include shared home environment, parental style, uterine environment, and so on. Unshared environmental variance is that not shared between twin pairs, and includes idiosyncratic experiences and also measurement error. In the present study the trait variances are standardised to equal 1, so A, C, D, and E parameters equal the proportion of variance accounted for by each source. Note that the proportion of variance in a trait accounted for by genetic factors (i.e., the sum of A and D) represents the heritability (h²) of the trait.
Partitioning of phenotypic variance into genetic and environmental components can be achieved because MZ twins share all their genes, while DZ twins share only half their genes on average. Thus, if A were the sole source of variance in a trait, we would expect a twin correlation of 1.0 for MZ pairs, and 0.5 for DZ pairs. If D were the sole source of variance in a trait, we would expect a twin correlation of 1.0 for MZ pairs, and 0.25 for DZ pairs (see Posthuma et al. (2003) for an explanation). By definition, if C were the sole source of variance in a trait, we would expect a twin correlation of 1 for both MZ and DZ pairs, and likewise if E were the sole source of variance in a trait we would expect a zero twin correlation for both MZ and DZ pairs.
In reality, observed MZ and DZ twin correlations generally reflect a combination of these genetic and environmental influences, and structural equation modelling allows us to determine the combination that best matches the observed data. A limitation of the design is that C and D cannot both be estimated for the same variable in the same model with twins reared together, as they are confounded: C influences push the DZ correlation up relative to the MZ correlation, whereas D influences push the DZ correlation down relative to the MZ correlation. For a variable where the DZ twin correlation is more than half the MZ twin correlation, C is estimated, and for a variable where the DZ twin correlation is less than half the MZ correlation, D is estimated.
Cross-twin cross-trait correlations allow us to partition covariance between traits into A, C or D, and E in the same way as we do for variance in a single trait. In this way we can calculate genetic correlation, a measure of the overlap in the genetic variation of two traits.
An assumption of the classical twin design is that trait-relevant environments are similar to the same extent in MZ and DZ twin pairs; tests of this assumption for personality traits (Loehlin 1992) suggest it is valid. Further details of the classical twin design can be found elsewhere (Neale and Cardon 1992; Posthuma et al. 2003).
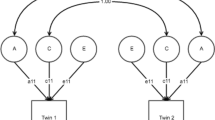
To test our hypothesis that the relationship between risky sexual behaviour and personality traits can be explained by genetic correlation between the traits, we fitted four bivariate Cholesky models (Fig. 1a), one for each personality variable with risky sexual behaviour. (As Eysenck’s Extraversion, Psychoticism, and Neuroticism scales are expected to be orthogonal, a computationally and conceptually complicated five-variable model would not be substantially more informative than separate bivariate models.) An alpha level of .002 was adopted as an approximate Bonferroni correction for the number of hypothesis tests performed. Significant influence of genes on trait variation was tested by dropping the genetic paths to each trait and comparing model fit. Significant overlap between the genetic (or environmental) variation in a pair of traits was tested by dropping the genetic (or environmental) crosspath in the constrained model. For ease of interpretation, the models were transformed from Cholesky forms into ‘correlated factors’ models (Fig. 1b) as suggested by Loehlin (1996). This yielded the proportion of variance in each trait accounted for by A, C or D, and E effects, as well as genetic and environmental correlations.
Bivariate models in Cholesky form (a) and ‘correlated factors’ form (b). Additive genetic (A), non-additive genetic (D), shared environmental (C), and unique environmental (E) influences are represented by circles, and the observed traits (a personality trait (one of Impulsivity, Extraversion, Psychoticism, or Neuroticism) along with risky sexual behaviour) are represented by boxes. In b the straight lines indicate parameters that when squared equal the proportion of variance in the trait the arrow points to that is accounted for by the latent factor that it points from. A curved line between A factors represents genetic correlation, and between E factors, environmental correlation
Results
Preliminary analyses: heterogeneity of thresholds across age, sex, and zygosity
Before modelling variance components, we tested for heterogeneity in thresholds and twin correlations across age, sex, and zygosity. This process involved many tests, but using a very low alpha level may have caused us to overlook assumption violations, so an α-level of 0.01 was employed. Extraversion, Impulsivity, Psychoticism, Neuroticism, and risky sexual behaviour all showed significant age effects on the thresholds (older people were lower on all traits), and the latter four variables also showed significant sex effects on the thresholds (males were higher in risky sexual behaviour, Impulsivity, and Psychoticism, but lower in Neuroticism). These effects were accounted for in subsequent modelling by including age as a covariate and allowing thresholds to differ between the sexes, in effect partialling out individual differences due to age and sex.
