Abstract
This study was designed to evaluate the effects of multi-strain probiotic (MSP) on growth, whole-body composition, digestive and antioxidant enzymes, hematology, blood biochemistry, and physiological stress responses in Clarias batrachus fingerlings. Five experimental diets were prepared with supplementation of MSP (composed of Bacillus subtilis, B. licheniformis, and Enterococcus faecalis) powder at 0.5, 1.0, 1.5, and 2.0 g/kg. Healthy fingerlings (N = 225) were procured from a fish hatchery and acclimatized to laboratory conditions for 15 days. After acclimation, the fingerlings (10.13 ± 0.01 g; mean ± SD) were randomly stocked into five experimental groups (15 fish/aquarium) with three replicates and provided with experimental diets at a 5% body weight ration five times a day for 90 days. Fish fed with 2.0 g/kg MSP diets showed significantly higher growth performance in terms of weight gain % (493.98 ± 6.3%) and specific growth rate (1.97 ± 0.01%/day) and the lowest feed conversion ratio (1.72 ± 0.01) in comparison to the control (299.73 ± 5.17%, 1.53 ± 0.01%/day, and 2.28 ± 0.05, respectively). Similarly, higher activities (p < 0.05) of amylase (1.96 ± 0.02 U/mg protein), protease (10.51 ± 0.06 U/mg protein) and lipase (2.26 ± 0.02 U/mg protein) were also observed in the group fed 2.0 g/kg MSP than in the control group (1.20 ± 0.01, 8.61 ± 0.01, and 1.55 ± 0.02 U/mg protein, respectively). Dietary supplementation significantly (p < 0.05) enhanced the activities of superoxide dismutase (5.86 ± 0.04 U/mg prot), catalase (85.54 ± 0.58 U/mg prot), and glutathione peroxidase (105.69 ± 0.17 μU/mg prot) and reduced the malondialdehyde content (2.13 ± 0.06 mg/g prot) in fish fed 2.0 g/kg MSP-supplemented diets compared to the control group (4.54 ± 0.08 U/mg prot, 70.24 ± 0.53 U/mg prot, 89.61 ± 0.32 μU/mg prot, and 2.77 ± 0.02 mg/g prot, respectively). Dietary MSP did not affect the survival rate and proximate composition of fish. Hematological parameters such as white blood cells, red blood cells, hemoglobin, and hematocrit were also significantly increased in response to MSP supplementation in diets. Moreover, an increasing trend in blood biochemistry was observed in the activities of alanine phosphatase, while a decrease in alanine aminotransferase and aspartate aminotransferase was observed. It is concluded that MSP supplementation up to 2.0 g/kg showed promising results on C. batrachus fingerlings. MSP supplementation significantly enhanced the growth performance, antioxidant and digestive enzyme activities, blood biochemistry, and hematological parameters of C. batrachus compared to the control. The physiological stress response did not show any significant alteration. Thus, MSP can be effectively recommended as an effective growth promotor for fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquaculture industry has a key role in human nutrition and in meeting the future demand for aquatic food products and currently accounts for 50% of global fish consumption. The rapid increase in the human population has compelled the aquaculture industry to intensify its efforts to sustainably meet growing fish demand (Boyd et al. 2020). However, culturing a large number of fish in limited space leads to frequent collisions, competition for food, and degradation of water quality, which lead to oxidative stress in fish. Stress causes fish to exhibit depressed growth performance, a weakened immune system, and vulnerability to pathogens, which leads to frequent disease occurrence and causes huge economic losses in aquaculture (Martos-Sitcha et al. 2020). In the past, antibiotics were utilized to prevent bacterial diseases; however, due to serious health concerns such as antibiotic resistance and bioaccumulation, antibiotics have been banned in aquaculture (Manage 2018). Therefore, researchers have been actively seeking cost-effective, promisingly safe, and environmentally friendly alternatives to replace antibiotics in aquafeed. To address these concerns, dietary intervention with additives such as probiotics is considered the best approach (Mishra et al. 2015).
Probiotics, whether in viable or nonviable states, refer to nonpathogenic bacteria administered at a specific duration and optimal concentration that confer health benefits to their host. In fish farming, the incorporation of probiotics into feed offers a valuable nutritional approach and has become vital for various purposes (Nayak 2010). Probiotics help to maintain a healthy microbial balance by establishing beneficial microbiota in the gut. Probiotics help in disease prevention, reduce stress, support immunomodulation, and contribute to bioremediation efforts (El-Saadony et al. 2021; Tachibana et al. 2020)
Aquaculture researchers have successfully evaluated the use of single-strain probiotics (SSPs) as valuable supplements (Kwoji et al. 2021). However, the effect of probiotics on the host immune response depends on various factors, such as their source, type, strain, and species, which means that supplementing SSPs to a specific host may not necessarily yield a beneficial impact on the host immune system. Therefore, combining diverse species and genera of probiotics as multi-strain probiotics (MSPs) can be used in harmony and synergistically strengthen the host’s immune system (Ouwehand et al. 2018). Currently, in the fish farming industry, there has been a growing trend in the utilization of a large range of bacterial species, such as gram-positive spore-forming bacteria belonging to the genus Bacillus and lactic acid bacteria (LAB) (Abdel-Tawwab et al. 2020; Ringø et al. 2018). Among all of the species, Bacillus species have gained much importance, especially Bacillus subtilis, which has shown efficiency in various fish species. It has been used to enhance the growth, nutritional profile, immunity, and overall health status of fish. It exhibits the ability to prevent pathogen colonization and promote a balanced gut microbial environment (Kuebutornye et al. 2019; Telli et al. 2014; Zaineldin et al. 2018). Likewise, Bacillus licheniformis has emerged as a highly promising probiotic known for its endospore production, which enhances the immunity of fish and promotes the production of enzymes and the synthesis of antimicrobial compounds (Darafsh et al. 2019; Dawood et al. 2018; Gobi et al. 2018). Furthermore, LAB such as Enterococcus faecium can be effectively used in aquaculture (Ringo et al. 2020; Ringø et al. 2018). E. faecium, commonly found in the gastrointestinal tract, is a potential probiotic because it acts as a growth promotor, performs immunomodulatory activities, and produces bacteriocins to counteract the risk of infection (Costa Sousa et al. 2019; Tarkhani et al. 2020).
