Abstract
The accelerated development of high-density brackishwater shrimp farming necessitates the importance of bioremediation. Seaweeds have the potential to reduce nutrients from aquaculture systems and provide extra income when species of economic importance are used. Identification of suitable seaweed species which is locally available in abundance with bioremediation capacity in brackishwater system is paramount, and the present study addresses this issue. An exploratory monthly survey was undertaken in three brackishwater systems in Chennai coast viz. Muttukadu lagoon, Vennangupattu Lake and Pulicat Lake from March 2018 to February 2019 which led to a focus on species of the family Gracilariaceae. Identification of the species through taxonomical and molecular observations confirmed that seaweed from Muttukadu lagoon and Vennangupattu Lake is Agarophyton tenuistipitatum and that from Pulicat Lake is Hydropuntia edulis. Evaluation of the bioremediation potential of these two species indicated that they were similar with respect to ammonia and phosphate reduction efficiency whereas the specific growth rate of A. tenuistipitatum was significantly higher than H. edulis. Furthermore, the nutrient reduction efficiency and specific growth rate was significantly higher at biomass density of 3.5 and 4.5 g L−1 compared to 1.5 and 2.5 g L−1. It could therefore be concluded that A. tenuistipitatum could be utilised for bioremediation as well as culture in brackishwater system at a biomass density ranging from 3.5 to 4.5 g L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture in India has evolved as a viable commercial farming practice from the level of traditional backyard activity over the past few decades with considerable diversification in terms of species and systems and has been showing an impressive annual growth rate of 6–7%. Indian crustacean farming is brackishwater aquaculture wherein shrimps account for more than 90% of Indian crustacean production. Shrimp cultivation has been on a surge especially since 2009, after the introduction of Penaeus vannamei with production levels of 10–12 t ha crop−1 within a 135-day duration (Laxmappa 2016). The production of this species has reached a level of 622,327 tonnes during 2017–2018 (MPEDA 2018). At present, the major brackishwater-cultivated species is Penaeus vannamei which contributed about 52.9% among crustaceans in world aquaculture production (FAO 2020).
The aquaculture of marine animals is not an environmentally friendly activity. One of the most serious problems of aquaculture of invertebrates and fish is the loading of excessive nutrients into the local waters. In general, 52–92% of the nitrogen and 85% of the phosphorus enters the aquatic environment through feed wastage, excretion and faeces, which may easily induce eutrophication, induces algal bloom leading to anoxia, but both are part of the process (Zhou et al. 2006).
The use of brackishwater macroalgae capable of bioremediation would help to sequestrate the nutrient nitrogen and phosphorus to a great extent. Earlier studies have shown that in their normal growth and development, macroalgae absorb and metabolise large amounts of nutrients into their tissues (Lobban and Harrison 1994). Gracilariaceaean algae have been identified as valid and effective agents for nutrient bioremediation from different IMTA systems (Neori et al. 2004). In seawater temperature of 20–23 °C at irradiance of 250 μmol quanta m−2 s−1, Hydropuntia edulis was able to remove around 95% of ammonium from shrimp farming system (Jones et al. 2001). At marine salinity regime and 9.5–19 °C temperature, Gracilaria chilensis was capable of removing 95% ammonium and 32% orthophosphate from a salmon culture system under natural daylight condition (Buschmann et al. 1996). In a mollusc-fish-seaweed–based IMTA studies under natural daylight, it was found that in seawater and 16–26.9 °C temperature condition, Gracilaria conferta removed 34% of ammonium and about 25% of orthophosphate (Neori et al. 1998). Martinez-Aragon et al. (2002) reported that Gracilaria gracilis with the condition of 18 °C and 240 μmol photon m−2 s−1 illumination in a flow-through system could remove around 93% and 62.2% of ammonium and orthophosphate respectively from sea bass (Dicentrarchus labrax) cultured effluent water within marine salinity regime. Apart from the bioremediation potential, Gracilariaceaean algae have economic importance as agarophyte and as food for humans and marine animals (Yang et al., 2015). Several Gracilariaceaean species are important in the multi-million dollar agar and agarose phycocolloid industry (Johnson et al. 2014).
Family Gracilariaceae has a worldwide distribution; Gracilariacean algae (or its representatives) grows in tropical and subtropical waters, and also found in temperate zone along the European coast up to Sweden and along the Asian coast up to Sea of Okhotsk. Among family Gracilariaceae, the genus Hydropuntia and Agarophyton include around 8 and 3 species, respectively (Gurgel et al. 2018; World Register of Marine Species (WoRMS) 2020). In India, the main species cultivated is Hydropuntia edulis (= Gracilaria edulis) (Krishnamurthy 1991); among thirty-two species of Gracilariacean algae have been reported for the country.
