Abstract
Planktonic ciliate composition and distribution together with physicochemical variables were investigated in a shallow hypersaline lagoon, Pulicat, India, during three seasons, i.e., pre-monsoon (PRM), monsoon (MON), and post-monsoon (POM). The low freshwater inflow, evaporation, and closure of the lake mouth were the main factors for the hypersaline conditions in Pulicat Lake. The average depth and salinity were 1.8 ± 0.12 m (0.8 to 2.8 m) and 35.3 ± 1.68 (12.5 to 61), respectively. A total of 29 ciliate taxa belonging to 18 genera and five classes were identified. Strombidium conicum (24%) was the dominant species followed by Euplotes sp. (10.7%) and Stenosomella sp. (7.02%). Spirotrichea (84%) was the dominant class followed by Oligohymenophorea (9.6%) and Heterotrichea (5.8%). Fabrea salina, a typical species in hypersaline systems, was abundant at locations where the salinity was more than 35. Multivariate analysis using the Bray–Curtis similarity, followed by SIMPROF (Similarity Percentage Analysis), on ciliate abundance data revealed three ciliate assemblages characterizing south, central, and north of the lake at 40% similarity (SIMPROF, cophenetic correlation = 0.622, P = 5%). Both ciliate abundance and chlorophyll-a were positively correlated with salinity. Species richness and evenness were higher in the south sector when compared with those in the other two sectors. Biotic-environmental interaction through canonical correspondence analysis (CCA) inferred that the combined effects of salinity, chlorophyll-a, and nutrient levels are the key factors responsible for the distribution of the ciliate species, suggesting that ciliates can be considered to be potential bioindicators of water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Planktonic ciliates are a part of the microzooplankton (MZP) and most are in the size range from 20 to 200 μm. They play an important role in aquatic environments by mediating the transfer of energy between the trophic levels of the microbial food web (Azam et al. 1983). Numerous studies on the ecology and distribution of MZP, particularly ciliated protozoans in coastal waters, have been initiated in the last few decades. Several such studies have reported the abundance, community structure, and diversity of ciliates with respect to changing environmental conditions in both temperate (Modenutti et al. 2000; Queimaliños et al. 1999) and tropical (Jyothibabu et al. 2006; Sahu et al. 2016) regions. Most of these studies have indicated the ecological importance of planktonic ciliates, particularly their grazing of phytoplankton in coastal waters (Landry and Calbet 2004; Schmoker et al. 2013). Although ciliates are important grazers of phytoplankton and serve as a food source for mesozooplankton in hypersaline systems (Sanders and Wickham 1993; Brown et al. 2002), information on their species composition and diversity are limited from such environments (Elloumi et al. 2006).
In general, environmental factors such as salinity, temperature, and availability of food control the ciliate community structure (Nche-Fambo et al. 2016; Pineda et al. 2019). The ecological and environmental variabilities generally influence the community attributes such as species richness which decreases with an increase in salinity (Pedrós-Alió et al. 2000). Ciliates require a basic source of energy for survival, and they can exist actively even in extreme conditions (Lynn 2008) such as anaerobic conditions, high temperatures, alkalinity, and hypersalinity (Hu 2014). A few organisms such as Artemia salina (crustacean), Dunaliella salina (microalgae), and Fabrea salina (ciliate) have been reported as common in hypersaline habitats (Elloumi et al. 2006). Fabrea salina, a typical hypersaline heterotrichous ciliate, can tolerate salinity concentrations higher than 60 (Giordano et al. 1994). Anaerobic ciliates are mostly present in the fresh and marine water sediments (Fenchel and Jørgensen 1977; Xu et al. 2011). Chilodon, Tetrahymena, Folliculopsis, etc. are some examples of ciliate genera, which can survive in temperatures higher than 40 °C (Corliss 1973; Kahan 1969, 1972; Noland and Gojdics 1967). Finlay et al. (1987) investigated alkaline–saline water bodies of East African Lakes (Lake Nakuru and Lake Simbi) and reported the occurrence of Frontonia sp., Spathidium sp., and Holophyra sp. Studies on ciliates occurring in hypersaline systems have mainly dealt with biomass contribution (Pandey et al. 2008; Elloumi et al. 2006; Lei et al. 2009; Nche-Fambo et al. 2016). Recent studies have considered ciliates as bioindicators because of their rapid responses to change in water quality (Zhang et al. 2012; Jiang et al. 2013; Wang and Xu 2015; Sikder et al. 2019). However, studies characterizing the spatiotemporal variations in ciliate community distribution in hypersaline systems, particularly from the Indian subcontinent, are lacking.
Pulicat Lake, a hypersaline lagoon and the second largest lagoon in India after Chilika Lake, shows a wide range of salinity varying from 2 to 55 during monsoon and post-monsoon, respectively (Radhakrishnan 1976). Earlier studies have reported the topography and hydrological conditions of Pulicat Lake and suggested that the broad variations in salinity might be due to the low freshwater inflow, high residence time, evaporation (summer), and closure of the lake mouth due to siltation (Dhinamala et al. 2015). The present study aimed to illustrate the composition and distribution of ciliates in Pulicat Lake using various univariate and multivariate biodiversity models. The environmental gradients structured through spatiotemporal changes determine the community structure and diversity of planktonic ciliates. Therefore, we hypothesize that monsoon and shallow environment along with local dynamics and salinity regimes are the key factors to differentiate ciliate assemblages.
