Abstract
This study was carried out to evaluate the effects of using superzist probiotic (a mixture of Lactobacillus spp., Bacillus subtilis, and Bifidobacterium bifidum) on growth performance and hematological and some immunological indices in fingerling Acipenser baerii. In total, 240 Acipenser baerii fingerlings with mean weight 10.5 ± 0.14 g−1 were stocked in 12 tanks, 20 per each tank and each treatment in triplicate. Diets were prepared by spraying slowly the mixture of 50 ml saline serum with 100, 200, and 300 mg probiotic powder per 1-kg diet to make the concentrations 1 × 106, 2 × 106, and 3 × 106 CFU g−1 of probiotic bacteria in diet. Results showed that there was a significant increase in the final weight, weight gain (WG), percentage weight gain, condition factor (CF), and specific growth rate (SGR) in fish fed PB300 treatment compared with the control at the 8th week (p < 0.05); also, the lowest feed conversion ratio (FCR) belonged to fish fed PB300 that showed a significant difference compared with fish fed control diet (p < 0.05). Except for neutrophil, lymphocyte, and hematocrit (p < 0.05), values of all hematological parameters of fish fed different concentrations of probiotic diet did not differ from the values of fish fed the control diet (p > 0.05), but there was a significant increase in lysozyme and IgM fish fed probiotic (PB300) and compliment (ACH50) (PB200 and PB300) compared with control treatment during 56 days. Therefore, results indicated that 300 mg kg−1 probiotic (Lactobacillus and Bifidobacterium) can be used as a proper probiotic in sturgeon aquaculture to enhance fish health and growth performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Siberian sturgeon, Acipenser baerii is one of the most important sturgeon species that is used for caviar and meat production in sturgeon aquaculture (Bronzi et al. 2011). However, high-density rearing seems to lead to poor physiological environment that adversely affects fish health and growth rate in fingerlings and Juvenile periods (Yazdani et al. 2010) and therefore lead to a high degree of mortality. In the past decades, heavy reliance on vaccines and antibiotics to combat these diseases is intensive culture (Broch et al. 2015), but adverse effects on the use of antibiotics to control diseases have created some problems. These effects include accumulation of antibiotics in the tissue, fish immune-suppression (Tukmechi et al. 2007; Nayak et al. 2007; El-Haroun et al. 2006), and ecological threat to coastal areas that caused by heavily exploited for industrial cultivation of fish and shell fish (Gildberg et al. 1997). The probiotic is alive or dead or is a component of a microbial cell that when included to the feed or to the rearing water has benefits for the host by improving its microbial balance of digestive tract or microbial balance of the ambient environment (Merrifield et al. 2010C); in addition, probiotic has beneficial effect on diseaces controls via enhancement of the non-specific innate immunity system (Gatesoupe 2008) and improve digestibility (Cruz et al. 2012) in fish that leads to the increase growth of the cultured organisms (Balcazar et al. 2006).
During the last decade, an improved understanding arose of the importance of commensal microbiota in the fish intestine and production of indigenous probiotics (Burr et al. 2005). Askarian et al. (2009) isolated two lactic acid bacteria (LAB) species, Lactobacillus curvatus and Leuconostoc mesenteroides, from the GI tract of Huso huso and A. baerii, respectively. In other study, Ghanbari et al. (2009) carried out isolation and characterization of cultivable allochthonous lactobacilli in this species. Also, Hoseinifar et al. (2016) addressed the effects of Lactococcus lactis JF831150 administration on intestinal microbiota (TVC and presumptive LAB levels) of A. baerii in a 56-day feeding trial, but little information is available on the use of commercial probiotics in sturgeon studies. For example, some studies have evaluated the effects of probiotic on sturgeon growth performance, physiology, and health status (Faramarzi et al. 2011, 2012a, b; Iranshahi et al. 2011) and modulation of the intestinal microbiota (Askarian et al. 2011). Therefore, the present study was undertaken to evaluate the potential effect of the dietary administration of the commercial aquaculture probiotic (superzist, Varena Co., Iran) Bacillus subtilis, Lactobacillus acidophilus, Lactobacillus delbrueckii, Lactobacillus rhamnosus, Lactobacillus plantarum, and Bifidobacterium bifidum on growth parameters, blood, biochemical, and immune indices in this species.