The main aim of this investigation was to identify the locally available brackishwater seaweed species (through morphological as well as molecular methods) which is abundant and has a commercial value along with its bioremediation potential, i.e. to estimate the minimum biomass density required for maximum sequestration of nutrients (N and P) with higher growth rate. From this point of view, the members of the Gracilariaceae are the most promising objects among brackishwater seaweeds. Therefore, three naturally available Gracilariacean algae collected from Pulicat Lake, Muttukadu lagoon and Vennangupattu Lake were studied.
Materials and methods
Identification of seaweeds
Studies on seaweed species of brackishwater system are meagre. Monthly exploratory survey on three brackishwater systems in Chennai coast viz. Muttukadu lagoon, Vennangupattu Lake and Pulicat Lake from March 2018 to February 2019 aided in selecting seaweeds with the help of local fishermen. In all locations, Gracilariacean algae were found abundant almost throughout the year. Seaweeds were collected from the shallow (0.4–1 m) water bodies in the Pulicat Lake (13° 28′ 59.4″ N and 80° 15′ 46.1″ E) of salinity ranged between 12.5 and 61 g L−1, and also a very shallow (< 0.5 m) zone of the Muttukadu lagoon (12° 48′ 42.1″ N and 80° 14′ 39″ E) and Vennangupattu Lake (12° 14′ 41″ N and 79° 58′ 58″ E) of salinity ranged from 10 to 27.4 g L−1 during the year at the low-tide period (Fig. 1). After collection, algae were transferred to the laboratory where epiphytes and encrusting organisms were removed. Thereafter, they were stored in a tank with seawater for further studies.
Morphological and anatomical methods
Fresh seaweeds were collected from the field and the genus were identified in the laboratory by studying morphological and anatomical features which were photographed using a Nikon DS-U3 DS-Fi2-U3 camera on a bright field microscope. For anatomical identification, the cross section of fresh thallus and cystocarp were mounted with 40% corn syrup.
Molecular protocol
Three samples (one per site) were collected and cleaned of surface epiphytes, debris and portions of each sample were kept separately for DNA extraction for which the Chelex protocol (Goff and Moon, 1993) as outlined in Zuccarello et al. (1999a) was followed. A fresh thallus tip of approximately 3–15 mm or 10–25 mg was used for each sample. Based on the earlier studies by Zuccarello et al. (1999a, b), two DNA markers from different cell organelles were used to identify the species. The first one was intergenic region between ribulose bisphosphate carboxylase/oxygenase large and small subunit viz. RuBisCo spacer region located in plastid and the second one was intergenic region between cytochrome oxidase subunit 2 and cytochrome oxidase subunit 3 viz. Cox2–3 spacer region located in mitochondria. RuBisCo spacer region was amplified using forward primer 5′-tatacttctacagacacagctga-3′ (rbcF1) and reverse primer 5′-atttcacacaggaaacagctatgacatgtcaaataatggtagtcccca-3′ (rbcR2-M2) and amplification of Cox2–3 spacer region was performed using forward primer (cox2-for) 5′-gtaccwtctttdrgrrkdaaatgtgatgc-3′ and a reverse primer (cox3-rev) 5′-ggatctacwagatgraawggatgtc-3′ (Zuccarello et al. 1999b; Byrne et al. 2002). Polymerase chain reaction (PCR) products were checked by electrophoresing in a 1.5% agarose gel, stained in ethidium bromide, visualised under UV light and photographed. Crude PCR products were prepared and sent to Eurofins Genomics India Pvt. Ltd. for sequencing. Sequenced data obtained from the two DNA markers for three specimens were analysed for similarity using nucleotide BLASTN programme online.
Bioremediation efficiency
Experiment design
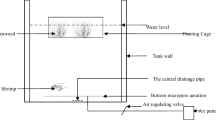
The experimental system was placed inside a poly-house with a natural photoperiod and the photon flux density of sunlight inside the room was ranged between 57 and 76 μmol photons m−2 s−1 during the daytime. Besides, temperature was simulated in the poly house with the help of a cellulose cooling pad, and the overall room temperature was maintained between 27 and 30 °C during the daylight period. A factorial design was employed analysing Factor 1 (two seaweeds species from three different locations) and Factor 2 (biomass density) in the following treatments: 0 g L−1 (without any seaweed), 1.5 g L−1 (310 g m−2), 2.5 g L−1 (517 g m−2), 3.5 g L−1 (724 g m−2) and 4.5 g L−1 (931 g m−2) as control, T1, T2, T3 and T4, respectively. Thirty plastic tanks each having a capacity of 30 L (triplicate) were filled with filtered sea water, and adequate amount of fresh water was added into it to maintain a constant salinity of 25 g L−1. Air stones were placed in the bottom of each tank to provide continuous aeration, and DO level was maintained between 5.5 and 6 mg L−1 ranges during the experiment. Initially, 5 mg L−1 of NH4-N and 2 mg L−1 of PO4-P level were administered to the treatments with the help of (NH4)2SO4 and KH2PO4 salts respectively to determine the bioremediation efficiency of the algae. The experiment was continued up to 96 h from the time of the addition of seaweed in the tanks.