As can be seen in “Appendix 2”, individual risky sex behaviours generally yielded MZ correlations greater than DZ correlations, suggesting that these specific behaviours are heritable. Twin correlations for the composite RSB scale, along with the personality scales, are displayed in Table 2. For each of these traits, MZ twin correlations were higher than DZ twin correlations for both male and female pairs; correlations were not significantly different for male versus female MZ pairs, nor for male versus female DZ pairs. Based on the size of the DZ correlations relative to the MZ correlations (Table 2), C was estimated as a specific influence on risky sexual behaviour in subsequent modelling, whereas D was estimated as a specific influence on each of the personality traits.
For the personality traits, opposite-sex DZ twin pair correlations could be equated to same sex-DZ twin pair correlations. However, for risky sexual behaviour, twin correlations were significantly lower for opposite-sex than same-sex DZ pairs. Initial modelling showing C effects acting in opposite directions in males and females suggested that the low opposite-sex DZ twin correlations could be due to different shared environmental influences acting on males and females. Thus, we allowed for sex difference in the source of C in subsequent modelling by allowing the genetic correlation of opposite-sex twin pairs to be free to be estimated, rather than fixing it at 1.0. The magnitudes of genetic and shared and unshared environmental effects were similar in males and females for all models, and were equated without significant loss of fit. Hypotheses were tested against this base model.
Hypothesis testing
Genetic modelling (Table 3) showed that risky sexual behaviour was significantly influenced by additive genetic factors as well as shared environmental factors. The heritability of risky sexual behaviour was estimated at 33%, with the shared environment contributing 29% and unshared environment the remaining 38%. Impulsivity, Extraversion, Psychoticism, and Neuroticism were also significantly influenced by genetic factors (Table 3). Table 4 shows maximum likelihood estimates of the A, D, and E components of variance in Impulsivity, Extraversion, Psychoticism, and Neuroticism. These estimates are from the base models where male and female parameters have been equated but A, C, D, and E parameters are retained whether or not they are significant. Estimates of broad heritability (which includes additive and non-additive genetic effects) were remarkably consistent across the different personality traits, ranging from 41 to 50%. The non-additive components of this genetic influence were substantial for each personality trait (15–35% of variance), but did not reach significance, suggesting probable non-additive genetic effects which cannot be confirmed with the current sample size or design.
In Tables 3 and 4 it can be seen that risky sexual behaviour correlated positively and significantly with Impulsivity, Extraversion, Psychoticism, and Neuroticism. For the former three personality traits, the correlation with risky sexual behaviour ranged from 0.20 to 0.27, and was primarily due to a significant overlap of genetic influences, though there was also a significant overlap of unshared environmental influences. The correlation between Neuroticism and risky sexual behaviour was weaker, and though there was some evidence for overlapping genetic influences (P = .003), there was no such evidence for overlapping unshared environmental influences.
Discussion
Our results indicate that risky sexual behaviour is significantly influenced by genetic factors (accounting for 33% of the variance) as well as by the shared environment (29%). The personality traits Impulsivity, Extraversion, Psychoticism, and Neuroticism were also significantly influenced by genetic factors (42 to 50%), but there was no evidence for a role of shared environment, in accordance with past research (Jang et al. 1996, 2002; Loehlin 1992). We found that risky sexual behaviour was significantly positively correlated to each personality trait, and that these correlations were largely due to overlapping genetic influences. Unshared environmental influences were found to play a minor role in the covariance of personality and risky sexual behaviour.