Clarias batrachus is an economically important freshwater air-breathing fish that belongs to the family Clariidae. Although it is considered an invasive species, its adaptability has led to its introduction in many countries beyond its native range for aquaculture purposes (Paul et al. 2015). In Asia, it is famous for its good nutritional content, taste, and consistent availability throughout the season and has gained immense popularity with high market demand among consumers (Gupta and Verma 2020; Sinha et al. 2014). Furthermore, it has a high growth rate, an efficient feed conversion ratio, and the capacity to survive in oxygen-depleted water conditions, which make it a desirable culture species. Therefore, this experiment was planned to assess the effect of MSP on growth performance, blood biochemistry, and digestive and antioxidant enzyme activities in C. batrachus.
Materials and methods
Preparation of probiotic-based diets
A commercial MSP powder (Compro NaproTM Aqua Probiotics (PR-24), China) was used, and each 1.0 kg of this product contained Bacillus subtilis (5.0 × 109 CFU/gram), Enterococcus faecalis (5.0 × 108 CFU/gram), and B. licheniformis (5.0 × 109 CFU/gram). Five experimental diets with 40% CP were formulated by incorporating different levels of MSP powder: 0.0, 0.5 g/kg, 1.0 g/kg, 1.5 g/kg, and 2.0 g/kg, labelled T0 (control diet), T1, T2, T3, and T4, respectively. Ingredients were taken from the local market in Lahore and analyzed for proximate composition following AOAC (2016). All the ingredients (Table 1) were weighed and ground to a particulate size of 0.05 mm and mixed in an electrical mixer (km 280, Kenwood) with the gradual addition of oil. Then, MSP powder was added according to the formulation described in Table 1. The ingredients were combined and mixed thoroughly by adding 15% distilled water to make a stiff dough. The dough was then passed through a meat mincer (Ag 3060, Anex) for pellet formation. Pellets were air dried with 10% moisture, and the experimental diets were packed in well-sealed plastic-labelled zipper bags in a refrigerator at 4 °C and then used throughout the research period. The samples of formulated feed were also analyzed for proximate analysis according to the method followed by AOAC (2016). The moisture content was estimated by using a hot-air oven (Wise Ven). Crude protein (CP) was analyzed using the micro-Kjeldahl method with an N percent of 6.25 by using the Kjeltec autoanalyzer (KjeltecTM 8100), crude fat (CF) was assessed through the use of a Soxhlet apparatus (Behro Test 901745) with diethyl ether extraction (40–60 °C), and the ash contents were determined by using a muffle furnace (Vulcan D-550) at 660 °C for 5 h.
Fish acclimatization
Two hundred twenty-five C. batrachus fingerlings (10.13 ± 0.01 g; mean + SD) were procured from the local fish farm of the Sindhwan fish hatchery, Head Balloki, Department of Fisheries, Government of Punjab, and transferred to the Fish Seed Rearing Unit, University of Veterinary and Animal Sciences, Ravi Campus, Pattoki , Pakistan. Fingerlings were placed in a KMnO4 bath (5 g/L) for 1–2 min and stocked into a cemented flow through circular tanks with 0.3 L/mint water exchange for acclimatization. Fish were fed a control diet (T0) during a 15-day acclimatization period.
Experimental design, fish rearing, and husbandry
At the beginning of the feeding trial, fingerlings were weighed and randomly distributed into glass aquaria (315 liter capacity) with dimensions 89 × 58 × 61 (cm) (length × width × depth) and a stocking density of 15 fish per aquarium. The fish were hand fed with test diets in triplicate at 5% of their body weight, and feed was given to fingerlings five times (every 4 h) a day for 90 days. On a daily basis, any uneaten feed was siphoned daily to calculate feed intake (g) and then feed conversion ratio. Physicochemical parameters such as dissolved oxygen, temperature, and pH (7.1 ± 0.2 mg/L, 28.5 ± 0.3 °C, and 7.1 ± 0.1, respectively) were monitored on a daily basis.
Growth indices
After the termination of the 90-day feeding trial, the fish weight was measured, and the number of fish in each replicate was also recorded. Growth parameters, such as weight gain percent (WG%), feed intake (FI), feed conversion ratio (FCR), specific growth rate (SGR), and survival rate (SR), were determined using the following formulae:
Whole-body composition
The four fish were arbitrarily selected for whole-body proximate analysis in terms of moisture content, crude protein, crude fat, and ash content following the protocol AOAC (2016) described above in the feed proximate analysis.
Digestive enzyme activities
Intestinal digestive enzyme analysis, such as amylase activity, was evaluated using starch solution as a substrate at 2% (w/v) (Bernfeld 1955). Lipase activity was measured using the spectrophotometric technique with the substrate p-nitro phenyl palmitate (pNPP) (Mahadik et al. 2002). The activity of protease was determined using the García-Carreño (1992) casein digesting technique. At 37 °C, the enzymatic unit hydrolyzes casein and produces a color identical to 1 mol/mint tyrosine (pH = 7.5).
Antioxidant enzyme activities
Each 2-g liver sample was mixed with 6 ml phosphate buffer (pH 7.4), homogenized, filtered using Whatman filter paper no. 1, and centrifuged at 10,000 × g for 15 min. The supernatant was separated, and all enzyme isolation steps were carried out at 4 °C. The activity of CAT was estimated by using the method of Maehly and Chance (1954). Briefly, the reduction in H2O2 concentration at a wavelength of 240 nm was measured using an Analytik Jena Specord 200 Plus UV/VIS spectrophotometer. SOD activity was measured as described by Giannopolitis and Ries (1977). The activity of SOD was determined by measuring its ability to inhibit the photoreduction of nitroblue tetrazole (NBT). The activity of GPx was determined by calculating its capacity to decrease the H2O2 concentration at a wavelength of 470 nm following the method of Civello et al. (1995).