Materials and methods
Study area
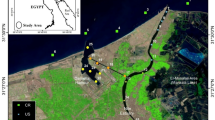
Pulicat Lake is located between 13° 26′–13° 43′ N latitude and 80° 03′–80° 18′ E longitude, parallel to the coastline of Bay of Bengal is 60 km long, and has a maximum width of about 20 km. The water-spread area during the monsoon period is 460 km2, whereas during the dry period, it is 250 km2 (Coulthard 2008). The average depth is about 1.8 m. It is separated from the Bay of Bengal by an inland spit called Sriharikota Island. The main freshwater sources for Pulicat Lake are the Arani River in the south and Kalangi and Swarnamukhi rivers at the mid-western and northern regions, respectively. These rivers discharge freshwater into the lake only during the rainy season, i.e., Northeast monsoon.
Environmental variables
Altogether, 39 samples were collected from thirteen GPS fixed sampling stations to cover the entire lake (Fig. 1). Sampling was carried out once in a month during September 2018, November 2018, and January 2019 representing the tropical climatic conditions such as pre-monsoon (PRM) (rainfall 47.3 mm; avg. 18 days), monsoon (MON) (rainfall, 449.6 mm; avg. 24 days), and post-monsoon (POM) (rainfall 3.1 mm; avg. 7 days), respectively (https://www.worldweatheronline.com/pulicat-weather-averages/tamil-nadu/in.aspx). Depth and water temperature were measured by using portable Citadel CTD-NV. Salinity (Knudsen’s method), total suspended matter (TSM), dissolved oxygen (DO) (Winkler’s method), nitrate–nitrogen (NO3−), nitrite–nitrogen (NO2−), ammonium–nitrogen (NH4+), reactive phosphate (PO4−), reactive silicate (SiO4−), total nitrogen (TN), and total phosphorus (TP) were estimated by following standard protocols (Grasshoff et al. 1999) and using a multi-chemistry AutoAnalyzer unit (SEAL Analytical, model AA3 HR). For phytoplankton biomass (chlorophyll-a), a known volume of the water was filtered through the GF/F filter with a gentle vacuum (of ≤ 80 mmHg) and then, the filter was wrapped with an aluminum foil and frozen until analysis. The filter was extracted with 90% acetone at 4 °C in dark for 24 h, and the pigment concentration was measured using a spectrophotometer (UV1800, Shimadzu) (Strickland and Parsons 1972).
Ciliate enumeration
One liter of subsurface water was collected with a Niskin’s water sampler and fixed with 2% acid Lugol’s iodine and stored in a cool dark place (UNESCO 1978). The fixed sample was allowed to stand for 48 h, and then, the supernatant was siphoned leaving 100 ml as the final volume (Utermöhl 1958). An aliquot of the sample (1 ml) was placed in a Sedgewick Rafter counting chamber, and ciliates were enumerated under ZEISS Axioscope-A1 upright microscope at × 100–× 400. Taxonomic identification and species description were made following the taxonomic keys of Kofoid and Campbell (1929), Corliss (1979), Curds (1982), Lynn (2008), and Gao et al. (2016). To measure the biovolume of each taxon, linear dimensions such as length, width, and diameter were measured for 15 to 20 cells per taxon by using the AxioVision Rel 4.8 software which was configured with AxioCam MRC, Carl Zeiss camera along with calibrated ocular micrometer and fitted into geometric models (Vadrucci et al. 2007). The total biovolume per taxon per liter was obtained by multiplying the mean cell biovolume by its abundance and converted to carbon biomass (pg C l−1) by applying the conversion factor (C: vol ratio of 0.19) proposed by Putt and Stoecker (1989) due to cell shrinking in Lugol’s iodine. For tintinnids, cell volume was considered ½ the lorica volume, and after measuring the lorica volume (LV) of the identified taxa, the carbon content was estimated using the conversion factor:
(Verity and Langdon 1984).
Statistical analyses
A series of multivariate analysis was adopted to address the spatial distribution of ciliate species in Pulicat Lake. Ciliate abundance data were subjected to the D’Agostino and Pearson omnibus normality test and revealed a non-normal distribution pattern. Hence, we used a non-parametric technique called PERMANOVA, with a pseudo-F statistic, and P value obtained from 999 random permutations on the resemblance matrix (Anderson 2001). Bray–Curtis similarity–based cluster analysis was performed on the square root–transformed ciliate abundance data (which contributed up to 86% of the total abundance) through group average linking for classifying the ciliate assemblages according to the standard protocols (Clarke and Green 1988; Clarke and Warwick 2001; Clarke and Gorley 2006). The contribution of each species and spatial differences in ciliate assemblages were summarized using SIMPROF (Similarity Percentage Analysis) (Clarke and Gorley 2006) and submodule CAP (canonical analysis of principal coordinates) of PERMANOVA+, respectively, to test statistical difference between sample clouds which were separated by two CAP axes (Anderson et al. 2008; Jiang et al. 2013).
The species–environmental relationship matrix between each dominant species (selected 15 species) and environmental variables was presented in a tabular format with statistical regression and probability strength obtained through paired Spearman’s rank correlations. Subsequently, a multivariate correlation test using the submodule biotic–environmental interactions (BIOENV) through the Spearman rank correlation–based permutation Mantel coefficient test (RELATE) protocol was employed to identify a potential relationship between the datasets (Clarke and Gorley 2006). The square root–transformed ciliate abundance data was used for the biotic–abiotic interaction study performed by using the unimodal canonical correspondence analysis (CCA) to explain variations in ciliate composition, as described by measured environmental gradients ter Braak (1986). The Monte Carlo randomization test (499 permutations under the reduced model with inter-species distance matrix) was carried out to assess the best probability of the observed patterns (ter Braak and Verdonschot 1995).
In order to emphasize the patterns of ciliate diversity across the assemblages, alpha (species number S, Margalef’s richness index d, Shannon–Weiner diversity H′, and Pielou’s evenness index J′), species turnover, beta (beta diversity index, beta dissimilarity based on Bray–Curtis), and total number of species, gamma diversity indices were measured following the proposed protocols (Whittaker 1960, 1972; Wilson and Shmida 1984; Koleff et al. 2003). A Venn diagram was used to represent the ciliate species turnover across different ciliate assemblages, and the numerical values in the diagram denote the number of species.
