Abstract
Whereas the majority of research on adolescent sexual initiation has focused solely on environmental factors, the present study used behavioral genetic analyses to investigate the relative contributions of genetic and environmental influences. Structural equation models were fitted to data from adoptive and non-adoptive sibling pairs (231 biologically related pairs and 169 unrelated pairs) from the Colorado Adoption Project. Information from censored individuals who had not yet experienced sexual initiation was maximized by adapting the twin survival analysis method of Pickles et al. (Behav Genet 24(5):457–468, 1994) to accommodate adoptive and non-adoptive siblings. Point estimates of variance components from an ACE model, including additive genetic (A), shared environmental (C), and non-shared environmental (E) influences were 28%, 24%, and 48%, respectively. Despite the lower point estimate for shared environmental effects than additive genetic effects, a CE model provided the best fit to the data. However, because adoptive siblings provide a direct estimate of shared environmental influences there is greater power to detect shared environmental effects in adoption designs. Evidence for genetic influences from our data were somewhat lower than those obtained in previous twin studies, possibly reflecting a return to more socially conservative sexual attitudes, changing sexual behaviors, or ambiguities in the wording of questions commonly used in research on adolescent sexuality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The initiation of sexual activity among adolescents has often been studied as a societal problem. Its importance is underscored by the fact that those who engage in sexual intercourse at an early age tend to engage in sex more frequently, have more partners (The Allen Guttmacher Institute 2001), and are less likely to use contraception (Abma and Sonenstein 2001). This exposes them to higher risks for pregnancy and sexually transmitted diseases such as human papillomavirus infection (Kahn et al. 2002), pelvic inflammatory disease, chlamydia, gonorrhea (Miller et al. 1995; Chacko et al. 2004), and HIV (CDC 1996).
Most studies of sexual initiation have investigated social (Day 1992; Lammers et al. 2000; Slonim-Nevo 1992;), family (Moore et al. 1986; Lammers 2000; Whitbeck et al. 1999), and peer influences (Babalola 2004; Kraft 1991; Miller et al. 1997). Often underlying these and similar studies are either a social control hypothesis, which emphasizes such inhibiting factors as parental monitoring and social norms that act to delay the initiation of sexual activity, or a socializing hypothesis emphasizing the past and present influence of family and peer models of behavior (Miller et al. 2001; Rodgers and Rowe 1990). In either case, it is often assumed that normal adolescent motivation and opportunity for sexual activity is uniform and constant.
A much smaller body of research, however, has suggested that individual differences in sexual motivation may be attributable to biological and social maturity. Biological influences are suggested by associations between the onset of sexual behavior and hormonal levels, especially among males (Halpern et al. 1994; Udry 1988, 1990; Udry and Billy 1987; Udry et al. 1986). Though hormone levels vary individually, they tend to increase during puberty and it is not surprising that early pubertal development has been associated with early sexual initiation (Flannery et al. 1993; Halpern et al. 1993; Miller et al. 1998). According to Udry (1988), the level of pubertal development may increase the likelihood of engaging in sexual intercourse by providing the opportunity (e.g., sexual attractiveness) and by increasing sexual motivation. There are probably sex differences, however, in the relative influence of such biological variables. For example, in one biosocial model predicting sexual intercourse, Udry (1988) found strong biological (e.g., androgen hormone levels) and weak sociological (e.g., church attendance) effects for boys, while only sociological effects were important for girls. A possible genetic explanation for this is suggested by Miller et al. (1999) who found an association between the age at sexual initiation and polymorphisms at dopaminergic receptor encoding genes that was stronger among males than females.
It is likely that individual differences in such biological factors as pubertal development and changes in hormonal levels are influenced by heritable factors (Mustanski et al. 2004; Dick et al. 2001; Harris et al. 1998). Genetically influenced personality traits also may affect an individual’s choice of social environment (Udry and Bearman 1998), which may, in turn, affect sexual behavior. In this study, we employed behavioral genetic methods to estimate the relative importance of genetic and environmental sources of variability in the initiation of sexual behavior. We are aware of only three previous behavioral genetic studies (Dunne et al. 1997; Martin et al. 1976; Rodgers et al. 1999) that have used similar methods. The earliest study (Martin et al. 1976) was based on 134 MZ and 112 DZ pairs, ranging in age from 16 to 54 (M=26.7) who responded to a mailed questionnaire regarding personality, sexual attitudes, and age of first intercourse. Martin et al. (1976) found that while none of the models tested adequately fit their data, non-shared environmental factors were most important, followed by genetic and shared environmental factors. They suggested that genetic effects were likely mediated through personality and culturally influenced sexual attitudes affecting the likelihood of early sexual initiation. More recently, Dunne et al. (1997) found age cohort and gender effects in a study of sexual initiation involving 2,540 Australian twin pairs responding to alcohol use-related (mail and follow-up telephone) surveys, which also included questions regarding sexual behavior. For their younger cohort (aged 27–40 years), males and females differed in the relative importance of genetic (72% vs. 49%) and shared environmental (0% vs. 25%) variance in the most parsimonious model. Rodgers et al. (1999) found somewhat similar results in a sample of 2,338 kinship pairs (i.e., cousins, half-siblings, full-siblings, and same-sex twins) from the National Longitudinal Survey of Youth (NLSY). They reported that heritability for age at sexual initiation was high for same-sex male pairs and low for same-sex female pairs (54% vs. 15%) while shared environmental variance showed the reverse pattern (9% for males vs. 27% for females). Across all kinship pairs heritability was estimated at 37% with 8% shared environmental variance.