Sample collection and analytical methods
Water pH and dissolved oxygen (DO) were determined and recorded using probes (Orion 9107 BNMD for pH and Lutron DO-5510 for dissolved oxygen) at every 12 h interval for 24 h. The water samples (triplicate) were collected and analysed for NH4-N (phenol hypochlorite method), NO2-N (sulphanilamide NED method), NO3-N (sulphanilamide NED method after reduction of NO3 to NO2 with cadmium) and PO4-P (phosphomolybdic acid-ascorbic acid method) for the same duration (APHA 1998).
Nutrient removal percentage (NR %) for ammonium (NH4-N) and orthophosphate (PO4-P) in the systems was estimated after Zhou et al. (2006):
where Ccnl is nutrient concentration in the control treatment (mg L−1) and Cp is nutrient concentration in the seaweed treatment (mg L−1) at a particular time since beginning.
At the end of the experiment (96 h), the seaweed in each tank was weighed and their growth was estimated as below (Rosenberg et al. 1984):
where W0 is the initial wet weight of algae (g); Wf is the final wet weight of algae (g) at time t since the beginning; and t is the number of days between initial and final sampling.
Statistical analysis
The parameters were computed and expressed as mean along with the standard error. Data concerning nutrient removal percentage and SGR were analysed by two-way ANOVA to determine the effect of species and biomass density on the treatments. The level of significance (α = 0.05) was used. Statistical analyses were performed with SPSS version 17.0 and graphics generated by GraphPad Prism version 5.
Results
Seaweeds identification
Based on morphological and anatomical features (Figs. 2 and 3, Table 1), algae from Muttukadu lagoon and Vennangupattu Lake were identified as Agarophyton tenuistipitatum (= Gracilaria tenuistipitata C. F. Chang & B.-M. Xia) and that from Pulicat Lake was identified as Hydropuntia edulis (= Gracilaria edulis (S. G. Gmelin) P. C. Silva). This identification was confirmed by genetic analysis (Fig. 4; Table 2).
Amplified Cox2–3 (left) and RuBisCo spacer region (right) separated by agarose gel electrophoresis (1.5%) and visualised following ethidium bromide staining. Lane d, molecular weight ladder. Left side of ladder shown amplified Cox2–3 spacer region for A. tenuistipitatum from Muttukadu (M) lagoon (lane a), Vennangupattu (V) Lake (lane b) and H. edulis from Pulicat (P) Lake (lane c). Right side of ladder shows amplified RuBisCo spacer region of A. tenuistipitatum from Muttukadu (M) lagoon (lane e), Vennangupattu (V) Lake (lane f) and H. edulis from Pulicat (P) Lake
Bioremediation efficiency
Abiotic parameters
The average water temperature ranged between 28.1 and 30.6 °C in all treatments during the study (Table 3). The average pH was insignificant between different biomass for both species during 0 h and 12 h duration, but it was significantly affected during 24 h of study. It was found higher as biomass of seaweed increased. Average pH ranged from 7.95 to 8.19 among seaweed treatments whereas it was 7.75 in control (Table 3). Initial water quality revealed that source water contained both the NO2-N and NO3-N. The variation with respect to NO2-N and NO3-N during the experimental trial is depicted in Table 3. Mean NO2-N concentration was 0.001 mg L−1 initially in all treatments for both species during the entire experimental period (Table 3). It was observed that mean NO3-N concentration was 0.30 mg L−1 initially in all treatments and in control during 12 h and 24 h. Mean NO3-N concentration reduced significantly for both species, and reduction was significantly lesser at 1.5 g L−1 than other higher biomass density during the experiment. Optimum NO3-N sequestration observed at 3.5 and 4.5 g L−1 biomass density was approximately 0.1 mg L−1 at 12 h and < 0.1 mg L−1 24 h for both species (Table 3).
Macroalgal performance
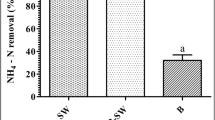
H. edulis and A. tenuistipitatum showed a significant difference with respect to mean NH4-N removal percentage in the water at 12 h (Table 4; Fig. 5a). Higher removal efficiency was observed in A. tenuistipitatum than H. edulis. However, at 24 h, there was no difference between the species in terms of mean NH4-N removal percentage (Table 4; Fig. 5b).