PRIMER v6.1 software package was used for all the univariate and multivariate procedures outlined above (Clarke and Gorley 2006). CANOCO v4.5 (ter Braak and Smilauer 2002) was used for canonical correspondence analysis (CCA). The box plots were built by using Origin v8. Microsoft Excel 2007 was used for data analysis including linear regression.
Results and discussion
Environmental variables
The environmental and biological variables recorded in the three sectors of the lake are presented with average values and standard errors in Table 1. The lake is shallow with an average depth of about 1.8 m. Secchi disc transparency ranged from 0.3 m (central) to 1.8 m (south) with an average of 0.7 m. Water temperature was a maximum of 32.8 °C during the PRM and a minimum of 26 °C in MON. Salinity differed significantly between the seasons ranging from 12.5 to 61 (mean ± SE, 35.3 ± 1.63), while the maximum (avg. 41.3 ± 4.3) was recorded in the month of September 2018 (PRM) followed by January 2019 (POM; avg. 36.9 ± 1.3) and November 2018 (MON; avg. 29.5 ± 2.3).
The comprehensive salinity-based classification (Venice System 1959) for coastal water bodies indicated that during the PRM and POM, the salinity of the lake was between meta-haline and hyperhaline, whereas during the MON, the lake exhibited mesohaline to meta-haline conditions due to considerable inputs of freshwater in the form of rainfall and increased river flow (Fig. 2). The salinity trends indicated no significant spatial disparity. Nevertheless, the spatial heterogeneity in salinity within the north sector was higher when compared with that of the other two regions. Earlier findings correlated the extreme salinity and temperature in Pulicat Lake, to the low rainfall, insufficient river water inflow, and closure of the lake mouth (Padma and Periakali 1999; Coulthard 2008). No significant spatial and seasonal trends in pH were seen, while the maximum pH (8.7) was noticed during the monsoon period. The total suspended matter differed significantly among the regions, being highest (237 ± 50.9 mg l−1) in the central sector followed by the north (99.8 ± 17.1 mg l−1) and south (61.5 ± 0.6 mg l−1) sectors.
The linear relationship between TSM, salinity, and chlorophyll-a was positively correlated (Supplementary Fig. 1a–d). Similar observations were reported by Pérez-Ruzafa et al. (2019) in Mar Menor, a hypersaline lagoon in the semi-arid region of Mediterranean Sea. In Bahía de los Angeles, Gulf of California, wind-induced vertical and horizontal circulation patterns were the factors that regulated phytoplankton biomass and productivity (Muñoz-Barbosa et al. 1991). Additionally, the above relationship between TSM, salinity, and chlorophyll-a could be due to the high salinity that tends to decompose the organisms followed by the breakdown of organic matter may be the reason for the increase in TSM along with salinity (Shadrin and Anufriieva 2018). However, in tropical coastal lagoons, e.g., Chilika Lake, nutrient availability and phytoplankton production generally depend on the denitrification, remineralization, and sediment–water coupling processes (Kanuri et al. 2013; Muduli et al. 2013; Ganguly et al. 2015). Although the ciliate abundance increased with an increase in salinity (Supplementary Fig. 1d), it was not significant. The regression analysis (moving average) (Supplementary Fig. 1e) between the biomass of ciliates and phytoplankton indicated that the ciliate biomass was high when chlorophyll was low and vice versa, suggesting the commonly observed top-down control of ciliates on phytoplankton (Landry and Calbet 2004).
The monthly averages of dissolved nutrient concentrations for the three regions are presented in Table 1. Nitrite, ammonium, silicate, and total nitrogen exhibited significant spatial trends across the three regions of the lake. The maximum concentrations of nitrite (0.7 μM), nitrate (4.9 μM), and phosphate (2 μM) were detected during the PRM. Silicate concentration was high (80.1 μM) during the MON and low (3.6 μM) in the POM. Total nitrogen levels ranged between 18.5 μM (POM) and 91.7 μM (PRM). Total phosphorus was high during the PRM (3.2 ± 0.42 μM) followed by MON (2.8 ± 0.6 μM) and POM (0.93 ± 0.09 μM). Ammonium (4.9–33.3 μM), phosphate (0.1–2.0 μM), and silicate (3.6–80.1 μM) showed significant seasonal variations. Chlorophyll-a varied from 0.6 mg m−3 (PRM) to 106.8 mg m−3 (MON) (Table 1). These results support the general conclusion that the seasonal variability in freshwater runoff and temperature are the key factors that regulate the physicochemical conditions in shallow hypersaline systems (Padma and Periakali 1999; Modassir and Ansari 2011).
Biological variables
Ciliate community structure and distribution
A total of 29 taxa belonging to 18 genera and five classes, namely Heterotrichea, Litostomatea, Oligohymenophorea, Prostomatea, and Spirotrichea (Choreotrichea, Hypotrichea, and Oligotrichea), were identified. The Bray–Curtis similarity–based cluster analysis on ciliate species abundance data revealed three main species associations at 26% similarity level (Fig. 3). The group I represents a set of 16 species, group II with 11 species, and the other two species formed as group III. Overall, 19 species were identified as common to all the sectors (south, central, and north) of Pulicat Lake, out of which 16 species belonged to Spirotrichea. In general, the composition and abundance of ciliates are regulated by salinity, food availability, and grazing activities (Al-Yamani et al. 2019). However, it is noteworthy that salinity was identified as an important factor that controls the distribution of ciliates (Jyothibabu et al. 2006; Urrutxurt et al. 2003).