Limitations in all of these previous studies included potential sampling biases, retrospective data collection, and biases introduced by methods used to account for censored individuals. Martin et al. (1976) suggested that poor sampling or a violation of the equal environments assumption between MZ and DZ twins may have affected their results. Sample bias was possible given that only 246 of the 776 pairs in the twin registry returned a survey. Finally, censored individuals (i.e., those who had not yet initiated sexual activity by their latest observation) were scored as having had sex at the age of their last testing. This method may have introduced an extraneous bias in modeling genetic influence since censored individuals represented approximately 20% of the sample and tended to be, on average, six years younger than those who had initiated. Dunne et al. (1997) addressed this issue by converting age of initiation to a 10-point ordinal scale and assigning the highest score to censored individuals. While this adjustment was adequate since the lower age bound of 27 was safely past the age of risk, age at initiation was retrospectively reported and likely less accurate than if reported closer to the age of risk. A sampling bias was also likely given that, by design, at least one twin in each pair in the available data had a history of alcohol dependence. Rodgers et al. (1999) also used retrospective reports; however, their sample was not past the age of risk. The distribution of age at first sex for females in the full NLSY dataset shows that about half initiated sex between 18 and 25 years old, which is the same age range of the full sample during their assessments in 1983 and 1984. Their analyses did not take into account censored individuals.
Some of these limitations may affect estimations of genetic and environmental variance for age at sexual initiation. For example, retrospective data collection likely leads to less accurate reports among both MZ and DZ pairs. This may increase measurement error, which is a part of non-shared environmental variance, and consequently result in an underestimation of genetic and shared environmental influences. It is unknown how the different treatments of censoring above influenced genetic and environmental parameters. However, studies that do not account for censoring at all are likely to underestimate heritability in certain cases. If a trait is influenced by genetic factors one would expect greater similarity among MZ pairs than DZ pairs, and thus more discordant pairs among DZ twins than MZ twins. When only using complete data (i.e., both individuals must experience the event to have complete age of onset data) one is more likely to discard discordant DZ pairs than MZ pairs.
The present study attempted to improve on these methodological limitations. Since the sample was derived from a long-term longitudinal study of adolescents transitioning into adulthood, the data used in this study were collected at multiple time points starting at age 17. Thus, for many individuals, there were relatively few intervening years between the event and its recollection, and accuracy could be verified by multiple previous and subsequent reports. Further, we used a sibling survival model (Pickles et al. 1994) that maximized the information provided from censored individuals by allowing for differential censoring by age at assessment. Finally, the present study used a sibling adoption design. The use of genetically unrelated sibling pairs provides a direct estimate of shared environmental influences not possible with twins reared together. Thus, the results of this study, in combination with those of previous twin and kinship studies, may provide a more comprehensive assessment of the environmental and genetic influences on age at sexual initiation.
Methods
Samples
Core CAP Sample
The subjects in this study are participants in the Colorado Adoption Project (CAP), an ongoing longitudinal study of genetic and environmental influences on behavioral development that has followed adoptive and non-adoptive children and their families from infancy through young adulthood (DeFries et al. 1994; Petrill et al. 2003; Plomin and DeFries 1985; Plomin et al. 1988). CAP families were recruited between 1975 and 1983 through two adoption agencies in Denver, Colorado. Data from a variety of cognitive, personality, and health-related measures were collected from the biological parents (mothers and approximately 20% of the biological fathers) and adoptive parents prior to the birth of the child. Adopted children and their unrelated siblings have been prospectively assessed approximately yearly, though not all subjects have participated in each assessment. The core CAP sample consists of an adoptive proband and the next youngest sibling closest in age to the proband. Adopted children were separated from their biological parents within a few days of birth and placed in their adoptive homes at 29 days of age, on average. The original CAP sample consisted of 245 adoptive families and 245 non-adoptive control families. Non-adoptive control families were recruited from hospital birth records in the same geographical area and matched to the adoptive families by sex of the adopted child, age of the father (±5 years), number of years of education of the father (±2 years), and the occupational status of the father (±8 points on the National Opinion Research Center occupational rating scale; Reiss et al. 1961). Details of the CAP design, including demonstrations of the representativeness of the sample and little or no evidence for selective placement, are provided in DeFries et al. (1994), Plomin and DeFries (1985), and Plomin et al. (1988).
Young Adult CAP Sample
In the most recent wave of assessments for the CAP, additional siblings between the ages of 18 and 30 were added to the original core CAP sample of probands and their first younger siblings. The extended sample of additional siblings (including both older and younger siblings to the proband) was included in the current study yielding a final sample of 847 subjects who participated in the interviews on the transition from adolescence to adulthood. All subjects had to be at least 17 years old to participate in these assessments. Forty-eight subjects were excluded from the current analysis because they provided inconsistent information regarding their age at first sexual initiation over the course of their various assessments, leaving 799 participants for the present study. These participants included 305 adoptive respondents (194 adopted probands and 111 unrelated siblings) and 494 non-adoptive respondents (214 non-adopted probands and 280 related siblings). Of these, 657 reliably reported age at sexual initiation and 142 indicated they had not initiated at the time of their latest assessment. These 142 individuals who reported that they had not yet initiated sexual activity were included in our analyses as censored cases. By ethnicity, the 799 individuals were predominantly (93%) Caucasian, with 4% Hispanic, less than 1% Asian, less than 1% Black, and 2% from other racial/ethnic groups.
Sibling Pair Sample
In order to determine the relative contributions of heredity and environment to individual differences in initiation of sexual behavior we utilized an adoptive sibpair design. Biologically related full sibling pairs and biologically unrelated pairs of siblings were identified among the 799 subjects described above. Pairs were formed from all possible combinations of two, three, or four siblings within families containing at least two siblings participating in the study. Table 1 describes how the sibling pair sample was constructed. For the most part, biologically related pairs were formed from non-adoptive families and unrelated pairs were formed from adoptive families. Exceptions occurred when a non-adoptive family (based on the parent–proband relationship) contained an adopted child, and when adoptive families contained two or more children who were biologically related to one another. Of the 654 paired individuals in our sibling pair sample, 537 reliably reported age at sexual initiation and 117 were reliably censored.
Our sibling pair sample excluded 101 singletons (i.e., only children with no participating sibling), 20 half-sibling pairs, and two twin pairs. Because these few pairs provide limited information their inclusion in the analyses was not warranted; however, they were included as a comparison group to determine the representativeness of our sample. Among the 145 individuals not included in the pairs sample for these reasons, 120 reported age at initiation and 25 were censored.