Materials and methods
Probiotics and preparation of diets
The probiotic powder was prepared from Zistyar Varena Co. (Rasht, Gilan Province, Iran) with 10 × 1010 CFU mixture of Bacillus subtilis, Lactobacillus acidophilus, Lactobacillus delbrueckii, Lactobacillus rhamnosus, Lactobacillus plantarum, and Bifidobacterium bifidum. A commercial diet composed of 47% crude protein, 11% lipid, 1.16% phosphorus, 11% moisture, 10% ash, and 3% fiber was used as a control diet (1.9-mm pellets, BioMar, France). Diets were prepared by spraying slowly the mixture of 50 ml saline serum with 100, 200, and 300 mg probiotic powder per 1-kg diet to make the concentrations 1 × 106, 2 × 106, and 3 × 106 CFU g−1 of probiotic bacteria in diet except for the control diet and named PR100, PR200, and PR300.
Fish and rearing conditions
The fish were obtained from the breeding of 5 Siberian sturgeon breeders (3 female and 2 male fish) in International Sturgeon Research Institute. One thousand larvae absorbed yolk sac were transferred to 15 fiberglass tanks (1.5 × 0.5 × 0.3 mm3). After larva transferring, feeding was done under the same culture conditions with Artemia (stage Instar I) and Daphnia (for 20 days); then for another 15 days, larvae were fed by dry diet mixed with different percentages of Gammarus for adaptation for commercial diet. During the adaptation period, the fish fed commercial diet well and had low mortality rate (3 to 5%). Fingerlings adapted to experimental diets for 7 days. During the adaptation period, the feed efficiency in fish fed by dry diet was high with low mortality rate (survival, 95%; and mortality, 3 to 5%). Also, fingerlings had fast swimming, had no lesions in the body morphologically, and fed dry diet well. After adaptation, a total of 240 fingerlings (average weight 10.5 ± 0.14 g−1) were stocked into 12 circular fiberglass tanks (diameter 200 cm, water volume 2000 l, water flow 4.75 l min−1) and fed with diet (1.9-mm pellets, BioMar, France) supplemented with varying inclusions of probiotic (100, 200, and 300 g kg−1) (PR100, PR200, and PR300) for 8 weeks. Fish were fed twice daily by hand in the rate of 3% of body weight for 8, 13, and 22 h. Each of 2 weeks, the weights of each experimental fish were determined using a digital balancer (Mahak, Iran). Water quality was monitored daily and was within acceptable limits throughout the experiment. Dissolved oxygen concentrations, temperature, and pH were ranged 6.0–6.5 mg/l−1, 20–23 °C, and 6.5–6.8, respectively. Growth performance of the A. baerii was calculated using the following formula (Hung et al. 1989):
where PI = Feed intake × % of protein in diet
Blood index assays and total bacterial count in the intestine and food
At the end of trial feeding, fish were fasted for 24 h immediately prior to blood sampling and 30% of fish per each tank were randomly chosen and anaesthetized with clove powder (150 mg l−1) (Hallajian et al. 2011). The blood samples were collected through a syringe by caudal vein and stored in non-heparinized tubes. For biochemical assays, blood samples were immediately centrifuged (3000 g, 10 min−1) at room temperature and then serum was separated and stored at − 20 °C until analysis (Anderson et al. 1997). The hematological indices included white blood cell (WBC), red blood cells (RBC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were measured by a spectrophotometer at 450 nm (UV/Vis-6505 N, Junway Company, England) using commercial kits (Pars Azmun Co. Ltd., Tehran, Iran). Lysozyme and compliment were measured by AutoAnalyzer Technicon (R.A.1000, Junway Company, England) using commercial kits (Pars Azmun Co. Model ISC and ILT., Tehran, Iran) described by Ellis 1990, and IgM were determined by the nephelometric method using the Binding Site Kit (Yousefi Jourdehi et al. 2014).