Ammonia reduction efficiency during 12 h (a) and 24 h (b) of H. edulis and A. tenuistipitatum for different biomass densities. Data represents mean ± SE (n = 3 replicates). Different small and capital letters indicate statistical significance (p < 0.05) among different seaweed biomass densities and species, respectively
With respect to different biomass densities, it was observed that the mean ammonia removal was significantly higher after 24 h than after 12 h (Table 4). Mean ammonia removal increased with biomass increase, reaching > 85% after 12 h (Fig. 5a) and 95% after 24 h (Fig. 5b) at highest biomass (4.5 g L−1).
Phosphate removal % did not reveal a difference between species both at 12 h and 24 h time intervals (Table 4; Fig. 6a, b). Similarly, with respect to different biomass densities, it was observed that mean phosphate removal was significantly higher after 24 h than after 12 h (Table 4). Mean phosphate removal increased with biomass increase, reaching 11–12% after 12 h (Fig. 6a) and about 30% after 24 h (Fig. 6b) at highest biomass (4.5 g L−1).
Phosphate reduction efficiency during 12 h (a) and 24 h (b) of H. edulis and A. tenuistipitatum for different biomass densities. Data represents mean ± SE (n = 3 replicates). Different small and capital letters indicate statistical significance (p < 0.05) among different seaweed biomass density and species, respectively
The SGR of both the species was significantly different with A. tenuistipitatum revealing a higher SGR than H. edulis at the experimental system (Table 4; Fig. 7). With respect to biomass density, a higher SGR was observed at 3.5 and 4.5 g L−1 treatment groups compared to 1.5 and 2.5 g L−1 treatment groups, but there was no significant difference between 3.5 and 4.5 g L−1 and also between 1.5 and 2.5 g L−1 biomass density (Fig. 7). The results showed that the optimum SGR achieved for A. tenuistipitatum was 1.43% day−1 whereas it was 0.43% day−1 for H. edulis at minimum 3.5 g L−1 biomass density.
Discussion
Studied species
The characterisation of the species is largely based on gross plant morphology and the developmental morphologies of vegetative and reproductive structures (Withell et al. 1994; Womersley 1996). In a genus like Gracilaria which is morphologically diverse, it is inevitable that the relatively few consistent features that classically distinguish the species overlap and ambiguity arises, particularly in cases where field populations are seldom represented by all life history stages. The ambiguous natures of the populations make the genus Gracilaria as well as the genera Hydropuntia and Agarophyton, recently segregated from Gracilaria (Gurgel and Fredericq 2004; Gurgel et al. 2018) difficult for identification throughout the world (Byrne et al. 2002). Often, cylindrical Gracilariacean algae in Asia were reported as Gracilaria verrucosa or were not identified, similarly to algae from Muttukadu lagoon and Pulicat Lake (Bharathan 1987; Kaliaperumal et al. 1995, Jayasankar et al. 2006). To exclude any ambiguities, we used combinations of morphological and molecular methods for identification of algae in this study. The analysis revealed the identity of the species as A. tenuistipitatum from Muttukadu lagoon and Vennangupattu Lake and H. edulis from Pulicat Lake region. Both species are widely distributed in the tropical zones. A. tenuistipitatum grows mainly in brackishwater conditions (Haglund and Pedersen 1993, Chaoyuan et al. 1993; Lee et al. 1999). In India, in brackishwater lagoons such as Muttukadu lagoon and Vennangupattu Lake, A. tenuistipitatum could be found throughout the year. In contrast, Hydropuntia edulis usually occurs in seawater salinity regime (> 30 g L−1) having a better growth under these conditions (Kaladharan et al. 1996; Jayasankar and Ramamoorthy 1997; Raikar et al. 2001; Ganesan et al. 2011). In the present study, it was found that Pulicat Lake has an average salinity of more than 30 g L−1 throughout the year (Dhinamala et al. 2015; Basuri et al. 2020).
Bioremedial potential and growth
In general NH4-N is cited as the preferred form of nitrogen for seaweed growth because NH4-N is the most reduced form of inorganic nitrogen, but simultaneously seaweeds also absorb other inorganic nitrogenous substances (Lobban and Harrison 1994). This observation corroborated the findings of the study of Marinho-Soriano et al. (2009), in which it was shown that G. caudata absorbed both NH4-N and NO3-N simultaneously, but removal of NO3-N was lower than that of removal of NH4-N. Different biomass densities of the seaweed was efficient to remove nitrite and nitrate as evident by the results on nitrite and nitrate concentration which were at a very negligible level throughout the experiment, i.e. below the harmful level for aquaculture practices. The study also revealed that irrespective of the seaweed biomass density, nitrite and nitrate concentration was found to be insignificant with different biomass densities whereas the reduction efficiency of ammonium and phosphorus was raised with biomass increase. A similar observation was reported in clam and mussel culture by Mao et al. (2009).