Measures
Age at Sexual Initiation
Because this sample is followed longitudinally, age at initiation was determined from a maximum of seven possible assessments per individual over potentially six annual testing sessions. The core CAP sample of probands and first younger siblings completed a maximum of three interviews via telephone and four paper-and-pencil questionnaires either mailed or in-person at ages 17 and older. Up to three of these sessions involved both an interview and a mailed questionnaire. The extended sample of additional siblings, part of a project that began when subjects were age 17 or older and under different protocols, completed a maximum of four assessments: three of the same interviews and one questionnaire.
Interview questions varied somewhat from occasion to occasion but included: “How old were you when you had intercourse for the first time?” and “How many different partners have you ever had intercourse with?” Questions at later waves asked, “How many partners have you had intercourse with since we spoke last (interview provides month/year of last contact)?” While some assessments asked about both lifetime and past-year experience, others asked only about “the first time you had sex” and the number of partners “in your life.”
Among those who reported age at initiation, 608 were determined by lifetime reports. In order to maximize the sample and the use of available information, we chose to include 49 subjects whose age at initiation was determined by comparing the last year of no reported sexual experience and the first year of reported sexual partners. This latter method was considered reliable only when the range between virginity (reliably reporting no sexual intercourse) and at least one new reported sexual partner could be narrowed to within two consecutive years. In these cases, age at initiation was estimated as the age of the subject when they first reported at least one sexual partner. If there was a discrepancy between reported dates of first sexual experience, we chose to use the report assessed closest to the reported age of the event. For example, if a subject reported at age 17 an age of sexual initiation during that year, but then at a later assessment reported having initiated at age 18, we considered the assessment at age 17 to be more reliable. Forty-eight individuals reporting discrepancies of more than two years were excluded from the analysis. Those who initiated sex prior to the mean age at initiation for the entire sample were considered to have initiated “early.”
Data Analysis
Sibling Threshold Model
Whereas all sibling pairs in our sample share a common family environment and have unique individual experiences, biologically related full siblings also share, on average, half their alleles identical-by-descent. Adoptive unrelated siblings do not share common genetic influences. This difference, assuming that common environmental influences operate similarly in related and unrelated pairs, allows one to compare observed correlations of related and unrelated siblings to decompose the relative contributions to individual differences in age at first sexual initiation into additive genetic effects (A), shared environmental influences (C), and non-shared environmental influences (E), which also includes measurement error. A path model describing sibling resemblance for initiation of sexual experience including these variance components is shown in Fig. 1.
Model fitting analyses were conducted using the ordinal approach to fitting raw data in the Mx statistical modeling package (Neale et al. 1999). Our approach assumes that there is a normal continuous liability distribution underlying the ordinal data (i.e., age at first sexual initiation). Because in our sample adopted individuals initiated sexual behavior earlier, on average, than non-adopted individuals, and there was evidence of an interaction by sex (see Fig. 2), we also estimated adoption status-, and sex-dependent thresholds. Thresholds are simply cut-points on the latent liability distribution that give rise to the observed ordinal age at initiation data. The thresholds are estimated directly from the data based on the prevalence of initiation at each age, taking into account the differences in prevalence among adopted and non-adopted individuals, and among males and females.
The full ACE model was compared to more restrictive models that constrained parameters to zero (e.g. AE, CE, and E only models). The fit of these nested models was compared to the full model using the Chi-square statistic, which is computed as twice the difference between the log-likelihood for the full model (LL0) and that for a reduced model, i.e., χ2=−2(LL0− LL1), with degrees of freedom equal to the difference in degrees of freedom between the two models being compared.
Sibling Survival Model
To accommodate censored data we used a biometrical survival analysis based on a method developed by Pickles et al. (1994) for twin pairs. Unlike same-age twin pairs, our paired siblings necessarily differ in age of assessment (i.e., generally older siblings have been assessed through later ages than their younger siblings, except in rare circumstances where an older sibling was missing at later assessments). In our approach we treated the sibling pair as the unit of censorship. We grouped subjects by the youngest assessment age of the siblings in a pair, and then fit the model as a multiple group problem across 8 assessment cohorts (i.e., those tested through age 17, through age 18, etc., through age 24 and above). There were insufficient numbers of sibling pairs in which the youngest assessment age was greater than 24, so we truncated our assessment groups at this point. This allowed for differential censoring by assessment age cohort, which is more informative than methods that assign all censored individuals a universal value. That is, given that the mean age of initiation in our sample was 17, one would expect greater censoring among individuals tested only up through age 17 than among individuals tested through age 24.
For siblings that differ in age at assessment we necessarily had to utilize the common assessment point at which we would have complete information on both members of the sibling pair. This is the youngest assessment age for a pair. For example, consider a pair of siblings where one has been assessed through age 17 and the other assessed through age 19. We cannot utilize the most recent data from the older sibling, who was assessed beyond age 17 (the common point at which both members of the pair have been assessed) without making some assumptions about how the younger sibling might score through age 19. If, for example, the older sibling initiated sex at age 18 or 19, and the younger sibling was abstinent through age 17, it is unclear whether this pair would be discordant for initiation of sexual behavior. What we do know for certain is that both siblings were abstinent through age 17 and are concordant-censored at that point in time. Similarly, for a sibling pair where the younger sibling was assessed through age 20 and the older sibling through age 24, data from the assessment through age 20 would be utilized for both individuals in the analysis. This treatment of censored information deals only with known information at a common assessment point, and requires no assumptions about future behavior. This approach results in the loss of some information from older siblings who initiated later than the common assessment age or who were still abstinent at a later age (i.e., these data would not be used in the analysis). However, we should point out that because the mean age of onset for initiation of sexual behavior in our sample was 17, and all subjects have been assessed through at least age 17, known data from older siblings was not used for only 8% of our sibling pairs.