Also for determination of viability and counting of bacteria in food and the intestine, at first, 10 g of enrichment food and the intestine were weighted separately; contents of the intestine were washed three times with physiological serum. Then, the weighted material was transferred to sterile glass containers and physiological serum was added to give the desired dilution. In the following, 100 μl of solution was cultured on a culture medium of MRS (Man Rogson Sharp) bacteria, respectively. Plates were incubated at 30 °C for 96 h in anaerobic conditions, and after incubation, bacteria were counted (Merrifield et al. 2011).
Statistical analysis
Statistical analyses were conducted using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Duncan’s test was used for data analysis after checking the normality of data and homogeneity of variance (Table 1). Mean values were considered significantly different at p < 0.05. Data are presented as mean values ± SD.
Results
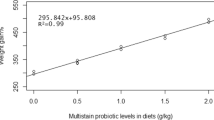
The growth performance of Acipenser baerii fed diets containing various probiotic levels is shown in Table 2. After 8-week feeding, fish fed the basal diet had a significant lower growth performance than those fed diet 4 (PB300). Fish fed with PB300 have the highest final weight (FW), weight gain (WG), and percentage weight gain (PWG). Also, the fish treated with PR300 were more interested in food than the control. Increase of commercial probiotic in diet leads to an increase of protein efficiency ratio. The highest parameter showed belonged to the fish fed with diet containing 300 mg kg−1 probiotic. Also, specific growth rate (SGR) in fish fed with dietary PB100 and PB300 were significantly higher than that in fish fed basal diet and PB200. Feed conversion ratio (FCR) in fish fed with basal diet showed no significant difference (p > 0.05) from that in those fed diets PB100 and PB200, but significantly different (p < 0.05) from that in fish fed PB300 (300 mg kg−1 probiotic) (Table 2). The best of protein efficiency ratio of fingerling Acipenser baerii during 8-week feeding belonged to diet 4 (PB300) (p < 0.05).
Blood samples collected at the end of the feeding trial during the experiment indicated that the hematocrit in fish fed with control diet has a significant difference (p < 0.05) compared with that in fish fed PB300 (300 mg kg−1) but no significant difference (p > 0.05) showed in PB100 and PB200 groups. There was no significant difference in WBC of fish fed diets PB0, PB100, PB200, and PB300. The red blood cell count (RBC) showed no significant difference (p > 0.05) in fish fed PB0, PB100, PB200, and PB300; however, with increasing probiotic in diet, WBC and RBC showed an increasing trend. The highest hemoglobin was measured in fish fed diet PB300, and the lowest was observed in fish fed with control diet without significant difference (p > 0.05). There were no significant differences (p > 0.05) in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), but the percentage of neutrophil in all fish fed had varying concentrations. In the other hand, at the end of 8-week fish feeding, the highest percentage of neutrophil was recorded for fish fed with PB300 and showed a significant difference in fish fed PB0, but no significant difference in fish fed PB100 and PB200. The lowest lymphocyte was recorded in fish fed with PB0 and the highest was recorded in fish fed PB300 (p < 0.05). RBC showed no significant difference in all diets (p > 0.05). There was no significant difference (p < 0.05) in the monocyte of all fish fed varying quantity of probiotic. At the end of the experimental period (8 weeks), the fish fed PB300 (300 mg kg−1 probiotic) had better secretion of lysozyme, compliment (ACH50), IgM and was higher than the fish fed the control diet (p < 0.05), whereas fish fed diets PB0, PB100, and PB200 had no significant difference in lysozyme, compliment (ACH50), and IgM levels (p > 0.05) (Table 2).
Mean values were considered significantly different at p < 0.05. Means within column with different superscript letters are significantly different (± SD) (p < 0.05)
The results of total bacterial count in the H. huso intestine fed with PB0, PB100, PB200, and PB300 were calculated which the highest bacterial count was observed in the intestine of fish fed the PB300 that showed a statistically significant difference with the bacterial intestine fed the PB200 and PB100 diets (p < 0.05). Also, the approximate total bacterial count in diets was being CFU/g−1 (Table 3).