Average ammonium reduction efficiency was found to be higher in A. tenuistipitatum than in H. edulis at 12 h of experiment whereas at 24 h, both species performed equally with respect to NH4-N absorption. Higher extracellular concentration of NH4-N primarily triggers the downhill transport and thus resultant nutrient uptake rate is proportional to the external concentration. Passive uptake has been shown to occur for ammonium uptake in Macrocystis, Gracilaria tikvahiae and Agardhiella subulata at ammonium concentrations greater than 25 μM (Lobban and Harrison 1994). At used in the experiments ammonia concentration more than 250 μM, the algae mainly uptake nutrients by passive transport. The factors affecting the nutrient uptake between different species are enigmatic. Although, the above-mentioned higher removal efficiency for A. tenuistipitatum may be explained according to its higher growth rate compared to H. edulis because increase of growth rate may increase the nutrient uptake (Lobban et al. 1985). Yu et al. (2013) reported a significantly higher growth rate for A. tenuistipitatum than H. edulis. Besides, for passive transport, the rate of diffusion varies with chemical potential gradient across the plasmalemma (Lobban et al. 1985). Ammonium and phosphate removal % increased proportionately to biomass density, indicating that the thallus density positively affected the capacity of nitrogen and phosphorus uptake. Overall studies regarding ammonium and phosphate removal indicated that biomass density of 3.5 g L−1 can efficiently remove optimum levels of nitrogen and phosphorus from the system. Higher seaweed biomass density increases the potential for nutrient uptake, mainly due to the increased biomass and the higher surface area of macroalgae (Samocha et al. 2015). Besides, the effect of different seaweed biomass density on nutrient uptake may be explained by the specific growth rate because growth has a direct effect on nutrient absorption, i.e. increase the growth rate and thus increase the nutrient uptake (Lobban et al. 1985). Biomass density at a minimum of 3.5 g L−1 was found to be optimum with respect to specific growth rate; hence, this biomass density could be considered appropriate for optimum nutrient absorption. A similar observation was reported by Sarkar et al. (2019a) for IMTA experiment with A. tenuistipitatum.
The bioremediation efficiency of NH4-N in this study was > 80% at 3.5 g L−1 algal density after 24 h. The NH4-N uptake was reported as 87% for H. edulis in a shrimp farm effluent water at 20 g L−1 density into an integrated system with oyster after 24 h (Jones et al. 2001); whereas, it was 59.5% with G. caudata at density of 5 g L−1 in a shrimp farm wastewater system within 4 h (Marinho-Soriano et al. 2009) and 60% with Gracilaria lemaneiformis in 8 days into a fed fish culture system in coastal waters (Zhou et al. 2006). Sarkar et al. (2019a) also reported a 95.71% removal for A. tenuistipitatum at 3.5 g L−1 density in shrimp culture system after 21 days of culture. The PO4-P removal % also followed a similar trend as that of NH4-N removal % for different seaweed biomass densities, but reduction efficiency was lower compared to NH4-N removal %. The 3.5-g L−1 algal density was found to be optimal for removal of PO4-P (> 20%). A reduction of PO4-P concentration of 12.3% in 4 h with G. caudata at 5 g L−1 density was reported by Marinho-Soriano et al. (2009) in a shrimp farm effluent system; whereas, a figure of 32% was recorded by Buschmann et al. (1996) in Gracilaria chilensis at 1.5 kg m−2 density from fish culture effluent during the cultivation cycle of 13 months; Troell et al. (1997) recorded 27% for G. chilensis in a co-culture system with fish during 2 months of cultivation; Jones et al. (2001) reported a 35% reduction for H. edulis in a shrimp farm effluent water at 20 g L−1 density after 24 h into an integrated system with oyster, and Sarkar et al. (2019a) also reported a 95.74% removal for A. tenuistipitatum at 3.5 g L−1 density in shrimp culture system after 21 days of cultivation. In our study, the removal efficiency was close to these earlier reported values, but these slight differences observed are a characteristic of different culture systems and may also be related to other factors such as light, temperature, nutritional status, algal density, duration of culture, nutrient uptake rate, associated epiflora, type of tissue and age of the alga (DeBoer 1981).