Results
Descriptive Analyses
Reliability of Measurement
Whereas 306 subjects had only one potentially reliable report of age at first sexual initiation, 351 had two or more potentially reliable reports. For those with more than 2 reports we used the two most discrepant to determine reliability of measurement. The test–retest Pearson correlation among reports was .93 (P<.01). More than two-thirds (68%) of these subjects reported the exact same age at initiation on separate testing occasions, while 26% were within one year, and 6% were within two years. On average the follow-up report occurred 1.1 years later with a range of between 0 (because some questionnaires were completed contemporaneously with interviews) and 6 years.
Because three different types of questions were asked about “sex”, “intercourse”, or “sex partners” we investigated whether reliability differed among reports based on the same versus different items. Among the 351 non-censored individuals with at least two assessments, 29.6% had the same assessment on both occasions and 70.4% had different assessments. Although the test–retest correlation was statistically different based on Fisher’s r to z transformations (r=.97, 95% CI: .95, .98, for same question pairs; and r=.90, 95% CI: .88, .93, for different question pairs), test–retest reliability was quite good (≥.90) in both cases, suggesting that any bias introduced by using these differently worded questions is likely to be minimal.
Sample Bias and Representativeness
On average, non-paired individuals were no different than paired siblings for age at initiation (M=16.7, SD=2.3 vs. M=17.0, SD=2.3, F (1, 655)=1.81, P>.05). The ratio of nonadoptees to adoptees was the same for singletons and paired individuals (1.50), though there was a discrepancy in the ratio of males to females (1.55 for singletons vs. 1.03 for paired individuals). While there were too few Asians and Blacks to make any meaningful comparisons, approximately 47% of non-Hispanic Caucasians and 50% of Hispanics had sex before age 17, and there was no statistical difference in the mean age at initiation between non-Hispanic Caucasians (M=16.9, SD=2.35) and Hispanics (M=16.3, SD=1.95, F (1, 630)=2.21, P>.05).
Individual-level Analysis
Table 2 describes the non-censored siblings (i.e., only those reporting an age at initiation) used in the pairs analyses. Adoptees first had sex, on average, 8 months earlier than non-adoptees. There were no overall significant mean sex differences in any of the measures of sexual initiation or age of testing. There were some interactions of adoption status by gender in age at initiation. Adopted males differed from non-adopted females (P<.05) and adopted females differed from both non-adopted males (P<.05) and non-adopted females (P<.05). More than half of the adopted females initiated sex early (i.e., before our sample mean age of 17), which is 10%–20% more than the other subgroups. The pattern for adoption status by gender subgroups can be seen in the survival curves in Fig. 2. While non-adoptees appear to differ from adoptees at each age, there were no significant differences for the main effect of gender. The percentage of non-adopted females “surviving” (i.e., not yet initiating sex) at each age is greater than that of non-adopted males, followed by adopted males, and adopted females.
For comparison, data from a nationally representative high school sample (CDC 1996) were also included in Fig. 2. There appears to be some variation around ages 15 and 16 between the CDC data and this Colorado sample composed predominantly of white, suburban adolescents. In order to make a proper comparison of survival rates with CDC data reported by grade level, data from the present study were converted into the proportion of subjects interviewed by a given age or older who had initiated sex a year prior to that age. In our Colorado sample, 20% of subjects interviewed by age 16 or later had had sex by age 15, compared with 36% of freshman in the CDC sample. Our prevalence estimates were closer to the CDC high school data for juniors and seniors.
Pairs Analyses
Table 3 compares sibling pairs on several measures of similarity in age at initiation. Evidence for heritable variation is found when differences between related pairs who share genes and family environment are less than those between unrelated sibling pairs who share only family environment. Related pairs, who differed by 1.7 years in age at initiation on average, resembled each other more closely than unrelated pairs who differed by 2.3 years on average, F (1, 398)=7.49, P<.01. A gender pair breakdown reveals no important differences between same-sex males, same-sex females, and opposite sex pairs; however, these subgroups are not distributed equally when further broken down by genetic relatedness. The average difference between siblings’ age at initiation in related and unrelated pairs is 6 months for those that are opposite sex, 7 months for same sex males, and 11 months for same sex females. While there is no statistically significant interaction of gender by relatedness, F (2, 394)=.672, P>.05, possibly due to the small cell sizes, a sex-constrained model was tested against an unconstrained model to determine which provided the better fit to the data.
Threshold Models
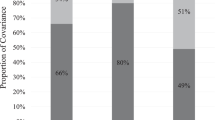
Given the different survival curves for sex by adoption subgroups, three different models were tested (Table 4) to determine whether it was necessary to include sex- and adoption-based thresholds as well. While the simplest model with only assessment age-dependent thresholds found moderate heritability for age at sexual initiation, models incorporating sex- and adoption status-dependent thresholds suggested a larger contribution of shared and non-shared environmental influences. The first model, which included assessment age-, adoption status- and sex-dependent thresholds, provided the best fit to the data. This model suggested 28% of the variability in sexual initiation could be explained by genetic factors and 24% by shared environmental influences, with the remaining 48% of the variance attributable to non-shared environment.
Based on previous findings suggesting that genetic and environmental factors for age at initiation may operate differently for males and females (Miller et al. 1999; Rodgers et al. 1999; Dunne et al. 1997), we tested for differences between same-sex male, same-sex female, and opposite-sex sibling correlations. In the sex-limited (unconstrained) model of Table 5, related and unrelated same-sex correlations are more similar for females (.46 and .48, respectively) than for males (.32 and .27), suggesting no heritability for females and minimal heritability for males. However, due to the small subsamples, there are broad standard errors around those correlations. We were able to equate sibling correlations across sex in the sex-constrained model. Thus, a more complex sex-limited model was not warranted for our data.