Thiobarbituric acid reactive substance contents in the liver and muscles were determined by following Gatta et al. (2000). Briefly, samples were homogenized in a solution of KCl and Tris-maleate, followed by the addition of ascorbic acid and incubation at room temperature. Thiobarbituric acid (TBA) and HCl were added to the samples, which were boiled for 25 min and then cooled and centrifuged after the addition of trichloroacetic acid. Finally, TBA values, expressed as l g malondialdehyde equivalents/mg tissue, were determined photometrically at 530 nm.
Blood analysis
Five live fish were randomly selected from each aquarium, and their blood was collected from the caudal vein by using 3-ml syringes and stored in EDTA vacutainers. Hematological parameters such as red blood cells (RBCs), white blood cells (WBCs), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), hemoglobin (HB), mean corpuscular hemoglobin (MCH), and platelets (PLT) were determined by an autohematological analyzer (Celltac α, MEK-6550 Ltd., Japan) in the general laboratory, Department of Fisheries and Aquaculture, UVAS Ravi Campus, Pattoki.
Aside from hematological testing, blood samples were also collected in Eppendorf tubes without anticoagulant and centrifuged at 3000 rpm for 15 min within 30 min of collection. The resulting samples were then stored at − 80 °C for analysis. Serum was used for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alanine phosphatase (ALP) by using the kit method (BioScien) followed by Hossain et al. (2016). Serum was also used for stress parameters such as cortisol and glucose assays for the determination of stress by an automatic biochemical analyzer (Hitachi 7600-110 Ltd., Japan).
Statistical analysis
Data presented in the description and tables are the means and standard deviation of the three replicates. Data obtained in this study were subjected to one-way ANOVA in SAS (version 9.1). Significant parameters (p < 0.05) were compared using Duncan’s multiple range (DMR) test (Duncan 1955). Linear regression analysis (best fitter model based on R2) was performed on WG% data.
Results
Growth and feed utilization
Dietary supplementation with MSP significantly (p < 0.05) enhanced the growth performance and feed utilization of C. batrachus compared to the control group (Table 2). Significantly higher (p < 0.05) weight gain % was observed in fish fed with 0.5 (340.04 ± 5.68), 1.0 (393.92 ± 6.42), 1.5 (430.56 ± 5.69), and 2.0 (493.98 ± 6.39) g/kg MSP-supplemented diets compared with the control group (299.73 ± 5.17). The highest weight gain was observed in fish fed 2.0 g/kg MSP based on linear regression analysis (295.842x + 95.808; R2 = 0.99) (Fig. 1). Similarly, significantly (p < 0.05) higher SGR (%/day) was observed in fish fed with 0.5 (1.64 ± 0.01), 1.0 (1.77 ± 0.01), 1.5 (1.85 ± 0.01), and 2.0 (1.97 ± 0.01) g/kg MSP-supplemented diets compared with the control group (1.53 ± 0.01). Dietary supplementation significantly reduced (p < 0.0.5) the FCR linearly in fish fed with 0.5–2.0 g/kg MSP-supplemented diets in comparison to the control group. The lowest FCR was observed in the 2.0 g/kg MSP treatment (1.72 ± 0.01), and the highest FCR was observed in the control group (2.28 ± 0.05). Dietary supplementation of MSP showed significantly higher (p < 0.05) feed intake (g/fish) of 0.5 (73.88 ± 0.58), 1.0 (81.78 ± 0.58), 1.5 (85.51 ± 0.51), and 2.0 (86.30 ± 0.56) g/kg compared with the control group (69.35 ± 0.57) after 90 days of the feeding trial. However, the highest feed intake was observed in the 1.5 and 2.0 g/kg MSP-supplemented groups, which showed nonsignificant (p > 0.05) differences.
Whole-body proximate analysis
Dietary supplementation of MSP in the diet did not influence (p > 0.05) the whole-body composition, as presented in Table 3. Values of proximate composition were observed as moisture (74.41–74.91%), crude protein (16.75–17.64%), crude fat (4.98–5.31%), and ash contents (3.69–3.93%).
Intestinal digestive enzyme activity
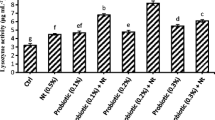
Dietary incorporation of MSP in the diets significantly improved (p < 0.05) the digestive enzyme activities (U/mg protein)) in the intestine of C. batrachus (Table 4). Amylase activities were significantly enhanced (p < 0.05) with increasing levels of MSP such as 0.5 g/kg (1.35 ± 0.03), 1.0 g/kg (1.55 ± 0.03), 1.5 g/kg (1.78 ± 0.03), and 2.0 g/kg (1.96 ± 0.02) compared with the control group (1.20 ± 0.01). A similar trend (p < 0.05) was observed in protease and lipase activities. The highest protease activities (p < 0.05) were observed in the 2.0 g/kg treatment group (10.51 ± 0.06), and the lowest activity was observed in the control group (8.61 ± 0.01). Similarly, fish fed the 2.0 g/kg MSP-supplemented diet showed the highest (p < 0.05) lipase activities (2.26 ± 0.02), and the lowest activities were observed in the control group (1.55 ± 0.02).