Seaweed-specific growth rate was found to be significantly higher in A. tenuistipitatum compared to H. edulis throughout the study. SGR is directly related to the concentration of extracellular substrate (Lobban et al. 1985). Therefore, significantly higher uptake of NH4-N during 12 h period could be a triggering factor for higher growth rate in the case of A. tenuistipitatum. Besides, salinity might be a factor for this difference because several authors from India viz. Kaladharan et al. (1996), Raikar et al. (2001) and Ganesan et al. (2011) demonstrated that seawater salinity regime is better for the growth of H. edulis in Indian climate whereas A. tenuistipitatum is euryhaline in nature in Indian water and grows well in brackishwater salinity regime (Sarkar et al. 2019b). Moreover, H. edulis was collected from Pulicat Lake which has an average salinity of more than 30 g L−1 throughout the year (Dhinamala et al. 2015; Basuri et al. 2020). Although the physiological studies of Yu et al. (2013) showed that H. edulis could be adapted in brackishwater salinity regime, according to our studies, a selection of A. tenuistipitatum would be a better choice for brackishwater aquaculture system (Sarkar et al. 2019b). Other observations have been reported by Luhan (1992) for Gracilaria heteroclada in the Philippines and by Msuya and Neori (2002) for Eucheuma denticulatum in Tanzania with loss of biomass and growth due to decrease of salinity. Studies with different biomass densities indicated that optimum specific growth rate was found at a density of 3.5 g L−1 after which it decreased. Zhou et al. (2006) reported that thallus density negatively affected growth. A similar observation was recorded by Mao et al. (2009) who opined that static water, lower temperature and light limitation may also affect seaweed growth. A possible reason for lower growth at greater biomass density (> 3.5 g L−1) could partly be attributed to limitation of light due to higher biomass density.
Conclusion
The accelerated development of high-density brackishwater shrimp farming necessitates the importance of bioremediation. In India, the methods for treating effluents from brackishwater mariculture systems with macroalgae could be initiated and simultaneously can be utilised to reduce the risk of eutrophication of the environment by introducing culture systems. Our results expressed that A. tenuistipitatum could be utilised as a potential candidate species to improve water quality at a biomass density ranging from 3.5 to 4.5 g L−1 as this species showed better growth in brackishwater salinity regime. Besides, A. tenuistipitatum is potentially a fast-growing species with higher SGR compared to H. edulis in brackishwater culture system and hence, multiple crops may be obtained from an IMTA system with lesser input. Therefore, this study gave a preliminary idea for its IMTA potential in brackishwater condition and further association between biomass production and agar content needs to be investigated in order to check the economic feasibility of A. tenuistipitatum. Currently, seaweed farming is gaining momentum along the southeast Indian coast owing to constant depletion of fishing resources and fish catches over the years. This will help fetch additional income besides supplying continuous source of raw material to the seaweed-based industries.
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, D.C.
Basuri CK, Pazhaniyappan E, Munnooru K, Chandrasekaran M, Vinjamuri RR, Karri R, Mallavarapu RV (2020) Composition and distribution of planktonic ciliates with indications to water quality in a shallow hypersaline lagoon (Pulicat Lake, India). Environ Sci Pollut Res 27:18303–18316. https://doi.org/10.1007/s11356-020-08177-6
Bharathan G (1987) Experimental culture of Gracilaria at the Mariculture Centre, Muttukadu, Tamil Nadu. J Mar Biol Ass India 29:54–59 http://mbai.org.in/php/journaldload.php?id=1272&bkid=68
Buschmann AH, Troell M, Kautsky N, Kautsky L (1996) Integrated tank cultivation of salmonids and Gracilaria chilensis (Gracilariales Rhodophyta). Hydrobiologia 326(327):75–82. https://doi.org/10.1007/BF00047789
Byrne K, Zuccarello GC, West J, Liao M, Kraft GT (2002) Gracilaria species (Gracilariaceae, Rhodophyta) from southeastern Australia, including a new species, Gracilaria perplexa sp. nov.: morphology, molecular relationships and agar content. Phycol Res 50:295–311. https://doi.org/10.1046/j.1440-1835.2002.00282.x
Chaoyuan W, Li R, Lin G, Wen Z, Dong L, Zhang J, Huang X, Wei S, Lan G (1993) Some aspects of the growth of Gracilaria tenuistipitata in pond culture. Hydrobiologia 260(261):339–343. https://doi.org/10.1007/978-94-011-1998-6_42