Mean values were considered significantly different at p < 0.05. Means within column with different superscript letters are significantly different (± SD) (p < 0.05)
Discussion
All fish were well fed with the given feed and no mortality was observed; also, the fish treated with PB300 were more interested in food than the control and had fast swimming. At the end of the experimental period (8 weeks), the fish fed 300 mg kg−1 probiotic grew better than the fish fed the control diet, whereas the final body weight, weight gain, percentage weight gain, and specific growth rate in fish fed diet PB300 were higher significantly (p < 0.05) than those in the fish fed diet control, but the lowest feed conversion ratio (FCR) was achieved in fish fed diet PB300 that indicated an increase of probiotic in diet leads to that growth rate and feed efficiency ratio was improved. Results of our study were in agreement with numerous studies that have shown the application of probiotics can improve feed conversion, growth rates, and weight gain of Salmonidae (Merrifield et al. 2010b) and available information in Acipenseridae (Hoseinifar et al. 2016). Application of B. subtilis and Bacillus licheniformis resulted in a significant improvement of Oncorhynchus mykiss in feed conversion ratio (FCR), specific growth rate (SGR), weight gain, and protein efficiency ratio (PER) after 2-month feeding trial (Bagheri et al. 2008). Similar results were reported by Askarian et al. (2011) after feeding A. persicus and H. huso with Chironomidae incorporated with Lactobacillus curvatus and Leuconostoc mesenteroides for 50 days. Results of other investigations about probiotic indicated that these organisms are promoting growth rate and increasing the efficiency of feed conversion (Young-Hyo et al. 2001) by competitive adhesion to the digestive tract wall to prevent colonization with pathogenic microorganisms (Ibrahem 2015) and enhancement of nutrition of host species through the production of supplemental digestive enzymes (Kesarcodi-Watson et al. 2008). Askarian et al. (2011) and Soltani et al. (2015) attributed growth improvement in A. persicus and H. huso on some vitamins like vitamin K, and B12, as well as Lara-Flores et al. (2003) indicated the role of probiotic that helps to improve feed utility and digestion of proteins and increase the digestibility of feed that led to increase of growth and feed efficiency in Oreochromis niloticus. Unfortunately, we did not measure the enzyme digestion of A. baerii fed different levels of probiotic superzist, but possibility seems that suitable intestinal microflora produced by probiotic (in diet of PB200 and PB300) leads to a good digestibility and a high growth performance (Adineh et al. 2013) in juvenile A. baerii.
RBC and hemoglobin level were improved in PB300 compared with those in control diet (PB0) (p > 0.05), but hematocrit of fish fed the control diet was significantly lower than that of fish fed PB300 (p < 0.05). Hemoglobin and erythrocyte count are good indicators for oxygen transportation capacity of fish (Lamas et al. 1994). On the other hand, increase of hemoglobin and hematocrit plays an important role for improving the well-being of fish and consequently enhancing the immunity and growth of fish (Talpur and Ikhwanuddin 2012). At a similar manner, WBC count of fish was increased (p > 0.05). But, the highest percentage of neutrophil and lymphocyte was recorded for fish fed with PB300 that was significantly higher than the percentage of neutrophil and lymphocyte of fish fed with PB0 (p < 0.05). Some researchers believed that higher counts (%) of phagocytic cells (neutrophils and monocytes) and lymphocytes are also indicative of infection in fish (Mohapatra et al. 2012); however, others suggested that probiotics actively stimulate the proliferation of B lymphocytes in fish such as O. mykiss (Panigrahi et al. 2004), L. rohita (Nayak et al. 2007), O. niloticus (Pirarat et al. 2011), and A. persicus (Soltani et al. 2015) and are a multiplier factor in leukocytes (neutrophils) and NK cells in O. mykiss and L. rohita that leads to enhancing innate immune responses (Irianto and Austin 2002; Nikoskelainen et al. 2001; Kumar et al. 2008). In this study, there was a significant increase in lysozyme and IgM in fish fed probiotic (PR300) and compliment (ACH50) (PR200 and PR300) compared with control treatment (p < 0.05) that supported the hypothesis that probiotic can modulate the non-specific immune responses and could increase the threshold of fish on disease and high density (Balcazar et al. 2006; Gatesoupe 2008).