Antioxidant enzyme activity
Dietary supplementation of MSP in diets of C. batrachus demonstrated a significant enhancement (p < 0.05) in liver SOD, CAT, and GPH-X activities (Table 5). Significantly higher (p < 0.05) activities of CAT (U/mg prot) were observed in fish fed with 0.5 g/kg (73.86 ± 0.56), 1.0 g/kg (79.0 ± 0.59), 1.5 g/kg (83.09 ± 0.57), and 2.0 g/kg (85.54 ± 0.58) compared with the control group (70.24 ± 0.53). Similarly, significantly higher (p < 0.05) activities of SOD (U/mg prot) were also observed in fish fed with 0.5 g/kg (4.98 ± 0.09), 1.0 g/kg (5.21 ± 0.08), 1.5 g/kg (5.64 ± 0.09), and 2.0 g/kg (5.86 ± 0.04) MSP-supplemented diets compared with the control group (4.54 ± 0.08). Dietary supplementation of MSP at 2.0 g/kg showed the highest (p < 0.05) activities (μU/mg prot) of GPH-x (85.54 ± 0.58), and the lowest activities were observed in the control group (89.61 ± 0.32). Supplementation with MSP significantly reduced (p < 0.05) the MDA concentrations (mg/g prot) with increasing levels of MSP such as 0.5 g/kg (2.56 ± 0.03), 1.0 g/kg (2.43 ± 0.02), 1.5 g/kg (2.28 ± 0.03), and 2.0 g/kg (2.13 ± 0.06) in comparison to the control group (2.77 ± 0.02).
Hematological parameters
The hematological parameters of C. batrachus in response to MSP in the diets are shown in Table 6. Values of WBC, RBC, HB, and HCT showed a significant linear increase (p < 0.05) with increasing levels of MSP in diets compared to the control group, and the highest values were observed in 2.0 g/kg MSP-supplemented diets. Moreover, MCH showed a significant decrease (p < 0.05) in response to increasing MSP supplementation in diets compared to the control group. Dietary supplementation with MSP did not influence (p > 0.05) the MCV, MCHC, or PLT values.
Serum biochemistry
Dietary MSP supplementation significantly (p < 0.05) increased the activities (U/L) of ALP and reduced the activities of AST and ALT in C. batrachus (Table 7). The activities of ALP were significantly (p < 0.05) increased in fish fed with 0.5 g/kg (82.82 ± 0.31), 1.0 g/kg (83.91 ± 0.16), 1.5 g/kg (86.90 ± 0.15), and 2.0 g/kg (90.30 ± 0.45) compared with the control group (80.79 ± 0.42). Significant (p < 0.05) increases in AST activities were observed in fish fed 0.5 g/kg (13.11 ± 0.23), 1.0 g/kg (12.10 ± 0.24), 1.5 g/kg (11.17 ± 0.24), and 2 g/kg (10.68 ± 0.29) MSP-supplemented diets compared with the control group (13.95 ± 0.23). Similarly, a significantly (p < 0.05) decreasing trend was also observed in activities, and the lowest activities were observed in the 2.0 g/kg (24.05±0.38) MSP group, while the highest activities were observed in the control group (36.23 ± 0.38).
Stress parameters
The effect of dietary MSP supplementation on the stress biomarkers is given in Table 8. Dietary supplementation with MSP decreased (p > 0.05) the glucose level with increasing levels of MSP, and the lowest glucose level was observed in the 2.0 g/kg (47.85 ± 0.84 g/dL) MSP group, while the highest glucose level was observed in the control group (47.26 ± 0.94 g/dL). Cortisol levels were observed to decrease (p > 0.05) with increasing supplementation of MSP, and the lowest level was observed in the 2.0 g/kg (8.33 ± 0.18 ng/mL-1) treatment group, while the highest level was observed in the control group (8.58 ± 0.10 ng/mL-1). Both glucose and cortisol showed nonsignificant responses (p > 0.05) with MSP supplementation.
Discussion
Single-strain probiotics are usually used in aquaculture; however, with advancements in aquaculture, multi-strain probiotics (MSPs) may provide additional benefits due to the synergistic effects of different probiotic strains (Mohammadi et al. 2021; Puvanasundram et al. 2021). In the current study, the supplementation of MSP in the diet significantly (p < 0.05) improved the growth performance in terms of FW, WG%, SGR, and FCR with increasing levels up to 2.0 g/kg. Consistent with our results, MSP has been reported to enhance growth performance, such as MSP consisting of B. subtilis, L. plantarum, and E. faecium in Pangasianodon hypophthalmus (Abdel-Latif et al. 2023); B. subtilis and B. licheniformis in O. niloticus (Tachibana et al. 2020); B. subtilis, B. licheniformis, and E. faecium in Oncorhynchus mykiss (Merrifield et al. 2010); Lactobacillus plantarum and Pediococcus pentosaceus in O. niloticus (Muhammad et al. 2022); and Lactobacillus and Bifidobacterium in Clarias gariepinus (Ayoola et al. 2013). Probiotics have been reported to enhance digestive enzyme activities in the fish gut, which leads to improved nutrient absorption and growth (El-Haroun et al. 2006). Therefore, the enhancement of growth might be due to improvements in growth factors, absorption, and assimilation in fish (Balami et al. 2022).
In the present study, a significant (p < 0.05) improvement in digestive enzyme activities (amylase, lipase, and protease) was observed in C. batrachus fed MSP-supplemented diets. The findings of this study are consistent with previous investigations in L. rohita (Mukherjee et al. 2019; Saravanan et al. 2021; Ullah et al. 2020), P. hypophthalmus (Abdel-Latif et al. 2023; Akter et al. 2019), O. mossambicus (Yaqub et al. 2021), O. niloticus (Liu et al. 2021), and O. mykiss (Adel et al. 2017). Probiotics help to boost the activation and secretion of digestive enzymes (Vazirzadeh et al. 2020). Moreover, bacteria have the ability to release protease enzymes to breakdown peptide bonds within proteins, facilitating the breakdown of proteins into component monomers and free amino acids, which can help the animal’s nutritional condition (Macfarlane and Cummings 1991). The majority of probiotics may secrete lipase enzymes, which stimulate the digestion of lipids, resulting in increased growth and immunity in fish (Sharma et al. 2010).