Given that the confidence interval for the estimated heritability of 28% includes 0, we tested three additional nested models in Table 6. By χ2 difference test the CE model provides the best fit to the data. However, we should point out that in adoption designs there is greater power to detect shared environmental influences than genetic effects. In addition, the survival model approach that we utilized was designed for use with sibling pairs. Some of our families contributed more than a single pair so that our sample was not comprised of independent sibling pairs. To address a reviewer’s concern regarding any bias that this approach may have introduced, we selected a subset of our original sample and performed a reanalysis on an independent sample of 305 sibling pairs. For those families contributing more than one sibling pair in our original sample, we randomly selected pairs for our revised sample with the limitation that one pair included the proband and that no sibling was included more than once in any additional pairs. Compared to the results based on the full sample presented in Table 6, in this analysis our estimate of heritability increased to 43% (95% CI: .02, .84), the shared environment estimate decreased to 21% (95% CI: .04, .36), and the non-shared environmental estimate decreased to 36% (95% CI: .07, .69). Taken together, these findings suggest important shared environmental and unique environmental influences contribute to individual differences in age at sexual initiation. The evidence for additive genetic influence is less compelling.
Discussion
The present study contributes to previous behavioral genetic studies of sexual initiation utilizing a longitudinal sibling adoption design that minimizes the limitations of retrospective responses. Though Rodgers et al. (1999) included a few adoptees in some of their analyses of kinship pairs, to our knowledge this is the first analysis of sexual initiation based on a sibling adoption design. Results in the full sample indicated non-significant genetic influences, while reanalysis on an independent pairs sub-sample suggested statistically significant genetic, shared, and unique environmental influences. Finally, a test of potential sex differences in the sibling correlations suggested that models including sex limitation were not required, though these analyses had limited power in this sample due to the small cell sizes when the sample was divided in this way.
In contrast to the three previous studies, we did not find compelling evidence for genetic influence. However, we should point out that in adoption designs there is greater power to test environmental effects. Our initial heritability estimate of 28% is approximately halfway between the estimates obtained by Dunne et al. (1997) in their older cohort (16%—including 0% for males and 32% for females) and the overall estimate obtained by Rodgers et al. (1999) using kinship pairs (37%).
It is useful to consider what factors might account for a lower estimate of heritability. One possibility is that heritability may be influenced by social attitudes about teen sexuality and teens’ own sexual attitudes and behaviors. Our low to moderate heritability estimates are in contrast to the large average estimate of 61% in Dunne et al.’s (1997) younger cohort (71% for males and 49% for females). Dunne et al. (1997) suggested that such differences in heritability of sexual initiation might reflect cohort differences in social attitudes so that more expressive environments with “fewer social controls on adolescent sexual behavior” lead to higher heritability estimates. While their older cohort (born between 1922 and 1952) had relatively low heritability estimates and moderate shared environmental variance, the younger cohort (born between 1952 and 1965) initiated sex in a “more laissez-faire social environment” and had relatively high heritability estimates with little shared environmental variance. Our subjects initiated sex during the 1990s in which social messages (e.g., abstinence pledges, Bearman and Bruckner 2001; Bersamin et al. 2005) as well as the fear of AIDS (Blinn-Pike 1999; Ebomoyi 1998; Sprecher and Regan 1996) may have contributed to sexual behaviors that were relatively more conservative than Dunne et al.’s (1997) younger cohort, though probably not as conservative as their older cohort. Such a change in adolescent sexual attitudes is also suggested by data on sexual behavior. Abma and Sonenstein (2001) found slight declines in the percentage who reported having sex by ages 15–17 between 1988 and 1995 for teenage males, and a leveling off for females. The years of sexual initiation for our study’s cohort also correspond with a 30% national decline in teenage pregnancy between 1991 and 2002. Among non-Hispanic white teenagers between 15 and 17 years old, this decline was 36% (Ventura et al. 1998). On the other hand, the greater availability and effectiveness of contraceptives and, due to treatment advances, the increasingly common view of HIV/AIDS as a treatable chronic condition (as opposed to a fatal disease) may mitigate this effect.
Another possible explanation for lower heritability estimates that is consistent with the data just presented is that adolescents in more recent generations have engaged in alternative sexual behaviors in order to delay what they perceived as “real sex.” Recently, Halpern-Felsher et al. (2005) found that, compared with vaginal sex, oral sex was perceived by a sample of 580 ninth graders as less risky, less morally and religiously objectionable, and more prevalent among same-aged peers than vaginal sex. In addition to avoiding pregnancy, it is also perceived erroneously by some as relatively without risk of sexually transmitted diseases. Furthermore, oral sex is not universally perceived as “sex.” Sanders and Reinisch (1999) found in a random, stratified sample of undergraduate students at a large Midwestern university that in considering whether various acts should be included in having “had sex,” 59% (CI, 54%–63%) of respondents did not include oral sex and 20% did not include anal intercourse.
Halpern-Felsher et al. (2005) found an overall prevalence for oral sex of 19.6% among racially diverse adolescents in California with a mean age of 14.5 years old, while Boekeloo and Howard (2002) found a prevalence of 18% among racially diverse 12- to 15-year-olds receiving a general health exam in the District of Columbia. In both cases, parental consent likely biased the composition of the sample, though it is not clear in what direction. In a retrospective study with a volunteer sample of mostly Caucasian college students, Schwartz (1999) found that 57% of females and 70% of males performed oral sex prior to their first experience of sexual intercourse.
Assuming that some percentage of subjects in our cohort engaged in such alternatives which they did not consider their first experience of “sex” or “sexual intercourse,” this may have lead to a delay in the reporting of their sexual initiation. This is consistent with the evidence above suggesting somewhat more conservative sexual behaviors among a national sample of adolescents in the same cohort as the subjects in this study (Abma and Sonenstein 2001).
Sex-limitation Model
While previous studies have found sex-based differences in the degree of hereditary and social environmental influence, we were unable to find such differences, possibly due to limited power. We caution against interpretations based upon correlations from the unrelated pairs, as the confidence intervals were so broad. Beginning with the values presented in Table 5, we were able to investigate various alternative models, which assumed all point estimates with the greatest sample size were fairly constant while those with the smallest sample sizes (i.e., same-sex males and females) could be adjusted within the bounds of their confidence intervals. Among the better-fitting models, which deviated the least from our relatively fixed correlations, one was very similar to Rodgers et al. (1999) who estimated heritability at approximately 50% for males and 15% for females, and shared environmental variance at about 10% for males and 30% for females. Of course, given the small subsamples in Table 5, other patterns of results involving different heritabilities also fit the data.