Lysozyme, one of the important bactericidal enzymes of innate immunity, is an indispensable tool of fish to fight against infectious agents (Lindsay 1986). Probiotics either single or in combination are found to trigger the lysozyme level in teleost fish (Ibrahem 2015). The enhancement of lysozyme level was recorded by various types of probiotics in O. mykiss (Panigrahi et al. 2004; Kim and Austin 2006), Miichthys miiuy (Song et al. 2006), and A. persicus (Soltani et al. 2015), respectively. In the teleost fish, compliment system is a component of the non-specific immune response, plays a key role in adaptive immune responses, involves in chemotaxis, opsonization, phagocytosis, and degradation of pathogens, and has effector mechanisms like direct killing of microorganisms by lysis (Ellis 1999). Probiotics can enhance the natural complement activity of teleost fish (Ellis 1999; Panigrahi et al. 2007). Similar results were reported in O. mykiss (Panigrahi et al. 2005), Epinephelus coioides (Son et al. 2009), Oplegnathus fasciatus (Harikrishnan et al. 2010), Rachycentron canadum (Geng et al. 2011), and A. persicus (Soltani et al. 2015). Also, the result of Soltani et al. (2015) showed an increase in the level of total IgM in the treated A. persicus by LAB compared with control. Also, stimulated lymphocyte population for IgM production as already reported by other researchers in Sparus aurata (Salinas et al. 2008) and orange-spotted grouper (Epinephelus coioides) (Sun et al. 2010) is similar to our results.
Finally, we concluded that adding 300 mg kg−1 superzist probiotic to diet had benefit effects on growth rate and some hematological and immunological indices in A. baerii, but further researches must focus on the effect of superzist probiotic on gut microflora, digestive enzymes, and challenge tests in A. baerii at different ages.
References
Adineh H, Jafaryan H, Sahandi J, Alizadeh M (2013) Effect of Bacillus spp. probiotic on growth and feeding performance of rainbow trout, Oncorhynchus mykiss larvae. Bulg J Veter Med 16(1):29–36
Anderson WG, McKinley RS, Colavecchia M (1997) The use of clove oil as an anaesthetic for rainbow trout and its effect on swimming performance. N Am J Fish Manag 17:301–307
Askarian F, Kousha A, Ringo E (2009) Isolation of lactic acid bacteria from the gastrointestinal tract of Beluga, Huso huso and Persian sturgeon, Acipenser persicus. J Appl Ichthyol 25:91–94
Askarian F, Kousha A, Salma W, Ringo E (2011) The effect of lactic acid bacteria administration on growth, digestive enzyme activity and gut microbiota in Persian sturgeon, Acipenser persicus and beluga, Huso huso fry. Aquacult Nut 17:488–497
Bagheri T, Hedayati SA, Yavari V, Alizadeh M, Farzanfar A (2008) Growth, survival and gut microbial load of rainbow trout, Oncorhynchus mykiss, fry given diet supplemented with probiotic during the two months of first feeding. Turk J Fish Aquat Sci 8:43–48
Balcazar JL, De Blas I, Zarzuela-Ruiz I, Cunningham D, Vendrell D, Mu Zquiz JL (2006) The role of probiotics in aquaculture (review). Vet Microbiol 114:173–186
Broch K, Pederson IE, Hogmo RO (2015) The use of probiotics in fish feed for intensive aquaculture to promote healthy guts. Inter Schol J 7:264–273
Bronzi P, Rosenthal H, Gessner J (2011) Global sturgeon aquaculture production: an overview. J Appl Ichthyol 27:169–1675
Burr G, Gatlin D, Ricke S (2005) Microbial ecology of the gastrointestinal tract of fish and the potential application of prebiotics and probiotics in finfish aquaculture. J World Aquacult Soc 36:425–436
Cruz PM, Ibanez AL, Hermosillo OAM, Ramirez Saad HC (2012) Use of probiotics in aquaculture. ISRN Microbiol 2012:916845
El-Haroun ER, Goda AS, Kabir AM, Chowdhury MA (2006) Effect of dietary probiotic Biogen supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia, Oreochromis niloticus (L.). Aquac Res 37:1473–1480
Ellis AE (1999) Immunity to bacteria in fish. Fish Shellfish Immunol 9:291–308