Most of the species from group I were found in all the three sectors except Strombidium oculatum and Leprotintinnus nordqvistii. Group II was divided into two subgroups with Strombidium conicum, Tintinnopsis tocantinensis, Strobilidium sp., Coleps sp., and Strombidium elongata as a major subgroup. Oligotrichs and choreotrichs were dominant when the salinity ranged from 27 to 35, which is close to that of general marine environments (Elloumi et al. 2006; Nche-Fambo et al. 2016). Fabrea salina was the only representative of the class Heterotrichea and appeared dominant in both the pre-monsoon and post-monsoon periods, particularly the locations where the salinity was higher than 40. Elloumi et al. (2006) reported that Fabrea salina has a wide tolerance of salinity and contributed to high ciliate biomass in the hypersaline salt works of Sfax, Tunisia. The second subgroup of group II comprised five species, i.e., Mesodinium sp., Favella ehrenbergii, Prorodon sp., Holosticha sp., and Epistylis sp., of which only Favella ehrenbergii was common. Amphirellopsis sp. and Tintinnopsis beroidea representing group III were restricted to the south and central sectors.
Ciliate assemblages, composition, and diversity
Multivariate techniques have proven to be the best for characterizing changes in planktonic communities and to explain their variations along with environmental gradients (Jiang et al. 2011). Henceforth, the Bray–Curtis similarity analysis was performed on the square root–transformed mean abundance data for 15 ciliate species which contributed to more than 2% of the total abundance (cumulative, 86%), namely Strombidium conicum (24.04%), Euplotes sp. (10.7%), Stenosomella sp. (7.02%), Strobilidium sp. (5.65%), Euplotes crassus (5.36%), Eutintinnus sp. (4.9%), Strombidium elongate (4.32%), Tintinnopsis tocantinensis (4.12%), Strombidium oculatum (3.6%), Fabrea salina (3.8%), Strombidium acutum (3.24%), Uronema sp. (2.7%), Prorodon sp. (2.5%), Tintinnopsis cylindrica (2.1%), and Strombidium spiralis (2%) (Plate 1). In contrast, three species contributed more than 50% of the ciliate biomass, i.e., Favella ehrenbergii (38.3%), Strombidium conicum (10.9%), and Eutintinnus sp. (8.03%). The dendrogram revealed three ciliate assemblages representing south (st. 1–3), central (st. 4–7, 9), and north (st. 8, 10–13) locations at 40% similarity (SIMPROF, cophenetic correlation = 0.622, P = 5%) (Fig. 4a). The resemblance matrix of the Bray–Curtis similarity was used to plot the canonical analysis of principal coordinates (CAP), and it revealed a similar spatial pattern to that of the ciliate communities (Fig. 4b). The first canonical axis separated the ciliate assemblages of the south sector from the other two groups at R = 0.941, P = 0.001, and the second canonical axis discriminated groups 2 (central sector) and 3 (north sector) from the south sector (R = 0.743 at P = 0.01). PERMANOVA test demonstrated a significant difference between each pair of groups (F = 3.96, P < 0.002).
Overall, the ciliate community was numerically dominated by Spirotrichea (84.4%) comprising three subclasses, namely Choreotrichea (22.9%), Oligotrichea (54.4%), and Hypotrichea (22.7%) (Fig. 5). The dominance of spirotrichous ciliates has been reported in many areas such as Western Mediterranean (Balkis 2004), Baltic Sea (Johansson et al. 2004), Cochin backwaters (Jyothibabu et al. 2006), and Gulf of Gabes (Kchaou et al. 2009). This group generally inhabits brackish environments and are filter feeders (Fenchel 1980; Lynn 2008; Esteban and Finlay 2003). Similar observations were reported from other hypersaline systems, for example salt works of Sfax, and Gulf of Gabes, Tunisia (Elloumi et al. 2006; Kchaou et al. 2009), where the ciliate abundance was largely dominated by Spirotrichea when salinity was low and Heterotrichea (mainly Fabrea salina) were dominated when the salinity was high (> 50). Regardless, Biswas et al. (2013) and Sivasankar et al. (2018) reported tintinnids as bioindicators in the wetlands and estuarine coastal waters of India depending on their abundance towards the upstream freshwater regions. In the South sector, Spirotrichea (87.5%) was largely represented by Strombidium conicum (54.3%), Euplotes crassus (11.5%), and T. tocantinensis (9.13%), whereas in the central sector, Spirotrichea was dominated by Euplotes sp. (22.5%) followed by Stenosomella sp. (16.5%) and Eutintinnus sp. (12%). Similarly, in the north sector, Spirotrichea (71.3%) > Heterotrichea (13.6%) > Oligohymenophorea (13%) were represented by Strobilidium sp., Fabrea salina, and Uronema sp., respectively.