Antioxidant enzymes (SOD, GPx, CAT) are involved in the defense mechanism of fish and play an important role in protecting fish from oxidative stress. Moreover, MDA serves as an indicator of lipid peroxidation in fish species (Hoseinifar et al. 2020). The increased SOD, CAT, and GPx activities and decreased MDA content in our study are consistent with previous research studies reported in different fish species fed probiotic-supplemented diets (Giannenas et al. 2015; Gobi et al. 2018; Kuebutornye et al. 2020; Wang et al. 2017). Probiotics are capable of producing metabolites such as glutathione, butyrate, folate, and exopolysaccharides, which are known for their antioxidant properties (Hoseinifar et al. 2020). Furthermore, probiotics also have the ability to produce or stimulate the release of GSH from the intestines, which has a favorable effect on the production of antioxidants in the liver of fish species (Mishra et al. 2015).
MSP supplementation showed nonsignificant (p > 0.05) results on the whole-body proximate composition of C. batrachus. These findings are aligned with previous studies on probiotic supplementation in O. mykiss (Ramos et al. 2015), P. hypophthalmus (Boonanuntanasarn et al. 2019), O. niloticus (El-Haroun et al. 2006), and Paralichthys olivaceus juveniles (Niu et al. 2019). In contrast, Mukherjee et al. (2019) reported a significant increase in the crude protein and crude lipid content of L. rohita fed a diet containing a Bacillus strain. The variation in the results could be attributed to differences in probiotic composition, fish species, experimental design, feed formulations, and rearing conditions.
The hematological parameters of fish are known to fluctuate in response to changes in physiological, nutritional, and environmental conditions and can serve as valuable indicators of the overall health of fish (Fazio 2019). The current results revealed a significant (p < 0.05) improvement in the hematological profile of C. batrachus. Similar to our study, previous investigations on MSP supplementation in fish diets have also reported enhanced levels of Hb, RBCs, MCH, and WBCs (Dahiya et al. 2012; Tabassum et al. 2021; Yaqub et al. 2021). Furthermore, previous research has also demonstrated that the use of MSP consisting of L. sporogenes, L. acidophilus, B. subtilis, B. licheniformis, and S. cerevisiae in C. mrigal (Sharma et al. 2010), as well as L. acidophilus and B. subtilis in O. niloticus (Aly et al. 2008), can lead to an increase in WBCs, which is also consistent with the findings of the present study. Furthermore, improvements in MCV, MCH, and MCHC suggest that fish fed the MSP diet were healthier, similar to the study reported by Gabriel et al. (2004). It can be inferred that MSP may play a role in the increase in HB and RBC content through the promotion of iron absorption in the gut. This could be attributed to the release of organic acid, which enhances the availability of iron, thus facilitating the production of more RBCs and HB (Dahiya et al. 2012). Furthermore, the observed increase in WBC levels may indicate improved innate immunity (Rajikkannu et al. 2015).
In serum biochemistry, AST, ALP, and ALT are key enzymes that serve as important biomarkers for evaluating liver and kidney functions. The reduced ALT and AST levels in our study are in line with previous studies, which demonstrated a significant reduction in AST and ALT levels in different fish species fed a combination of probiotics, i.e., B. subtilis, B. licheniformis, and E. faecalis (Liu et al. 2021; Wang et al. 2017). Dietary supplementation enhanced ALP activities in the current study, which is consistent with previous studies on probiotics (Gobi et al. 2018; Panigrahi et al. 2004; Sangma and Kamilya 2015; Sheikhzadeh et al. 2012). ALP activity is commonly associated with the increased production of enzymes by macrophages.
The blood glucose level is thought to be a sensitive indicator of stress (Abdel Rahman et al. 2020). Cortisol is classified as a chronic stress hormone, and its level has been used to measure stress in aquatic animals (Sadoul and Geffroy 2019). In the current study, no significant differences (p > 0.05) in the levels of glucose and cortisol were observed in fish fed MSP, which indicates the absence of any stress condition in fish during the rearing period. Our results are in line with the study of Tachibana et al. (2020).