Faramarzi M, Jafaryan H, Patimar R, Iranshahi F, Boloki ML, Farahi A (2011) The effects of different concentrations of probiotic Bacillus spp. and different bioencapsulation times on growth performance and survival rate of Persian sturgeon, Acipenser persicus larvae. World J Fish Mar Sci 3:145–150
Faramarzi M, Jafaryan H, Roozbehfar R, Jafari M, Biria M (2012a) Influences of probiotic Bacilli on ammonia and urea excretion in two conditions of starvation and satiation in Persian sturgeon Acipenser persicus larvae. Glob Vet 8:185–189
Faramarzi M, Jafaryan H, Roozbehfar R, Jafari M, Rashidi Y, Biria M (2012b) Influences of probiotic Bacilli via bioencapsulated Daphnia magna on resistance of Persian sturgeon larvae against challenge tests. Glob Vet 8:421–425
Gatesoupe FJ (2008) Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J Mol Microbiol Biotechnol 14:107–114
Geng X, Dong X, Tan B, Yang Q, Chi S, Liu H, Liu X (2011) Effects of dietary chitosan and Bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Fish Shellfish Immunol 31:400–406
Ghanbari M, Rezaei M, Jami M, Nazari RM (2009) Isolation and characterization of Lactobacillus species from intestinal contents of beluga, Huso huso and Persian sturgeon, Acipenser persicus. Iran J Vet Res Shiraz Univ 10:152–157
Gildberg A, Mikkelsen H, Sandaker E, Ringo E (1997) Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod (Gadus morhua). Hydrobiology 352:279–285
Hallajian A, Kazemi R, Yousefi Jourdehi A (2011) Effect of clove, Caryophillium aromaticus powder on anesthesia and recovery time on farmed 4 years old beluga, Huso huso. J Fish 5(2):133–140
Harikrishnan R, Balasundaram C, Heo MS (2010) Lactobacillus sakei BK19 enriched diet enhances the immunity status and disease resistance to streptococcosis infection in kelp grouper, Epinephelus bruneus. Fish Shellfish Immunol 29:1037–1043
Hoseinifar SH, Ringo E, Shenavar Masouleh A, Esteban MA (2016) Probiotic, prebiotic and symbiotic supplements in sturgeon aquaculture: a review. Rev Aquac 8:89–102
Hung SS O, Groff JM, Lutes PB, Kofi-Fynn-Aikinis F (1998) Hepatic and intestinal histology of juvenile white sturgeon fed different carbohydrate. Aquaculture 87:349–360.
Ibrahem MI (2015) Evolution of probiotics in aquatic world: potential effects, the current status in Egypt and recent prospective. J Adv Res 6:765–791
Iranshahi F, Faramarzi M, Kiaalvandi S, Boloki ML (2011) The enhancement of growth and feeding performance of Persian sturgeon, Acipenser persicus larvae by Artemia urmiana nauplii bioencapsulated via baker’s yeast, Saccharomyces cerevisiae. J Anim Vet Adv 10:2730–2735
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25:333–342
Kesarcodi-Watson A, Haspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14
Kim DH, Austin B (2006) Innate immune responses in Rainbow trout, Oncorhynchus mykiss induced by probiotics. Fish Shellfish Immunol 21:513–524
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo Rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol 24:168–172
Lamas J, Santos Y, Bruno DW, Toranzo AE, Anadon R (1994) Non-specific cellular responses of rainbow trout to Vibrio anguillarium and its extracellular products (ECPs). J Fish Biol 45(5):839–854
Lara-Flores M, Olvera-Novoa MA, Guzman-Mendez BE, Lopez-Madrid W (2003) Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia ,Oreochromis niloticus. Aquaculture 216:193–201
Lindsay GJH (1986) The significance of chitionlytic enzyme and lysozyme in rainbow trout, Salmo gairdneri defense. Aquaculture 51:169–173
Merrifield DL, Bradley G, Baker RTM, Davies SJ (2010b) Probiotic applications for Rainbow trout, Oncorhyncus mykiss (Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria post-antibiotic treatment. Aquac Nutr 16:496–503