DeBoer JA (1981) Nutrients. In: Lobban CS, Wynne MJ (eds) The biology of seaweeds. University of California Press, Berkeley, pp 356–392
Desikachary TV, Krishnamurthy V, Balakrishnan MS (1998) Rhodophyta, vol II-Part-II B. Madras Science Foundation, Chennai
Dhinamala K, Pushpalatha M, Samuel T, Raven R (2015) Spatial and temporal variation in the water quality parameters of Pulicat Lake, Tamil Nadu, India. Int J Fish Aquat Stud 3(2):255–259
FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action Rome. https://doi.org/10.4060/ca9229en
FAO/NACA (1996) Regional Study and Workshop on the Taxonomy, Ecology and Processing of Economically Important Red Seaweeds. In: NACA Environment and Aquaculture Development Series No. 3. Network of Aquaculture Centres in Asia-Pacific, Bangkok
Ganesan M, Sahu N, Eswaran K (2011) Raft culture of Gracilaria edulis in open sea along the south-eastern coast of India. Aquaculture 321:145–151. https://doi.org/10.1016/j.aquaculture.2011.08.040
Goff LJ, Moon DA (1993) PCR amplification of nuclear and plastid genes from algal herbarium specimens and algal spores. J Phycol 29(3):381–384. https://doi.org/10.1111/j.0022-3646.1993.00381.x
Gurgel CFD, Fredericq S (2004) Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): a critical assessment based on rbcL sequence analysis. J Phycol 40:154–159. https://doi.org/10.1111/j.0022-3646.2003.02-129.x
Gurgel CFD, Norris JN, Schmidt WE, Le HN, Fredericq S (2018) Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera, and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa 374(1):001–023. https://doi.org/10.11646/phytotaxa.374.1.1
Haglund K, Pedersen M (1993) Outdoor pond cultivation of the subtropical marine red alga Gracilaria tenuistipitata in brackish water in Sweden. Growth, nutrient uptake, co-cultivation with rainbow trout and epiphyte control. J Appl Phycol 5(3):271–284. https://doi.org/10.1007/BF02186230
Jayasankar R, Ramamoorthy N (1997) Propagation of Gracilaria edulis (Gmelin) Silva by reproductive method. Indian J Fish 44(4):353–360
Jayasankar R, Seema C, Leelabhai KS, Kanagam A (2006) Pond based grow out system of Gracilaria verrucosa. J Aquacult Trop 21:161–167
Jha B, Reddy CRK, Thakur MC, Rao MU (2009) Seaweeds of India, the diversity and distribution of seaweeds of the Gujarat coast. Developments in Applied Phycology 3:1–216. https://doi.org/10.1007/978-90-481-2488-6
Johnson RB, Kim JK, Armbruster LC, Yarish C (2014) Nitrogen allocation of Gracilaria tikvahiae grown in urbanized estuaries of Long Island Sound and New York City, USA: a preliminary evaluation of ocean farmed Gracilaria for alternative fish feeds. Algae 29:227–235. https://doi.org/10.4490/algae.2014.29.3.227
Jones AB, Dennison WC, Preston NP (2001) Integrated mariculture of shrimp effluent by sedimentation, oyster filtration and macroalgal absorption: a laboratory scale study. Aquaculture 193(1–2):155–178. https://doi.org/10.1016/S0044-8486(00)00486-5
Kaladharan P, Vijayakumaran K, Chennuhhotla VSK (1996) Optimisation of certain physical parameters for the mariculture of Gracilaria edulis (Gmelin) Silva in Minicoy lagoon (Laccadive archipelago). Aquaculture 139:265–270
Kaliaperumal N, Kalimuthu S, Ramalingam JR (1995) Economically important seaweeds. CMFRI special publication 62:1–35. http://eprints.cmfri.org.in/id/eprint/3476/
Krishnamurthy V (1991) Gracilaria resources of India with particular reference to Tamil Nadu Coast. Seaweed Resources Utilization 14(1):181–185
Laxmappa B (2016) Status and prospects: shrimp aquaculture in India. International Aquafeed 19:50–51. https://issuu.com/international_aquafeed/docs/iaf1605_w1/52/
Lee TM, Chang YC, Lin YH (1999) Differences in physiological responses between winter and summer Gracilaria tenuistupitata (Gigartinales, Rhodophyta) to varying temperature. Bot Bull Acad Sin 49:93–100
Lobban, C.S., Harrison, P.J., 1994. Seaweed ecology and physiology. Cambridge University Press, Cambridge https://doi.org/10.1017/CBO9780511626210
Lobban CS, Harrison PJ, Duncan MJ (1985) The physiological ecology of seaweeds. Cambridge University Press, Cambridge
Luhan MRJ (1992) Agar yield and gel strength of Gracilaria heteroclada collected from Iloilo, Central Philippines. Bot Mar 35(2):169–172. https://doi.org/10.1515/botm.1992.35.2.169
Mao Y, Yang H, Zhou Y, Ye N, Fang J (2009) Potential of the seaweed Gracilaria lemaneiformis for integrated multi-trophic aquaculture with scallop Chlamys farreri in North China. J Appl Phycol 21:649–656. https://doi.org/10.1007/s10811-008-9398-1
Marinho-Soriano E, Panucci RA, Carneiro MAA, Pereira DC (2009) Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour Technol 100(24):6192–6198. https://doi.org/10.1016/j.biortech.2009.06.102