Conclusion
In conclusion, MSP supplementation up to 2.0 g/kg significantly enhanced the growth performance and feed utilization of C. batrachus. Furthermore, MSPs also enhanced hematology, serum biochemistry, and digestive enzyme activities in fish fed MSP-supplemented diets, which indicates better health conditions. Moreover, antioxidant enzyme activities were also enhanced with the MSP-supplemented diet, indicating better stress coping capabilities. Therefore, it is recommended to supplement the diet of C. batrachus with 2.0 g/kg MSP to enhance growth performance and health.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdel Rahman AN, Mohamed AA-R, Mohammed HH, Elseddawy NM, Salem GA, El-Ghareeb WR (2020) The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquac 517:734777. https://doi.org/10.1016/j.aquaculture.2019.734777
Abdel-Latif HMR, Chaklader MR, Shukry M, Ahmed HA, Khallaf MA (2023) A multispecies probiotic modulates growth, digestive enzymes, immunity, hepatic antioxidant activity, and disease resistance of Pangasianodon hypophthalmus fingerlings. Aquac 563:738948. https://doi.org/10.1016/j.aquaculture.2022.738948
Abdel-Tawwab M, Khalil RH, Nour AM, Elkhayat BK, Khalifa E, Abdel-Latif HMR (2020) Effects of Bacillus subtilis-fermented rice bran on water quality, performance, antioxidants/oxidants, and immunity biomarkers of White leg shrimp (Litopenaeus vannamei) reared at different salinities with zero water exchange. J Appl Aquac 34(2):332–357. https://doi.org/10.1080/10454438.2020.1844110
Adel M, Lazado CC, Safari R, Yeganeh S, Zorriehzahra MJ (2017) Aqualase®, a yeast-based in-feed probiotic, modulates intestinal microbiota, immunity and growth of rainbow trout Oncorhynchus mykiss. Aquac Res 48(4):1815–1826. https://doi.org/10.1111/are.13019
Akter MN, Hashim R, Sutriana A, Siti Azizah MN, Asaduzzaman M (2019) Effect of Lactobacillus acidophilus supplementation on growth performances, digestive enzyme activities and gut histomorphology of striped catfish (Pangasianodon hypophthalmus Sauvage, 1878) juveniles. Aquac Res 50(3):786–797. https://doi.org/10.1111/are.13938
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25(1-2):128–136. https://doi.org/10.1016/j.fsi.2008.03.013
AOAC (2016) Official methods of analysis of AOAC International, 20th edn. AOAC International, Rockville, MD. Available: http://www.directtextbook.com/isbn/9780935584875, [Consulted: September 22, 2016]
Ayoola SO, Ajani EK, Fashae OF (2013) Effect of Probiotics (Lactobacillus and Bifidobacterium) on growth performance and hematological profile of Clarias gariepinus Juveniles. World J Fish Mar Sci 5(1):01–08
Balami S, Paudel K, Shrestha N (2022) A review: Use of probiotics in striped catfish larvae culture. Int J Fish aquat Stud 10:41–49
Bernfeld P (1955) Amylase, α and β. Methods Enzymol 1:149–158. https://doi.org/10.1016/0076-6879(55)01021-5
Boonanuntanasarn S, Ditthab K, Jangprai A, Nakharuthai C (2019) Effects of microencapsulated Saccharomyces cerevisiae on growth, hematological indices, blood chemical, and immune parameters and intestinal morphology in striped catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob Proteins 11(2):427–437. https://doi.org/10.1007/s12602-018-9404-0
Boyd CE, D'Abramo LR, Glencross BD, Huyben DC, Juarez LM, Lockwood GS, McNevin AA, Tacon AGJ, Teletchea F, Tomasso JR, Tucker CS, Valenti WC (2020) Achieving sustainable aquaculture: historical and current perspectives and future needs and challenges. J World Aquacult Soc 51(3):578–633. https://doi.org/10.1111/jwas.12714
Civello PM, Martínez GA, Chaves AR, Añón MC (1995) Peroxidase from strawberry fruit (Fragaria ananassa Duch.): partial purification and determination of some properties. J Agric Food Chem J 43(10):2596–2601. https://doi.org/10.1021/jf00058a008
Costa Sousa N, Couto MVS, Abe HA, Paixão PEG, Cordeiro CAM, Monteiro Lopes E, Ready JS, Jesus GFA, Martins ML, Mouriño JLP, Carneiro PCF, Maria AN, Fujimoto RY (2019) Effects of an Enterococcus faecium-based probiotic on growth performance and health of Pirarucu, Arapaima gigas. Aquac Res 50(12):3720–3728. https://doi.org/10.1111/are.14332
Dahiya T, Sihag RC, Gahlawat SK (2012) Effect of probiotics on the haematological parameters of Indian magur (Clarius batrachus L.). J Fish Aquat Sci 7(4):279–290. https://doi.org/10.3923/jfas.2012.279.290
Darafsh F, Soltani M, Abdolhay HA, Shamsaei Mehrejan M (2019) Improvement of growth performance, digestive enzymes and body composition of Persian sturgeon (Acipenser persicus) following feeding on probiotics: Bacillus licheniformis, Bacillus subtilis and Saccharomyces cerevisiae. Aquac Res 51(3):957–964. https://doi.org/10.1111/are.14440
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10(4):950–974. https://doi.org/10.1111/raq.12209
Duncan DB (1955) Multiple Range and Multiple F Tests. Biom 11(1):1–42. https://doi.org/10.2307/3001478
El-Haroun ER, Goda AMAS, Kabir Chowdhury MA (2006) Effect of dietary probiotic Biogen supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquac Res 37(14):1473–1480. https://doi.org/10.1111/j.1365-2109.2006.01584.x
El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MAO, Dhama K, Abdel-Latif HMR (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52. https://doi.org/10.1016/j.fsi.2021.07.007
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquac 500:237–242. https://doi.org/10.1016/j.aquaculture.2018.10.030
Gabriel UU, Ezeri GNO, Opabunmi OO (2004) Influence of sex, source, health status and acclimation on the haematology of Clarias gariepinus (Burch,1822). Afr J Biotechnol 3(9):463–467. https://doi.org/10.5897/ajb2004.000-2090
García-Carreño FL (1992) Protease inhibition in theory and practice. Biotechnol Educ 3(4):145–150