The alpha diversity indices measured based on abundance data for the three sectors were presented in the form of box plots (Fig. 6). Only the species number (S) exhibited significant spatial differences between the sectors (P = 0.05). Seasonally, the species number/richness (S) (1–15, 7.17 ± 3.23), abundance (N) (96–25,546, 2242 ± 677 ind. l−1), and Margalef’s richness (d′) (0.17–1.91, 0.87 ± 0.42) did not change significantly. In contrast, the Pielou (J′) (0.44–0.96, 0.75 ± 0.14) and Shannon–Wiener evenness (H′) (1.0–2.21, 1.41 ± 0.08) showed significant differences between the seasons at P < 0.02 and P < 0.04, respectively. In general, species richness and evenness are used to estimate water quality status; i.e., less polluted areas are usually associated with high values of species richness and evenness, and vice versa (Jiang et al. 2011). In this study, the species richness and evenness were high in the south sector when compared with that in the other two sectors, suggesting the ecological importance of ciliates to assess water quality. Similar findings were reported from Daya Bay, China, by Wu et al. (2016) and Sikder et al. (2019). In addition to alpha diversity, beta and gamma diversity (total number of species, 29) were also investigated on ciliate species using beta dissimilarity (based on the Bray–Curtis dissimilarity) and beta diversity index, β. The β-dissimilarity index was a maximum of 65.42% in the south and central sectors (sectors/zones I and II) and a minimum of 62.57% in the central and north sectors (sectors/zones II and III). In contrast, the beta diversity index ranged from 0.84 (south and north sectors, sectors/zones I and III) to 0.86 (south and central sectors, sectors/zones I and II) (Fig. 7). The beta diversity measures revealed a gradient distribution of species turnover between the assemblages; however, the difference was not noticeable. The turnover rates may be due to various environmental factors such as the salinity gradients, nutrients, and chlorophyll-a. It is observed that the species richness patterns of plankton are mostly driven by the influence of environmental heterogeneity (Jackson and Blois 2015; Rakhesh et al. 2015).
Biotic and abiotic relationships
The correlation between the ciliate abundance and environmental data was examined through a multivariate biota–environment (BIOENV) analysis. The best combination (R = 0.406, P = 0.008) of environmental variables with ciliate abundance was temperature, total suspended matter, silicate, nitrite–nitrogen, and nitrate–nitrogen, and the second best combination was offered by Secchi disc transparency, total suspended matter, silicate, nitrite–nitrogen, and nitrate–nitrogen (R = 0.404, P = 0.008). Overall, total suspended matter, silicate, nitrite–nitrogen, and nitrate–nitrogen were the most common variables included in the correlation. Spearman’s correlation analysis (two-tailed and non-parametric) (Table 2) performed on select ciliate species (used for cluster analysis) and environmental data revealed that the overall dominant species i.e., Strombidium conicum, Strobilidium sp., Euplotes crassus, and Tintinnopsis tocantinensis, were positively correlated with Secchi disc transparency and negatively correlated with salinity and total suspended matter, whereas Fabrea salina showed a positive relationship with temperature and salinity.
The canonical correspondence analysis (CCA) in this study aimed at explaining the multiple linear relationships between the species and environmental variables (Table 3 and Fig. 8). Canonical axis 1 showed the highest eigenvalue of 0.365 representing the main environmental gradient in the study area. On this axis, positive correlation existed for water quality variables such as salinity, nitrite, nitrate, and silicate, represented the central sector, whereas ammonium and phosphate are separated the south sector with negatively correlated axis values. The distributions of temperature, dissolved oxygen, pH, and chlorophyll-a were roughly homogenous between the three sectors during the study period; consequently, the correlations did not vary much. The variance of species–environment relationship was observed high at axis 1 (40.4%) supported the ciliate species such as Strombidium acutum, Euplotes sp., Eutintinnus sp., Tintinnopsis cylindrica, Strobilidium sp., Stenosomella sp., and Strombidium oculatum signifying the central sector. In contrast, the distributions of Euplotes crassus, Tintinnopsis tocantinensis, Prorodon sp., Strombidium elongatum, Strombidium conicum, and Strombidium sp. with high correlation values characterized the south sector of Pulicat Lake. The classification of ciliate assemblages revealed through the Bray–Curtis analysis was superimposed on CCA. Overall, the first two axes together explained more than the two-thirds of the spatial variation in ciliate community, which was explained by the measured variables (i.e., 62.8% of the total 89.9% constrained variability). Mieczan (2008) and Liu et al. (2016) reported similar results on the CCA of ciliate and environmental relationship from Lake Piaseczno (84% variance for all four axes) and Maowei Sea (72% for the first two axes), respectively. The influence of canonical axis 1 showed more strength (F = 1.147, P = 0.034) on the ciliate distribution, which was justified through the Monte Carlo permutation test (499 permutations under the reduced model). The trace static test of significance of all canonical axes revealed an overall relationship (F = 2.859, P = 0.006) between the ciliate species and environmental variables measured.
The biotic–abiotic relationship study conferred that the variations in physicochemical parameters play an important role in the abundance, distribution, and diversity of ciliates (Amblard et al. 1993; Kchaou et al. 2009; Wu et al. 2016). Several investigations demonstrated that the community structure and ecological patterns of ciliates mainly depend on water quality (Zhang et al. 2012; Jiang et al. 2013; Wang and Xu 2015; Anjusha et al. 2018). The results emphasize that salinity, chlorophyll-a, and dissolved nutrients are the key factors that control the ciliate composition particularly the dominance of Spirotrichea. Several authors also reported the dominance of choreotrich, hypotrich, and oligotrich ciliates in terms of species number, abundance, and biomass (Kchaou et al. 2009; Santoferrara and Alder 2009; Jiang et al. 2011, 2012). The present findings are consistent with the studies carried out elsewhere, e.g., Kuwait Bay (Al-Yamani et al. 2019), St. Lucia estuarine lake (Nche-Fambo et al. 2016), Daya Bay (Wu et al. 2016), Gulf of Gabes (Kchaou et al. 2009), solar salt works of Sfax, Tunisia (Elloumi et al. 2006), and the Baltic Sea (Johansson et al. 2004).
In conclusion, the present study offers an account of the ecology and distribution of ciliates with respect to spatial and seasonal changes in water quality of Pulicat Lake, situated on the east coast of India. The spirotrichous ciliates mostly dominated by oligotrichs were concentrated in the brackish and nutrient-rich areas of the lake (central sector). The indicator species, Fabrea salina, exhibited a positive correlation with temperature indicating that the existence of high saline conditions in Pulicat Lake (Padma and Periakali 1999) may be due to the temperature-mediated evaporation (Sima et al. 2013). Apart from salinity, food availability (chlorophyll-a) is another key factor controlling the distribution of ciliate species (Esteban and Finlay 2003; Elloumi et al. 2006; Pandey et al. 2008). Altogether, the biotic–abiotic interactions in the present study suggest that the community organization of planktonic ciliates accurately reflects the brackish to hypersaline condition in Pulicat Lake and ciliates can be considered to be bioindicators for assessing the water quality.