Sexual Initiation in Adopted Females
Compared to all other gender by adoption status subgroups, a larger percentage of adopted females in our sample initiated sex earlier in the mid to late adolescent age range (15–18) of the survival analysis curve (Fig. 2). This is in contrast to our non-adopted sample in which males initiated earlier than females. Data from the CDC (Abma and Sonenstein 2001; CDC 1996) suggest a convergence around the mid-1990s of male and female rates of sexual initiation, especially among white adolescents. While the effect size for the adoption-status difference (.26) is small, the effect size for the difference between adopted and non-adopted females (.42) is moderate (Cohen 1988). Such a difference is unlikely due to such factors as socioeconomic status, as the adoptive and control samples were matched on the father’s occupational status and years of education. There are several possible explanations for this finding. If adopted female’s mothers also had sex at an early age, this tendency might reflect genetically influenced personality traits (e.g., impulsivity, novelty seeking, or depression) or an earlier onset of puberty leading to early initiation. In the current sample, biological mothers of the adopted females were, on average, 20.0 (SD=3.7) years old when they gave birth (based on 70% of the 140 mothers for whom data were available) suggesting possible early initiation. This is significantly different from the average age of Colorado first-time mothers (M=25.3, SD=5.1) who gave birth between 1976 and 1983 (the birth years for this study’s cohort), t (102)=14.57, P<.01.
While adopted females in this sample may be genetically influenced in their early sexual initiation by a similar tendency in their biological mothers, one might also speculate on indirect pathways leading to a similar result. For example, perhaps pressures associated with being adopted lead to psychological problems that disproportionately affect female adoptees. This in turn might make them more vulnerable to or perhaps more likely to seek out sexual intimacy at an earlier age.
It has been suggested that, compared with the general population of children and adolescents, adoptees are more prone to symptoms related to hyperactivity (Brodzinsky et al. 1987; Simmel et al. 2001; Wierzbicki 1993), possibly due to such factors as having a younger birth mother, prenatal drugs and alcohol exposure, and low birth weight (Simmel et al. 2001). Similarly, in some studies depression and depressive symptoms have been linked to adoption status with small to moderate effect sizes (Brodzinsky et al. 1987; Miller et al. 2000; Whitten 2002). This may reflect a birth mother’s own genetic tendency toward depression as well as some adopted children’s struggle with identity issues during early adolescence. Finally, there is also some evidence of an association between early sexual initiation and impulsivity (White 1988; Breakwell et al. 1996), and with depressive symptoms (Lammers et al. 2000; Longmore et al. 2004; Ramrakha et al. 2000; Whitbeck et al. 1999). These studies, however, do not consistently favor an earlier onset for females as compared with males.
While these hypothetical pathways are possible, there is little consensus regarding the link between adoptive status and psychological problems in general. One meta-analysis of 66 studies (Wierzbicki 1993) found “higher levels of psychological difficulties” among adoptees in non-clinical group comparisons as well as adoptees’ relative representation in clinical samples. While there was a high mean effect size (average Cohen’s d) for studies based on clinical representation (mean d = 1.38, SD = 2.01), the mean group comparison effect size was low (mean d = .11, SD = .22). Plomin and DeFries (1985) have argued that studies based on group comparisons are more convincing, easier to interpret, and generally find few group differences. In a recent literature review of 98 studies, Juffer and Ijzendoorn (2005) found more behavioral problems (mean d = .18, CI = .13–.24) and clinical referrals (mean d = .72, CI = .57–.86) among combined samples of domestic and international adoptees compared to controls. Finley’s (1999) review of the literature suggested that adoption status-based differences often result from such methodological flaws as referral bias among wealthier, more educated adoptive parents who are more likely to seek psychological treatment for their adopted child. Other explanations include a negative association with the adoption label, a publication bias favoring higher effect sizes among studies with small sample sizes, and a failure to account for age at adoption. For example, differences among adoptees are more frequently found for later adoptees than when children are adopted early as in our CAP sample (Plomin and DeFries 1985). However, Wierzbicki (1993) and Juffer and Ijzendoorn (2005) found the latter explanation did not account for group differences among the studies reviewed.
Limitations
Some studies have suggested that recollection for the timing of past events is affected by the time elapsed since the event (Janson 1990; Bachman and O’Malley 1981; Sudman and Bradburn 1973). A “telescoping” effect occurs when, compared to more recent events, distant events are recalled as having occurred somewhat closer to the time of recall. To test for the possibility of telescoping in the present study, secondary reports of age at initiation were compared to primary reports used in the pairs analysis for the subset of cases where subjects reported on two or more separate occasions. Secondary reports occurred between 0 and 6 years following primary reports and the corresponding ages of initiation differed by two years. While 68% of secondary reports were identical to primary reports, 15% were one or two years later and 10% were one or two years earlier than primary reports, suggesting no telescoping biases in subjects’ recollections.
Another potential bias is the extent to which correlations among parents (e.g., assortative mating) influence the resemblance of siblings for age at initiation. Unfortunately, these data are not available on the parents of the subjects in this study. However, assortative mating tends to underestimate heritability in twin studies and overestimate heritability in sibling-adoption designs. The difference in heritability estimates reported here and in previous twin studies, therefore, is unlikely to be explained by non-random mating for age at initiation.
As discussed above, there is some evidence of ambiguity in the interpretation of the terms “sex” and “sexual intercourse.” To be more certain of adolescent sexual perceptions, it will be necessary to clarify the terms used in future studies. Most studies, including this one, have not used specific sexual descriptions, so it is very likely that they are insensitive to changing trends in adolescent sexual behaviors and perceptions. The subjects in our study may have also perceived some ambiguity in the phrase “the first time you had sex,” with some responses that included oral sex and others that excluded it. Finally, our measure made no distinction between heterosexual and homosexual sex, nor whether the experience was consensual. To the extent that these ambiguities existed, estimation of non-shared environmental effects, which include measurement error, may have been overestimated.