Gatta, Pirini, Testi, Vignola, Monetti (2000) The influence of different levels of dietary vitamin E on sea bass Dicentrarchus labrax flesh quality. Aquaculture Nutrition 6(1):47–52. https://doi.org/10.1046/j.1365-2095.2000.00127.x
Giannenas I, Karamaligas I, Margaroni M, Pappas I, Mayer E, Encarnacao P, Karagouni E (2015) Effect of dietary incorporation of a multistrain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 41(1):119–128. https://doi.org/10.1007/s10695-014-0010-0
Giannopolitis CN, Ries SK (1977) Superoxide Dismutases. Plant Physiol 59(2):315–318. https://doi.org/10.1104/pp.59.2.315
Gobi N, Vaseeharan B, Chen JC, Rekha R, Vijayakumar S, Anjugam M, Iswarya A (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Gupta P, Verma SK (2020) Impacts of herbicide pendimethalin on sex steroid level, plasma vitellogenin concentration and aromatase activity in teleost Clarias batrachus (Linnaeus). Environ Toxicol Pharmacol 75:103324. https://doi.org/10.1016/j.etap.2020.103324
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29(2):198–217. https://doi.org/10.1080/23308249.2020.1795616
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM (2016) Dietary effects of adenosine monophosphate to enhance growth, digestibility, innate immune responses and stress resistance of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol 56:523–533. https://doi.org/10.1016/j.fsi.2016.08.009
Kuebutornye FKA, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol 87:820–828. https://doi.org/10.1016/j.fsi.2019.02.010
Kuebutornye FKA, Wang Z, Lu Y, Abarike ED, Sakyi ME, Li Y, Xie CX, Hlordzi V (2020) Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol 97:83–95. https://doi.org/10.1016/j.fsi.2019.12.046
Kwoji ID, Aiyegoro OA, Okpeku M, Adeleke MA (2021) Multi-strain probiotics: synergy among isolates enhances biological activities. Biol 10(4):322. https://doi.org/10.3390/biology10040322
Liu Q, Wen L, Pan X, Huang Y, Du X, Qin J, Zhou K, Wei Z, Chen Z, Ma H, Hu T, Lin Y (2021) Dietary supplementation of Bacillus subtilis and Enterococcus faecalis can effectively improve the growth performance, immunity, and resistance of tilapia against Streptococcus agalactiae. Aquac Nutr 27(4):1160–1172. https://doi.org/10.1111/anu.13256
Macfarlane GT, Cummings JH (1991) The colonic fora, fermentation and large bowel digestive function. In: Phillips SF, Pemberton JH, Shorter RG (eds) The Large Intestine: Physiology, Pathophysiology and Disease. Raven Press, New York, pp 51–92
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424. https://doi.org/10.1002/9780470110171.ch14
Mahadik ND, Puntambekar US, Bastawde KB, Khire JM, Gokhale DV (2002) Production of acidic lipase by Aspergillus niger in solid-state fermentation. Process Biochem 38(5):715–721. https://doi.org/10.1016/S0032-9592(02)00194-2
Manage PM (2018) Heavy use of antibiotics in aquaculture: emerging human and animal health problems – A review. Sri Lanka J Aquat Sci 23(1):13–27. https://doi.org/10.4038/sljas.v23i1.7543
Martos-Sitcha JA, Mancera JM, Prunet P, Magnoni LJ (2020) Editorial: Welfare and stressors in fish: challenges facing aquaculture. Front Physiol 11:162. https://doi.org/10.3389/fphys.2020.00162
Merrifield DL, Bradley G, Baker RTM, Davies SJ (2010) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac Nutr 16(5):496–503. https://doi.org/10.1111/j.1365-2095.2009.00688.x
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63(14):3615–3626. https://doi.org/10.1021/jf506326t
Mohammadi G, Rafiee G, Tavabe KR, Abdel-Latif HMR, Dawood MAO (2021) The enrichment of diet with beneficial bacteria (single- or multi strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquac 539:736640. https://doi.org/10.1016/j.aquaculture.2021.736640
Muhammad Z, Anjum MZ, Akhter S, Irfan M, Amin S, Jamal Y, Khalid S, Ghazanfar S (2022) Effect of Lactobacillus plantarum and Pediococcus pentosaceus on the growth performance and morphometry of the genetically improved farmed tilapia (Oreochromis niloticus). Pak J Zool. Online first article. https://doi.org/10.17582/journal.pjz/20220703220755
Mukherjee A, Chandra G, Ghosh K (2019) Single or conjoint application of autochthonous Bacillus strains as potential probiotics: effects on growth, feed utilization, immunity and disease resistance in Rohu, Labeo rohita (Hamilton). Aquac 512:734302. https://doi.org/10.1016/j.aquaculture.2019.734302
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Niu K-M, Khosravi S, Kothari D, Lee W-D, Lim J-M, Lee B-J, Kim K-W, Lim S-G, Lee S-M, Kim S-K (2019) Effects of dietary multistrain probiotics supplementation in a low fishmeal diet on growth performance, nutrient utilization, proximate composition, immune parameters, and gut microbiota of juvenile olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 93:258–268. https://doi.org/10.1016/j.fsi.2019.07.056
Ouwehand AC, Invernici MM, Furlaneto FAC, Messora MR (2018) Effectiveness of multi-strain versus single-strain probiotics. J Clin Gastroenterol 52(Supplement 1):S35–S40. https://doi.org/10.1097/mcg.0000000000001052
Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopathol 102(4):379–388. https://doi.org/10.1016/j.vetimm.2004.08.006
Paul P, Adikesavalu H, Banerjee S, Abraham TJ (2015) Antibiotic resistant motile aeromonads induced septicemia in Philippine catfish Clarias batrachus (Linnaeus, 1758) FINGERLINGS. Croatian J Fish 73(4):170–175. https://doi.org/10.14798/73.4.844
Puvanasundram P, Chong CM, Sabri S, Yusoff MS, Karim M (2021) Multi-strain probiotics: Functions, effectiveness and formulations for aquaculture applications. Aquac Rep 21:100905. https://doi.org/10.1016/j.aqrep.2021.100905
Rajikkannu M, Natarajan N, Santhanam P, Deivasigamani B, Ilamathi J, Janani S (2015) Effect of probiotics on the haematological parameters of Indian major carp (Labeo rohita). Int J Fish Aquat Sci 2(5):105–109