Martinez-Aragon JF, Hernandez I, Perez-Llorens JL, Vazquez R, Vergara JJ (2002) Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste water: 1. Phosphate J Appl Phycol 14:365–374
MPEDA, 2018. Total tiger shrimp, L. vannamei and scampi production. https://www.mpeda.gov.in/MPEDA/cms.php?%20id=eWVhci13aXNlLXNwZWNpZXMtd2lzZS1zdGF0ZS13aXNl. Accessed 18 April 2020
Msuya FE, Neori A (2002) Ulva reticulata and Gracilaria crassa: macroalgae that can biofilter effluent from tidal fishponds in Tanzania. WIO Journal of Marine Science 1(2):117–126
Neori A, Ragg NLC, Shpigel M (1998) The integrated culture of seaweed, abalone, fish and clams in modular intensive land-based systems: II. Performance and nitrogen partitioning within an abalone (Haliotis tuberculata) and macroalgae culture system. Aquac Eng 15:215–239. https://doi.org/10.1016/S0144-8609(98)00017-X
Neori A, Chopin T, Troell M, Buschmann AH, Kraemer GP, Halling C, Shpigel M, Yarish C (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 231:361–391. https://doi.org/10.1016/j.aquaculture.2003.11.015
Raikar SV, Lima M, Fujita Y (2001) Effect on temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J. Mar Sci 30:98–104
Rosenberg G, Probyn TA, Mann KH (1984) Nutrient uptake and growth kinetics in brown seaweeds: response to continuous and single additions of ammonium. J Exp Mar Biol Ecol 80(2):125–146. https://doi.org/10.1016/0022-0981(84)90008-X
Samocha TM, Fricker J, Ali AM, Shpigel M, Neori A (2015) Growth and nutrient uptake of the macroalga Gracilaria tikvahiae cultured with the shrimp Litopenaeus vannamei in an integrated multi-trophic aquaculture (IMTA) system. Aquaculture 446:263–271. https://doi.org/10.1016/j.aquaculture.2015.05.008
Sarkar S, Rekha PN, Balasubramanian CP, Ambasankar K (2019a) Bioremediation potential of the brackishwater macroalga Gracilaria tenuistipitata (Rhodophyta) co-cultured with Pacific white shrimp Penaeus vannamei (Boone). J. Coast. Res. 86:248–254. https://doi.org/10.2112/SI86-036.1
Sarkar S, Rekha PN, Balasubramanian CP, Ambasankar K, Vijayan KK (2019b) Culture of the seaweed, Gracilaria tenuistipitata in brackishwater pond as well as lagoon of Muttukadu, Chennai, Tamil Nadu. Indian J. Fish 66(4):60–68. https://doi.org/10.21077/ijf.2019.66.4.90176-08
Troell M, Halling C, Nilsson A, Buschmann AH, Kautsky N, Kautsky L (1997) Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 156(1–2):45–61. https://doi.org/10.1016/S0044-8486(97)00080-X
Withell AF, Millar AJK, Kraft GT (1994) Taxonomic studies of the genus Gracilaria (Gracilariales, Rhodophyta) from Australia. Aust Syst Bot 7(4):281–352. https://doi.org/10.1071/SB9940281
Womersley, H.B.S., 1996. The marine benthic Flora of southern Australia. Rhodophyta - part IIIB. Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales. Canberra: Australian biological resources study. https://data.environment.sa.gov.au/Content/Publications/Womersley3B.pdf. Accessed on 23 July 2019
World Register of Marine Species (WoRMS) 2020. Flanders Marine Institute. http://www.marinespecies.org/aphia.php?p=taxdetails&id=144188#sources.
Yang Y, Chai Z, Wang Q, Chen W, He Z, Jiang S (2015) Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res 9:236–244. https://doi.org/10.1016/j.algal.2015.03.017
Yu CH, Lim PE, Phang SM (2013) Effects of irradiance and salinity on the growth of carpospore-derived tetrasporophytes of Gracilaria edulis and Gracilaria tenuistipitata var liui (Rhodophyta). J Appl Phycol 25:787–794. https://doi.org/10.1007/s10811-012-9960-8
Zhou Y, Yang H, Hu H, Liu Y, Mao Y, Zhou H, Xu X, Zhang F (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of North China. Aquaculture 252:264–276. https://doi.org/10.1016/j.aquaculture.2005.06.046
Zuccarello GC, West JA, Kamiya M, King RJ (1999a) A rapid method to score plastid haplotypes in red seaweeds and its use in determining pa rental inheritance of plastids in the red alga Bostrychia (Ceramiales). Hydrobiologia 401:207–214. https://doi.org/10.1023/A:1003706931897
Zuccarello GC, Burger G, West JA, King RJ (1999b) A mitochondrial marker for red algal intraspecific relationships. Mol Ecol 8(9):1443–1447. https://doi.org/10.1046/j.1365-294x.1999.00710.x
Acknowledgements
The authors are thankful to the Department of Science and Technology, Govt. of India for financial support and the Director, ICAR-Central Institute of Brackishwater Aquaculture, Chennai for providing the necessary facilities, support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, S., Rekha, P.N., Ambasankar, K. et al. Bioremediation efficiency of indigenous seaweeds of Chennai coast in brackishwater system. Aquacult Int 29, 233–251 (2021). https://doi.org/10.1007/s10499-020-00621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00621-1