Future Directions
Our findings support social and possibly biological (genetic) contributions to individual differences in age at sexual initiation. The goal of future research will be to determine which specific genetic and environmental factors are most important and to determine how they might interact. It is likely that social variables will be moderated by genetic factors and vice versa. In combination with twin studies, designs that include specific social variables may offer a more complete picture of these influences than has been previously considered in most psychological research on sexual initiation.
References
Abma JC, Sonenstein FL (2001) Sexual activity and contraceptive practices among teenagers in the United States, 1988 and 1995. Vital Health Statistics 23(21). National Center for Health Statistics, Hyattesville, MD
The Allen Guttmacher Institute (2001) Can more progress be made? Teenage sexual and reproductive behavior in developed countries. Occasional Report No. 3
Bachman JG, O’Malley PM (1981) When four months equal a year. Public Opin Quart 45:536–548
Babalola S (2004) Perceived peer behavior and the timing of sexual debut in Rwanda: a survival analysis of youth data. J Youth Adolesc 33(4):353–363
Bearman PS, Bruckner H (2001) Promising the future: virginity pledges and first intercourse. Am J Sociol 106:859–912
Bersamin MM, Walker S, Waiters ED, Fisher DA, Grube JW (2005) Promising to wait: virginity pledges and adolescent sexual behavior. J Adolesc Health 36:428–436
Blinn-Pike L (1999) Why abstinent adolescents report they have not had sex: understanding sexually resilient youth. Fam Relat 48:295–301
Boekeloo BO, Howard DE (2002) Oral sexual experience among young adolescents receiving general health examinations. Am J Health Behav 26(4): 306–314
Breakwell G (1996) Risk estimation and sexual behaviour: a longitudinal study of 16- to 21-year-olds. J Health Psychol 1(1):79–91
Brodzinsky DM, Radice C, Huffman L, Merkler K (1987) Prevalence of clinically significant symptomatology in a nonclinical sample of adopted and nonadopted children. J Clin Child Psychol 16(4):350–356
Centers for Disease Control and Prevention (1996) CDC surveillance summaries, September 27, 1996. MMWR 45(No. SS-4)
Chacko MR, Wiemann CM, Smith PB (2004) Chlamydia and gonorrhea screening in asymptomatic young women. J Pediatr Adolesc Gynecol 17(3):169–178
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Earlbaum Associates, Hillsdale, NJ
Day RD (1992) The transition to first intercourse among racially and culturally diverse youth. J Marriage Fam 54:749–762
DeFries JC, Plomin R, Fulker DW (1994) Nature and nurture during middle childhood. Blackwell Publishers, Cambridge, MA
Dick DM, Rose RJ, Pulkkinen L, Kaprio J (2001) Measuring puberty and understanding its impact: a longitudinal study of adolescent twins. J Youth Adolesc 30(4):385–400
Dunne MP, Martin NG, Statham DJ, Slutske WS, Dinwiddie SH, Bucholz KK, Madden PAF, Heath AC (1997) Genetic and environmental contributions to variance in age at first sexual intercourse. Psychol Sci 8(3):211–216
Ebomoyi EW (1998) Campus courtship behavior and fear of human immunodeficiency virus infection by university students. J Natl Med Assoc 90(7):395–399
Finley GE (1999) Children of adoptive families. In: Silverman WK, Ollendick TH (eds) Developmental issues in the clinical treatment of children. Allyn & Bacon, Boston, pp 358–370
Flannery DJ, Rowe DC, Gulley BL (1993) Impact of pubertal status, timing, and age of adolescent sexual experience and delinquency. J Adolesc Res 8(1):21–40
Halpern CT, Udry JR, Campbell B, Suchindran C (1993) Testosterone and pubertal development as predictors of sexual activity: a panel analysis of adolescent males. Psychosom Med 55:436–447
Halpern CT, Udry JR, Campbell B, Suchindran C, Mason GA (1994) Testosterone and religiosity as predictors of sexual attitudes and activity among adolescent males: a biosocial model. J Biosoc Sci 26:217–234
Halpern-Felsher BL, Cornell JL, Kropp RY, Tschann JM (2005) Oral versus vaginal sex among adolescents: perceptions, attitudes, and behavior. Pediatrics 115:845–851
Harris JA, Vernon PA, Boomsma DI (1998) The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behav Genet 28(3):165–171
Janson CG (1990) Retrospective data, undesirable behavior and the longitudinal perspective. In: Magnusson D, Bergman LR (eds) Data quality and longitudinal research. CUP, Cambridge, pp 100–121
Juffer F, Ijzendoorn MH (2005) Behavior problems and mental health referrals of international adoptees: a meta-analysis. J Am Med Assoc 293:2501–2515
Kahn JA, Rosenthal SL, Succop PA, Ho GYF, Burk RD (2002) Mediators of the association between age of first sexual intercourse and subsequent human papillomavirus infection. Pediatrics 109(1): 132–133
Kraft P (1991) Age at first intercourse among Norwegian adolescents: a lifestyle perspective. Soc Sci Med 33(2):207–213
Lammers C, Ireland M, Resnick M, Blum R (2000) Influences on adolescents’ decision to postpone onset of sexual intercourse: a survival analysis of virginity among youths aged 13 to 18 years. J Adolesc Health 26:42–48
Longmore MA, Manning WD, Giordano PC, Rudolph JL (2004) Self-esteem, depressive symptoms, and adolescents’ sexual onset. Soc Psychol Quart 67(3):279–295
Martin NG, Eaves LJ, Eysenck HJ (1976) Genetical, environmental, and personality factors influencing the age of first sexual intercourse in twins. J Biosoc Sci 9:91–97
Miller BC, Benson B, Galbraith KA (2001) Family relationships and adolescent pregnancy risk: a research synthesis. Dev Rev 21:1–38