Ramos MA, Gonçalves JFM, Batista S, Costas B, Pires MA, Rema P, Ozório ROA (2015) Growth, immune responses and intestinal morphology of rainbow trout (Oncorhynchus mykiss) supplemented with commercial probiotics. Fish Shellfish Immunol 45(1):19–26. https://doi.org/10.1016/j.fsi.2015.04.001
Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK (2018) Lactic acid bacteria in finfish—an update. Front Microbiol 9:1818. https://doi.org/10.3389/fmicb.2018.01818
Ringo E, Van Doan H, Lee SH, Soltani M, Hoseinifar SH, Harikrishnan R, Song SK (2020) Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J Appl Microbiol 129(1):116–136. https://doi.org/10.1111/jam.14628
Sadoul B, Geffroy B (2019) Measuring cortisol, the major stress hormone in fishes. J Fish Biol 94(4):540–555. https://doi.org/10.1111/jfb.13904
Sangma T, Kamilya D (2015) Dietary Bacillus subtilis FPTB13 and chitin, single or combined, modulate systemic and cutaneous mucosal immunity and resistance of catla, Catla catla (Hamilton) against edwardsiellosis. Comp Immunol Microbiol Infect Dis 43:8–15. https://doi.org/10.1016/j.cimid.2015.09.003
Saravanan K, Sivaramakrishnan T, Praveenraj J, Kiruba-Sankar R, Haridas H, Kumar S, Varghese B (2021) Effects of single and multi-strain probiotics on the growth, hemato-immunological, enzymatic activity, gut morphology and disease resistance in Rohu, Labeo rohita. Aquac 540:736749. https://doi.org/10.1016/j.aquaculture.2021.736749
Sharma P, Kumar V, Sinha AK, Ranjan J, Kithsiri HMP, Venkateshwarlu G (2010) Comparative fatty acid profiles of wild and farmed tropical freshwater fish rohu (Labeo rohita). Fish Physiol Biochem 36(3):411–417. https://doi.org/10.1007/s10695-009-9309-7
Sheikhzadeh N, Heidarieh M, Karimi Pashaki A, Nofouzi K, Ahrab Farshbafi M, Akbari M (2012) Hilyses®, fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 32(6):1083–1087. https://doi.org/10.1016/j.fsi.2012.03.003
Sinha M, Mahapatra B, Saha D, Maitra N (2014) Mass scale seed production of Magur, Clarias batrachus at farm level through improvised modifications. Int J Fish Aquat Sci 2(2):210–214
Tabassum T, Sofi Uddin Mahamud AGM, Acharjee TK, Hassan R, Akter Snigdha T, Islam T, Alam R, Khoiam MU, Akter F, Azad MR, Al Mahamud MA, Ahmed GU, Rahman T (2021) Probiotic supplementations improve growth, water quality, hematology, gut microbiota and intestinal morphology of Nile tilapia. Aquac Rep 21:100972. https://doi.org/10.1016/j.aqrep.2021.100972
Tachibana L, Telli GS, Dias DC, Gonçalves GS, Guimarães MC, Ishikawa CM, Cavalcante RB, Natori MM, Fernandez Alarcon MF, Tapia-Paniagua S, Moriñigo MÁ, Moyano FJ, Araújo ERL, Ranzani-Paiva MJT (2020) Bacillus subtilis and Bacillus licheniformis in diets for Nile tilapia (Oreochromis niloticus): effects on growth performance, gut microbiota modulation and innate immunology. Aquac Res 52(4):1630–1642. https://doi.org/10.1111/are.15016
Tarkhani R, Imani A, Hoseinifar SH, Sarvi Moghanlou K, Manaffar R (2020) The effects of host-associated Enterococcus faecium CGMCC1.2136 on serum immune parameters, digestive enzymes activity and growth performance of the Caspian roach (Rutilus rutilus caspicus) fingerlings. Aquac 519:734741. https://doi.org/10.1016/j.aquaculture.2019.734741
Telli GS, Ranzani-Paiva MJT, Dias DC, Sussel FR, Ishikawa CM, Tachibana L (2014) Dietary administration of Bacillus subtilis on hematology and non-specific immunity of Nile tilapia Oreochromis niloticus raised at different stocking densities. Fish Shellfish Immunol 39(2):305–311. https://doi.org/10.1016/j.fsi.2014.05.025
Ullah S, Shao Q-J, Ullah I, Zuberi A, Khattak MN, Dawar FU, Imran M, Ullah A, Shah AB (2020) Commercially available probiotic enhanced growth, digestion and immune response of Rohu (Labeo rohita) reared in earthen pond. Isr J Aquacult-Bamid 72:1–10
Vazirzadeh A, Roosta H, Masoumi H, Farhadi A, Jeffs A (2020) Long-term effects of three probiotics, singular or combined, on serum innate immune parameters and expressions of cytokine genes in rainbow trout during grow-out. Fish Shellfish Immunol 98:748–757. https://doi.org/10.1016/j.fsi.2019.11.023
Wang L, Ge C, Wang J, Dai J, Zhang P, Li Y (2017) Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aquac Int 25(6):2091–2099. https://doi.org/10.1007/s10499-017-0175-5
Yaqub A, Awan MN, Kamran M, Majeed I (2021) Evaluation of potential applications of dietary probiotic (Bacillus licheniformis SB3086): effect on growth, digestive enzyme activity, hematological, biochemical, and immune response of Tilapia (Oreochromis mossambicus). Turk J Fish Aquat Sci 22(5):TRJFAS19882. https://doi.org/10.4194/trjfas19882
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AMS, Dawood MAO, Dossou S, Wang W, Yukun Z (2018) Bacillus subtilis as probiotic candidate for red sea bream: growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312. https://doi.org/10.1016/j.fsi.2018.05.035
Author information
Authors and Affiliations
Contributions
Ayesha Tanveer: Investigation, data curation, writing—original draft. Noor Khan: Supervision, methodology. Mahroze Fatima: Conceptualization, supervision. Wazir Ali: Formal analysis, writing—review and editing. Sadia Nazir: writing—review and editing. Sheeza Bano: writing—review and editing. Muhammad Asghar: writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
This study was performed after approval from the Ethical Review Committee of the University of Veterinary and Animal Sciences, Lahore.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanveer, A., Khan, N., Fatima, M. et al. Effect of multi-strain probiotics on the growth, hematological profile, blood biochemistry, antioxidant capacity, and physiological responses of Clarias batrachus fingerlings. Aquacult Int 32, 1817–1833 (2024). https://doi.org/10.1007/s10499-023-01245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01245-x