References
Al-Yamani F, Madhusoodhanan R, Skryabin V, Al-Said T (2019) The response of microzooplankton (tintinnid) community to salinity related environmental changes in a hypersaline marine system in the northwestern Arabian Gulf. Deep-Sea Res II Top Stud Oceanogr. https://doi.org/10.1016/j.dsr2.2019.02.005
Amblard C, Sime-Ngando T, Rachiq S, Bourdier G (1993) Importance of ciliated protozoa in relation to the bacterial and phytoplanktonic biomass in an oligo-mesotrophic lake, during the spring diatom bloom. Aquat Sci 55(1):1–9
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58(3):626–639
Anderson M, Gorley RNRN, Clarke K, Anderson MJ, Gorley RN, Clarke KR, Andersom MJ (2008) PERMANOVA+ for PRIMER. Guide to software and statistical methods. Primer-E, Plymouth, p 214
Anjusha A, Jyothibabu R, Jagadeesan L (2018) Response of microzooplankton community to the hydrographical transformations in the coastal waters off Kochi, along the southwest coast of India. Cont Shelf Res 167:111–124
Azam F, Fenchel T, Field J, Gray J, Meyer-Reil L, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10(3):257–263
Balkis N (2004) Tintinnids (Protozoa: Ciliophora) of the Büyükçekmece Bay in the Sea of Marmara. Sci Mar 68(1):33–44
Biswas SN, Godhantaraman N, Rakshit D, Sarkar SK (2013) Community composition, abundance, biomass and production rates of Tintinnids (Ciliata: Protozoa) in the coastal regions of Sundarban Mangrove wetland, India. Indian J Geo-Mar Sci 42(2):163–173
Brown S, Landry M, Christensen S, Garrison D, Gowing M, Bidigare R, Campbell L (2002) Microbial community dynamics and taxon-specific phytoplankton production in the Arabian Sea during the 1995 monsoon seasons. Deep-Sea Res II Top Stud Oceanogr 49(12):2345–2376
Clarke K, Gorley R (2006) PRIMER v6: user manual/tutorial. Primer-E, Plymouth, p 192
Clarke KR, Green RH (1988) Statistical design and analysis for a “biological effects” study. Mar Ecol Prog Ser 46(1):213–226
Clark KR, Warwick RM (2001) Change in marine communities: an approach to statistical and interpretation. Plymouth Marine Laboratory, Plymouth
Corliss JO (1973) Protozoan ecology: a note on its current status. Am Zool 13(1):145–148
Corliss JO (1979) The ciliated protozoa. Characterization, classification and guide to literature, 2nd edn. Pergamon Press. ISBN: 9781483154176, Oxford, p 472
Coulthard S (2008) Adapting to environmental change in artisanal fisheries—insights from a South Indian lagoon. Glob Environ Chang 18(3):479–489
Curds CR (1982) British and other freshwater ciliate protozoa. Published for The Linnean Society of London and The Estuarine and Brackish Water-Sciences Association, Cambridge University Press, Cambridge
Dhinamala K, Pushpalatha M, Samuel T, Raveen R (2015) Spatial and temporal variations in the water quality parameters of Pulicat Lake, Tamil Nadu, India. Int J Fish Aquat Stud 3(2):255–259
Elloumi J, Carrias J-F, Ayadi H, Sime-Ngando T, Boukhris M, Bouaïn A (2006) Composition and distribution of planktonic ciliates from ponds of different salinity in the solar saltwork of Sfax, Tunisia. Estuar Coast Shelf Sci 67(1–2):21–29
Esteban GF, Finlay BJ (2003) Cryptic freshwater ciliates in a hypersaline lagoon. Protist 154(3–4):411–418
Fenchel TOM (1980) Relation between particle size selection and clearance in suspension-feeding ciliates. Limnol Oceanogr 25(4):733–738
Fenchel T, Jørgensen BB (1977) Detritus food chains of aquatic ecosystems: the role of bacteria. In: Advances in microbial ecology. Springer, Boston, MA, pp 1–58
Finlay BJ, Curds CR, Bamforth SS, Bafort JM (1987) Ciliated protozoa and other microorganisms from two African soda lakes (Lake Nakuru and Lake Simbi, Kenya). Arch Protistenkd 133(1–2):81–91
Ganguly D, Patra S, Muduli PR, Vardhan KV, Abhilash K, Robin R, Subramanian B (2015) Influence of nutrient input on the trophic state of a tropical brackish water lagoon. J Earth Syst Sci 124(5):1005–1017
Gao F, Warren A, Zhang Q, Gong J, Miao M, Sun P, Xu D, Huang J, Yi Z, Song W (2016) The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Sci Rep 6:24874
Giordano M, Davis JS, Bowes G (1994) Organic carbon release by Dunaliella Salina (Chlorophyta) under different growth conditions of CO2, nitrogen, and salinity 1. J Phycol 30(2):249–257
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis. John Wiley & Sons, New York, p 600
Hu X (2014) Ciliates in extreme environments. J Eukaryot Microbiol 61(4):410–418
Jackson ST, Blois JL (2015) Community ecology in a changing environment: perspectives from the Quaternary. Proc Natl Acad Sci 112(16):4915–4921
Jiang Y, Xu H, Hu X, Zhu M, Al-Rasheid KA, Warren A (2011) An approach to analyzing spatial patterns of planktonic ciliate communities for monitoring water quality in Jiaozhou Bay, northern China. Mar Pollut Bull 62(2):227–235
Jiang Y, Zhang W, Zhu M, Al-Rasheid KA, Xu H (2012) Are non-loricate ciliates a primary contributor to ecological pattern of planktonic ciliate communities? A case study in Jiaozhou Bay, northern China. J Mar Biol Assoc U K 92(6):1301–1308