Miller BC, Norton MC, Curtis T, Hill EJ, Schvaneveldt P, Young MH (1997) The timing of sexual intercourse among adolescents: family, peer, and other antecedents. Youth Soc 29(1):54–83
Miller BC, Norton MC, Fan X, Christopherson CR (1998) Pubertal development, parental communication, and sexual values in relation to adolescent sexual behaviors. J Early Adolesc 18:27–52
Miller BC, Fan X, Christensen M, Grotevant HD, van Dulmen M (2000) Comparison of adopted and nonadopted adolescents in a large, nationally representative sample. Child Dev 71(5):1458–1473
Miller HG, Rodgers SM, Gribble JN, Turner CF (1995) Correlates of sexually transmitted bacterial infections among U.S. women in 1995. Fam Plan Perspect 31(1):4–9
Miller WB, Pasta DJ, MacMurray J, Chiu C, Wu H, Comings DE (1999) Dopamine receptor genes are associated with age at first sexual intercourse. J Biosoc Sci 31:43–54
Moore KA, Peterson JL, Furstenberg FF (1986) Parental attitudes and the occurrence of early sexual activity. J Marriage Fam 48(4):777–782
Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ (2004) Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Dev Psychol 40(6):1188–1198
Neale MC, Boker SM, Xie G, Maes HH (1999) Mx statistical modeling, 5th edn. VCU Box 900126, Department of Psychiatry, Richmond, VA 23298
Petrill SA, Plomin R, DeFries JC, Hewitt JK (2003) Nature, nurture, and the transition to early adolescence. Oxford University Press, Oxford
Pickles A, Neale M, Simonoff E, Rutter M, Hewitt J, Meyer J, Crouchley R, Silberg J, Eaves L (1994) A simple method for censored age-of-onset data subject to recall bias: mother’s report of age of puberty in male twins. Behav Genet 24(5):457–468
Plomin R, DeFries JC (1985) Origins of individual differences in infancy: The Colorado Adoption Project. Academic Press, New York, pp 312–325
Plomin R, DeFries JC, Fulker DW (1988) Nature and nurture during infancy and early childhood. Cambridge University Press, New York
Ramrakha S, Caspi A, Dickson N, Moffit TE, Paul C (2000) Psychiatric disorders and risky sexual behaviour in young adulthood: cross-sectional study in birth cohort. Br Med J 321:263–266
Reiss AJ, Duncan OD, Hatt PK, North CC (1961) Occupations and social status. Free Press, Glencoe, IL
Rodgers JL, Rowe DC (1990) Adolescent sexual activity and mildly deviant behavior: sibling and friendship effects. J Fam Issues 11(3):274–293
Rodgers JL, Rowe DC, Buster M (1999) Nature, nurture and first sexual intercourse in the USA: fitting behavioural genetic models to NLSY kinship data. J Biosoc Sci 31:29–41
Sanders SA, Reinisch JM (1999) Would you say you “Had Sex” if...? J Am Med Assoc 281(3):275–277
Schwartz IM (1999) Sexual activity prior to coital initiation: a comparison between males and females. Arch Sex Behav 28(1): 63–69
Simmel C, Brooks D, Barth RP, Hinshaw S (2001) Externalizing symptomatology among adoptive youth: prevalence and preadoption risk factors. J Abnormal Child Psychol 29(1): 57–69
Slonim-Nevo V (1992) First premarital intercourse among Mexican-American and Anglo-American adolescent women: interpreting ethnic differences. J Adolesc Res 7(3):332–351
Sprecher S, Regan PC (1996) College virgins: how men and women perceive their sexual status. J Sex Res 33(10):3–15
Sudman S, Bradburn NM (1973) Effects of time and memory factors on response in surveys. J Am Stat Soc 68:344, Appl Sect. 805–815
Udry JR, Bearman PS (1998) New methods for new research on adolescent sexual behavior. In: Jessor R (ed) New perspectives on adolescent risk behavior. Cambridge University Press, New York, pp 241–269
Udry JR (1990) Biosocial models of adolescent problem behaviors. Soc Biol 37:1–10
Udry JR (1988) Biological predispositions and social control in adolescent sexual behavior. Am Soc Rev 53:709–722
Udry JR, Billy JOG (1987) Initiation of coitus in early adolescence. Am Sociol Rev 52:841–855
Udry JR, Talbert LB, Morris NM (1986) Biosocial foundations for adolescent female sexuality. Demography 23(2):217–230
Ventura SJ, Mathews TJ, Curtin SC (1998) Decline in teenage birth rates 1991–1999; Update of national and state trends. October 25, 1998; National Vital Statistics Reports. Centers for Disease Control and Prevention, Atlanta, GA: No. 20
Whitbeck LB, Yoder KA, Hoyt DR, Conger RD (1999) Early adolescent sexual activity: a developmental study. J Marriage Fam 61:934–946
White H (1988) Risk taking as a predictor of adolescent sexual activity and use of contraception. J Adolesc Res 3(3–4):317–331
Whitten KL (2002) The healthy development of adopted adolescents: the role of family, school and community. Diss Abs Int: Sect B: Sci Eng 63(3-B):1592
Wierzbicki M (1993) Psychological adjustment of adoptees: A meta-analysis. J Clinic Child Psychol 22:447–454
Acknowledgments
This research is supported by grants HD010333, HD036773, DA011015, DA05131. We also greatly appreciate the many families who continue to participate and Jan L. Handke of the Colorado Department of Public Health and Environmental, Health Statistics and Vital Records Department, who compiled comparative vital statistics data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Richard Rose
Rights and permissions
About this article
Cite this article
Bricker, J.B., Stallings, M.C., Corley, R.P. et al. Genetic and Environmental Influences on Age at Sexual Initiation in the Colorado Adoption Project. Behav Genet 36, 820–832 (2006). https://doi.org/10.1007/s10519-006-9079-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-006-9079-2