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RMT, Bogwald J, Castex M, Ringo E (2010c) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Merrifield DL, Bradley G, Harper GM, Baker RTM, Munn CB, Davies SJ (2011) Assessment of the effects of vegetative and lyophilized Pediococcus acidilacticion growth, feed utilization, intestinal colonization and health parameters of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Nutr 17:73–79
Mohapatra S, Chakraborty T, Prusty AK, Das P, Pani Prasad K, Mohanta KN (2012) Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effect on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal micro-flora. Aquacult Nut 1:1–11
Nayak SK, Swain B, Mukherjee SC (2007) Effect of dietary probiotic and vitamin C on the immune response of India major carp Labeo rohita (Ham). Fish Shellfish Immunol 23:892–896
Nikoskelainen S, Ouwehand A, Salminen S, Bylund G (2001) Protection of rainbow trout, Oncorynchus mykiss from frunculosis by Lactobacillus rhamnosus. Aquaculture 198:229–236
Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H (2004) Immune response in the rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol 102:379–388
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout, Oncorhynchus mykiss. Aquaculture 243:241–254
Panigrahi A, Kiron V, Satoh S, Hirono I, Kobayashi T, Sugita H (2007) Immune modulation and expression of cytokine genes in rainbow trout, Oncorhynchus mykiss upon probiotic feeding. Dev Comp Immunol 31:372–382
Pirarat N, Pinpimai K, Endo M, Katagiri T, Ponpornpisit A, Chansue N (2011) Modulation of intestinal morphology and immunity in Nile tilapia, Oreochromis niloticus by Lactobacillus rhamnosus GG. Res Vet Sci 91(3):92–97
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead Seabream, Sparus aurata (L.). Fish Shellfish Immunol 25:114–123
Soltani M, Shenavar Masouleh A, Ahmadi M, Pourkazemi M, Taherimirghaed A (2015) Antibacterial activity, antibiotic susceptibility and probiotic use of lactic acid bacteria (LAB) in Persian sturgeon, Acipenser persicus. Iran J Aquat Anim Health 2(1):54–65
Son VM, Chang C, Wu M, Guu Y, Chiu C, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol 26(5):691–698
Song Z, Wu T, Cai L, Zhang L, Zheng X (2006) Effects of dietary supplementation with Clostridium butyricumon the growth performance and humoral immune response in Miichthys miiuy. J Zhejiang Univ Sci B 7(7):596–602
Sun Y, Yang H, Ma R, Lin W (2010) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol 29:803–809
Talpur AD, Ikhwanuddin MHD (2012) Dietary effects of garlic, Allium sativum on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 364-365:6–12
Tukmechi A, Morshedi A, Delirezh N (2007) Changes in intestinal microflora and humoral immune response following probiotic administration rainbow trout Oncorhyncus mykiss. J Anim Vet Adv 6(10):1183–1189
Yazdani MA, Pourkazemi M, Shakorian M, Pourali HM, Paykaran mana N, Sayed Hassani MH, Yeganeh H, Poursafar M (2010) Beluga rearing for meat and caviar production. Iranian Fisheries Research Organization (IFRO), 59 pp
Young-Hyo C, Jong-Keun K, Hong-Jong K, Won-Yong K, Young-Bae K, Yong-Ha P (2001) Selection of a potential probiotic Lactobacillus strain and subsequent in-vivo studies. A V Leeuwenhoek 80:193–199
Yousefi Jourdehi A, Sudagar M, Bahmani M, Hoseini SA, Dehghani MA, Yazdani MA (2014) Comparative study of dietary soy phytoestrogens genistein and equol effects on growth parameters and ovarian development in farmed female beluga sturgeon, Huso huso. Fish Physiol Biochem. https://doi.org/10.1007/s10695-013-9829-z
Acknowledgments
The authors thank the experts and directors in International Sturgeon Research Institute (Iran) for cooperation, especially Alireza Alipour, Reza Ghorbani, Ali Hoshyar, and Arash Ghorbani.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayed Hassani, M.H., Jourdehi, A.Y., Zelti, A.H. et al. Effects of commercial superzist probiotic on growth performance and hematological and immune indices in fingerlings Acipenser baerii. Aquacult Int 28, 377–387 (2020). https://doi.org/10.1007/s10499-019-00468-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00468-1