Jiang Y, Xu H, Hu X, Warren A, Song W (2013) Functional groups of marine ciliated protozoa and their relationships to water quality. Environ Sci Pollut Res 20:5272–5280
Johansson M, Gorokhova E, Larsson U (2004) Annual variability in ciliate community structure, potential prey and predators in the open northern Baltic Sea proper. J Plankton Res 26(1):67–80
Jyothibabu R, Madhu N, Jayalakshmi K, Balachandran K, Shiyas C, Martin G, Nair K (2006) Impact of freshwater influx on microzooplankton mediated food web in a tropical estuary (Cochin backwaters–India). Estuar Coast Shelf Sci 69(3–4):505–518
Kahan D (1969) The fauna of hot springs: With 1 table in the text. Internationale Vereinigung für theoretische und angewandte Limnologie. Verhandlungen 17(2):811–816
Kahan D (1972) Cyclidium citrullus Cohn, a ciliate from the hot springs of Tiberias (Israel). J Protozool 19(4):593–597
Kanuri VV, Muduli PR, Robin R, Kumar BC, Lovaraju A, Ganguly D, Patra S, Rao GN, Raman A, Subramanian B (2013) Plankton metabolic processes and its significance on dissolved organic carbon pool in a tropical brackish water lagoon. Cont Shelf Res 61:52–61
Kchaou N, Elloumi J, Drira Z, Hamza A, Ayadi H, Bouain A, Aleya L (2009) Distribution of ciliates in relation to environmental factors along the coastline of the Gulf of Gabes, Tunisia. Estuar Coast Shelf Sci 83(4):414–424
Kofoid C, Campbell A (1929) A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with description of the suborder Tintinnoinea, with description of new species principally from Agassiz expedition to the eastern tropical Pacific, 1904–1905. Univ Calif Publ Zool 34:1–403
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence–absence data. J Anim Ecol 72(3):367–382
Landry MR, Calbet A (2004) Microzooplankton production in the oceans. ICES J Mar Sci 61(4):501–507
Lei Y, Xu K, Choi JK, Hong HP, Wickham SA (2009) Community structure and seasonal dynamics of planktonic ciliates along salinity gradients. Eur J Protistol 45(4):305–319
Liu H, Chen M, Shen P, Huang H, Dai M, Qi Z (2016) A first description of ciliate assemblages in a subtropical, eutrophic bay, South China Sea: species assemblage and environmental correlates-ciliate variation in a subtropical bay. Aquat Living Resour 29(3):304
Lynn D (2008) The ciliated protozoa: characterization, classification, and guide to the literature. Springer Science and Business Media, New York, pp 1–605
Mieczan T (2008) Diversity and vertical distribution of planktonic ciliates in a stratified mesotrophic lake: relationship to environmental conditions. Oceanol Hydrobiol Stud 37(1):83–95
Modassir Y, Ansari A (2011) Plankton community of the hypersaline salterns of Goa, India. In Biol Forum 3:78–81
Modenutti BE, Balseiro EG, Queimalinos CP (2000) Ciliate community structure in two South Andean lakes: the effect of lake water on Ophrydium naumanni distribution. Aquat Microb Ecol 21(3):299–307
Muduli PR, Kanuri VV, Robin R, Kumar BC, Patra S, Raman A, Rao GN, Subramanian B (2013) Distribution of dissolved inorganic carbon and net ecosystem production in a tropical brackish water lagoon, India. Cont Shelf Res 64:75–87
Muñoz-Barbosa A, Gaxiola-Castro G, Segovia-Zavala J (1991) Temporal variability of primary productivity, chlorophyll and seston in Bahía de los Ángeles, Gulf of California. Cienc Mar 17(4):47–68
Nche-Fambo FA, Tirok K, Scharler UM (2016) Hypersaline conditions cause distinct ciliate community structure in a South African estuarine lake system. J Plankton Res 38(4):878–887
Noland LE, Gojdics M (1967) Ecology of free-living protozoa. Res Protozool 2:215–266
Padma S, Periakali P (1999) Physico-chemical and geochemical studies in Pulicat lake, east coast of India. Indian J Mar Sci 28:434–439
Pandey BD, Yeragi SG, Reddy AK, Hadiwara A (2008) Life strategies and aquacultural usabilities of a hypersaline ciliate, Fabrea salina. Curr Sci 97(3):307–309
Pedrós-Alió C, Calderón-Paz JI, MacLean MH, Medina G, Marrasé C, Gasol JM, Guixa-Boixereu N (2000) The microbial food web along salinity gradients. FEMS Microbiol Ecol 32(2):143–155
Pérez-Ruzafa A, De Pascalis F, Ghezzo M, Quispe-Becerra JI, Hernández-García R, Muñoz I, Vergara C, Pérez-Ruzafa IM, Umgiesser G, Marcos C (2019) Connectivity between coastal lagoons and sea: asymmetrical effects on assemblages’ and populations’ structure. Estuar Coast Shelf Sci 216:171–186
Pineda A, Peláez Ó, Dias JD, Segovia BT, Bonecker CC, Machado Velho LF, Rodrigues LC (2019) The El Niño Southern Oscillation (ENSO) is the main source of variation for the gamma diversity of plankton communities in subtropical shallow lakes. Aquat Sci 81:49–15. https://doi.org/10.1007/s00027-019-0646-z
Putt M, Stoecker DK (1989) An experimentally determined carbon: volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnol Oceanogr 34(6):1097–1103
Queimaliños CP, Modenutti BE, Balseiro EG (1999) Symbiotic association of the ciliate Ophrydium naumanni with Chlorella causing a deep chlorophyll a maximum in an oligotrophic South Andes lake. J Plankton Res 21(1):167–178
Radhakrishnan S (1976) Some aspects of the distribution and seasonal abundance of macrophytic flora in the brackish lake Pulicat, India. Aquatic weeds in south-east Asia. W. Junk, The Hague, pp 107–117