Previous reviews of the determinants of risky sexual behaviour (Aral 2001; Marston and King 2006) have focused on social and cultural influences, and have not considered the possibility of genetic influences. Recent studies, though, showed significant heritability for age of first intercourse and lifetime number of opposite-sex sexual partners in our own data (Dunne et al. 1997; Zietsch et al. 2008) and those of others (Bricker et al. 2006; Mustanski et al. 2007). In our data, Verweij et al. (2009) found heritability in a broad measure of risky sexual behaviours, and the present study further found that the specific sexual risk behaviours making up this measure each appeared to be influenced by genetic factors.
Shared environment also had a significant impact on levels of risky sexual behaviour. We cannot determine specifically what these environmental influences are, but as they are shared between co-twins they likely derive from the family environment or peer group; this could be in the form particular parenting styles or strategies (e.g., age when parental sex education takes place, if at all), or broader factors such as the family’s socioeconomic status. It also appears that the shared environment influences males and females in different ways. Initial modelling suggested that either the same shared environmental influences were acting in different directions in males and females, or different shared environmental influences were acting on males and females. Sex differences in the mode of action of shared environmental influence are unsurprising given the different social pressures and values regarding men and women’s sexual behaviour.
Another aspect of the etiology of risky sexual behaviour that has been relatively overlooked in epidemiology literature is the role of dispositional factors. The psychology literature contains numerous studies showing a link between personality traits and risky sexual behaviour (see Hoyle et al. (2000) for a review, and Trobst et al. (2002) and Miller et al. (2004) for more recent studies), but to our knowledge there has been no research toward elucidating the mechanisms underlying this link. Our findings suggest that the basis of the link is primarily genetic; that is, genetic factors underlying a person’s personality also predispose him or her to taking more or less risk in their sexual behaviour. There are also indications that unshared environmental influences make a lesser but significant contribution to the relationship of risky sexual behaviour with Impulsivity, Extraversion, and Psychoticism. Again, what these environmental influences may be cannot be determined from the data, but as they are unshared between twins, they are unlikely to derive from the family environment.
Our findings are broadly consistent with Eysenck’s predictions regarding personality and sexual behaviour based on his psychobiological theory of personality (Eysenck 1976). According to this theory, extroverts require greater external stimulation than normal in order to attain an optimum level of arousal, while introverts require less stimulation than normal. Thus, those high in Extraversion are predicted to seek stronger stimulation than those low on Extraversion and hence engage in more and more varied sexual behaviours, with less adherence to socially prescribed behaviours such as safe sex. Our evidence accords with this prediction. Psychoticism is thought to be related to testosterone, and high scorers tend to disregard risk, engage in socially disapproved acts, and have little concern for the wellbeing of others. This would seem to predict riskier sexual behaviour in high scorers on Psychoticism, and this is borne out in our results. Impulsivity, an ‘extra’ trait purported to lie on a plane formed by Extraversion and Psychoticism (Eysenck and Eysenck 1977), would accordingly be predicted to be associated with riskier sexual behaviour, and this is the case in our results. Neuroticism is purported to be based on the autonomic nervous system. A neurotic person’s strong, labile and lasting emotions of fear and anxiety in even mildly stressful situations could be thought to, if anything, decrease sexual interactions and hence risky sexual behaviour. Our data reveals a small effect to the contrary, that neuroticism is slightly positively correlated with risky sexual behaviour, in accordance with Trobst et al. (2002). It is unclear why this might be the case. More generally though, our results lend support to Eysenck’s approach of linking sexual behaviour with personality via biological mechanisms. They also demonstrate the importance of heritable differences in our personality in terms of predisposing to risk behaviour.
The finding in our data of genetic correlations between personality traits and risky sexual behaviour should be interpreted with caution, as it does not necessarily mean that pleiotropic genetic factors are at work. Other causal relationships could also manifest as genetic correlations. For example, if variation in personality traits caused variation in risky sexual behaviour at the phenotypic level, then the genetic and environmental variation underlying personality differences would also contribute to variation in risky sexual behaviour. However, the finding of much greater genetic correlations (0.21–1.0) between risky sexual behaviour and personality than environmental correlations (.04–.19) suggests at least some role of pleiotropic genetic factors, but our sample is insufficient in both size and data characteristics (non-normal variables, twins only, magnitudes of genetic and environmental influences too similar) to directly test competing causal hypotheses (Duffy and Martin 1994).