Rakhesh M, Madhavirani KSVKS, Kumar BC, Raman AV, Kalavati C, Rao YP, Subramanian BR (2015) Trophic–salinity gradients and environmental redundancy resolve mesozooplankton dynamics in a large tropical coastal lagoon. Reg Stud Mar Sci 1:72–84
Sahu BK, Srichandan S, Panigrahy R (2016) A preliminary study on the microzooplankton of Chilika Lake, a brackish water lagoon on the east coast of India. Environ Monit Assess 188(1):69. https://doi.org/10.1007/s10661-015-5062-9
Sanders RW, Wickham SA (1993) Planktonic protozoa and metazoa: predation, food quality and population control. Mar Microbial Food Webs 7(2):197–223
Santoferrara LF, Alder VA (2009) Morphological variability, spatial distribution and abundance of Helicostomella species (Ciliophora: Tintinnina) in relation to environmental factors (Argentine shelf; 40-55 S). Sci Mar 73(4):701–716
Schmoker C, Hernández-León S, Calbet A (2013) Microzooplankton grazing in the oceans: impacts, data variability, knowledge gaps and future directions. J Plankton Res 35(4):691–706
Shadrin N, Anufriieva E (2018) Ecosystems of hypersaline waters: structure and trophic relations. Zh Obshch Biol 79(6):418–427
Sikder MNA, Al MA, Xu G, Hu G, Xu H (2019) Spatial variations in trophic-functional patterns of periphytic ciliates and indications to water quality in coastal waters of the Yellow Sea. Environ Sci Pollut Res 26(3):2592–2602
Sima S, Ahmadalipour A, Tajrishy M (2013) Mapping surface temperature in a hyper-saline lake and investigating the effect of temperature distribution on the lake evaporation. Remote Sens Environ 136:374–385
Sivasankar R, Ezhilarasan P, Kumar PS, Naidu S, Rao G, Kanuri VV, Rao VR, Ramu K (2018) Loricate ciliates as an indicator of eutrophication status in the estuarine and coastal waters. Mar Pollut Bull 129(1):207–211
Strickland JD, Parsons TR (1972) A practical handbook of seawater analysis. 2nd ed. Bull Fish Res Bd Can 167:1–310
Ter Braak CJ (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67(5):1167–1179
Ter Braak CJF, Smilauer P (2002) CANOCO Reference manual and CanoDraw for Windows User’s guide: Software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
Ter Braak CJ, Verdonschot FM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57(3):255–289
Unesco TD (1978) Intergovernmental conference on environmental education. Final report. UNESCO ED/MD/49, Paris
Urrutxurt I, Orive E, de la Sota A (2003) Seasonal dynamics of ciliated protozoa and their potential food in an eutrophic estuary (Bay of Biscay). Estuar Coast Shelf Sci 57:1169–1182
Utermöhl H (1958) Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Verh Int Ver Theor Angew Limnol Mitt 9(1):1–38
Vadrucci M, Cabrini M, Basset A (2007) Biovolume determination of phytoplankton guilds in transitional water ecosystems of Mediterranean Ecoregion. Transit Waters Bull 1(2):83–102
Venice System (1959) Final resolution. The Venice System for the classification of marine waters according to salinity. 8-14 April 1958 Venice, Italy. In: Ancona, D. (Ed.), Symposium on the classification of brackish waters. Archives oceanography and limnology 11: 243–248
Verity PG, Langdon C (1984) Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. J Plankton Res 6:859–868
Wang Q, Xu H (2015) Colonization dynamics in the tropical-functional patterns of biofilm-dwelling ciliates using two methods in coastal waters. J Mar Biol Assoc UK 95:681–689
Whittaker RH (1960) Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr 30(3):279–338
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21(2–3):213–251
Wilson MV, Shmida A (1984) Measuring beta diversity with presence-absence data. J Ecol 72(3):1055–1064
Wu F, Huang J, Dai M, Liu H, Huang H (2016) Using ciliates to monitor different aquatic environments in Daya Bay, South China Sea. Can J Zool 94(4):265–273
Xu H, Jiang Y, Al-Rasheid KA, Al-Farraj SA, Song W (2011) Application of an indicator based on taxonomic relatedness of ciliated protozoan assemblages for marine environmental assessment. Environ Sci Pollut Res 18(7):1213–1221
Zhang W, Xu H, Jiang Y, Zhu M, Al-Reshaid KAS (2012) Colonization dynamics in trophic-functional structure of periphytic protist communities in coastal waters. Mar Biol 159:735–748
Acknowledgments
The authors thank the Secretary, Ministry of Earth Sciences (MoES), Government of India; and Director, National Centre for Coastal Research (NCCR), MoES, Government of India, for the financial support and facilities during the study period. This study is a part of the MoES-NCCR comprehensive project on “Ecosystem Based Services of Pulicat Lake.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Vedula VSS Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basuri, C.K., Pazhaniyappan, E., Munnooru, K. et al. Composition and distribution of planktonic ciliates with indications to water quality in a shallow hypersaline lagoon (Pulicat Lake, India). Environ Sci Pollut Res 27, 18303–18316 (2020). https://doi.org/10.1007/s11356-020-08177-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08177-6