Similarly, it should be noted that assortative mating, gene–environment correlation, and gene–environment interaction may play roles in a more complicated etiology than our results suggest, but a twins only design affords us negligible power to model these mechanisms. As such, part of the genetic influence in our results may be due to gene–environment correlation, and part of the shared environmental influence may actually be due to gene–environment interaction or assortative mating.
A further limitation of the present study, and one which affects most research based on self-report questionnaires, is the potential for socially desirable responding. This could have been particularly concerning here, given the sensitive nature of the items on risky sexual behaviour, but the problem was minimised by ensuring the responses were completely anonymous.
Another consideration is the possibility of participation bias. Females and MZ twins were overrepresented, as is common for community twin samples, but this is unlikely to seriously affect modelling results (Heath et al. 1998). Also, Dunne et al. (1997) found that the twins who explicitly consented to participate in this study (52%) had an earlier age at first sexual intercourse and had less conservative sexual attitudes than those who did not participate, but the effect sizes were small. As such, this participation bias probably did not seriously distort the results.
A broader limitation of this study is that the sources of variance identified are quite nebulous; we cannot point to a specific gene or set of genes, nor to specific social or cultural factors that moderate risky sexual behaviour. Similarly, epidemiological studies on specific social and cultural factors are inadequate without accounting for heritable dispositional factors. Together, though, the different types of approaches can paint a very useful picture of what affects the likelihood of a person engaging in risky sexual behaviour. It is therefore important that the research approach to risky sexual behaviour is multidisciplinary, and that future reviews of its etiology incorporate both external influences and dispositional factors.
In summary, we found overlap between genetic influences on risky sexual behaviour and genetic influences on Impulsivity, Extraversion, Psychoticism, and Neuroticism. This genetic correlation primarily drives the observed phenotypic correlation between personality traits and risky sexual behaviour. These results lend support to Eysenck’s (1976) perspective of linking personality with sexual behaviour via biological mechanisms. The results also reinforce the importance of considering heritable differences in disposition when investigating the causes of risky sexual behaviour.
References
Abma JC, Sonenstein FL (2001) Sexual activity and contraceptive practices among teenagers in the United States, 1988 and 1995
Aral SO (2001) Sexually transmitted diseases: magnitude, determinants and consequences. Int J STD AIDS 12(4):211–215
Bricker JB, Stallings MC, Corley RP, Wadsworth SJ, Bryan A, Timberlake DS et al (2006) Genetic and environmental influences on age at sexual initiation in the Colorado Adoption Project. Behav Genet 36(6):820–832
Cook RL, Clark DB (2005) Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sex Transm Dis 32(3):156–164
Darroch JE, Frost JJ, Singh S (2001) Can more progress be made? Teenage sexual and reproductive behavior in developed countries. The Alan Guttmacher Institute, New York
Delgado-Rodriguez M, Gomez-Olmedo M, Bueno-Cavanillas A, Galvez-Vargas R (1997) Unplanned pregnancy as a major determinant in inadequate use of prenatal care. Prev Med 26(6):834–838
Duffy DL, Martin NG (1994) Inferring the direction of causation in cross-sectional twin data: theoretical and empirical considerations. Genet Epidemiol 11(6):483–502
Dunne MP, Martin NG, Statham DJ, Slutske WS, Dinwiddie SH, Bucholz KK et al (1997) Genetic and environmental contributions to variance in age at first sexual intercourse. Psychol Sci 8(3):211–216
Eysenck HJ (1976) Sex and personality. Open Books, London
Eysenck SBG, Eysenck HJ (1977) Place of impulsiveness in a dimensional system of personality description. British J Soc Clin Psychol 16(FEB):57–68
Eysenck SBG, Eysenck HJ, Barrett P (1985) A revised version of the psychoticism scale. Pers Individ Differ 6(1):21–29
Fullerton D, Dickson R, Eastwood AJ, Sheldon TA (1997) Preventing unintended teenage pregnancies and reducing their adverse effects. Qual Health Care 6(2):102–108
Geller PA (2004) Pregnancy as a stressful life event. Cns Spectr 9(3):188–197
Heath AC, Madden PAF, Martin NG (1998) Assessing the effects of cooperation bias and attrition in behavioral genetic research using data-weighting. Behav Genet 28(6):415–427
Hoyle RH, Fejfar MC, Miller JD (2000) Personality and sexual risk taking: a quantitative review. J Pers 68(6):1203–1231
Jang KL, Livesley WJ, Vernon PA (1996) Heritability of the big five personality dimensions and their facets: a twin study. J Pers 64(3):577–591
Jang KL, Livesley WJ, Angleitner A, Riemann R, Vernon PA (2002) Genetic and environmental influences on the covariance of facets defining the domains of the five-factor model of personality. Pers Individ Differ 33(1):83–101
Loehlin JC (1992) Genes and environment in personality development. Sage, Newbury Park
Loehlin JC (1996) The Cholesky approach: a cautionary note. Behav Genet 26(1):65–69
Marston C, King E (2006) Factors that shape young people’s sexual behaviour: a systematic review. Lancet 368(9547):1581–1586
Martin NG, Martin PG (1975) The inheritance of scholastic abilities in a sample of twins: I. Ascertainment of the sample and diagnosis of zygosity. Ann Hum Genet 39:213–218
Miller JD, Lynam D, Zimmerman RS, Logan TK, Leukefeld C, Clayton R (2004) The utility of the five factor model in understanding risky sexual behavior. Pers Individ Differ 36(7):1611–1626
Mustanski B, Viken RJ, Kaprio J, Winter T, Rose RJ (2007) Sexual behavior in young adulthood: a population-based twin study. Health Psychol 26(5):610–617
Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer, Boston
Neale MC, Eaves LJ, Kendler KS (1994) The power of the classical twin study to resolve variation in threshold traits. Behav Genet 24(3):239–258
Neale MC, Boker SM, Xie G, Maes HH (2006) Mx: statistical modeling, 7th edn. Department of Psychiatry, Richmond
Ooki S, Yamada K, Asaka A, Hayakawa K (1990) Zygosity diagnosis of twins by questionnaire. Acta Genet Med Gemellol 39:109–115
Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I et al (2003) Theory and practice in quantitative genetics. Twin Res 6(5):361–376
Sato T (2005) The Eysenck Personality Questionnaire brief version: factor structure and reliability. J Psychol 139(6):545–552
Schmitt DP (2004) The big five related to risky sexual behaviour across 10 world regions: differential personality associations of sexual promiscuity and relationship infidelity. Eur J Pers 18(4):301–319
Trobst KK, Herbst JH, Masters HL, Costa PT (2002) Personality pathways to unsafe sex: personality, condom use, and HIV risk behaviors. J Res Pers 36(2):117–133
Verweij KJH, Zietsch BP, Bailey JM, Martin NG (2009) Shared aetiology of risky sexual behaviour and adolescent misconduct: genetic and environmental influences. Genes Brain Behav 8:107–113
Zietsch BP, Morley KI, Shekar SN, Verweij KJH, Keller MC, Macgregor S et al (2008) Genetic factors predisposing to homosexuality may increase mating success in heterosexuals. Evol Hum Behav 29:424–433
Acknowledgments
This research was funded by a small grant (R03) to J.M.B. from the US Institute of Mental Health (USA) and a small Commonwealth AIDS Research Grant to N. G. M. Twins participating in this study were drawn from the Australian NHMRC Twin Registry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Michael Lyons.
Appendices
Appendix 1
See Table 5.
Appendix 2
See Table 6.
Rights and permissions
About this article
Cite this article
Zietsch, B.P., Verweij, K.J.H., Bailey, J.M. et al. Genetic and Environmental Influences on Risky Sexual Behaviour and its Relationship With Personality. Behav Genet 40, 12–21 (2010). https://doi.org/10.1007/s10519-009-9300-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-009-9300-1