Abstract
The dietary supplementation of acidifier in Cirrhinus mrigala juvenile was evaluated in terms of change in growth, autochthonous intestinal microbiota and haemato-biochemical parameters for period of 60 days. Acidifier was added at a level of 0 (control diet), 0.5, 1 and 1.5% to the practical pellet diet. At the end of the experiment, growth parameters, survival, bacillus bacterial population, haematological and immunological parameters were analysed. Higher WG%, SGR and PER and lower FCR (P < 0.05) was found in group fed 1.5% OA. Lysozyme activity and respiratory burst activity (nitroblue tetrazolium) were significantly affected by dietary 1.5% acidifier (P < 0.05). In fish fed on the diet with 1.5% acidifier showed a significant increase of total heterotrophic autochthonous bacterial and Bacillus levels (P < 0.05) compared with those fed on the diets supplemented with organic acid. In addition to increase in WBC, RBC, haemoglobin and platelets count levels were observed in this group. Relative % survival rate was found to be higher in the fish fed on 1.5% acidifier after challenging with Aeromonas hydrophilla bacteria. These results indicated that dietary supplementation of acidifier at a dose of 1.5% improved growth performance and enhanced the beneficial intestinal microbiota and stimulate immune response of Cirrhinus mrigala juvenile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The awareness campaigning and constant criticism by socio-environmentalists promotes the aquaculture industry to adopt environment-friendly practices in all fields of fish and shrimp production, if sustainability is to be achieved (Williams et al. 2000). Growing awareness from consumers and producers has enhanced the desires for responsible and sustainable aquaculture.
One of many strategies to improve fish health and performance in aquaculture is by including feed additives to produce functional feeds. Feed additives are non-nutritive ingredients that are included in diet formulations to influence physical and chemical properties of the feed for improving fish performance (NRC 2011). Organic acids and their salts have been used as feed additives functioning as acidifiers of animal feeds. Such organic acids, including acetic, butyric, citric, formic, lactic, malic, propionic and sorbic acid have been shown to improve health and growth performance of livestock and poultry by altering the gastrointestinal tract function and energy metabolism, increasing the availability of nutrients and inhibiting the growth of pathogenic bacteria (Lückstädt 2008). These organic acids and their salts have been approved by the European Union to be suitable alternatives of AGP for livestock for enhancing performance of particular organisms.
In general, organic acids comprise short-chain fatty acids (C1-C7), volatile fatty acids and weak carboxylic acids with one or more carboxyl groups in their structure. The mechanism of action of these weak acids in limiting microbial growth had been reviewed by Booth and Stratford (2003). Organic acidifier can improve the general health status of cultured animals by its stronger antimicrobial effect towards gram-negative bacteria, such as Escherichia coli and Salmonella sp., than towards lactobacilli (Zhou et al. 2009). The undissociated form of an organic acid is lipophilic and can passively diffuse through the cell wall of pathogenic bacteria. In the more alkaline cytoplasm of bacteria, organic acid dissociates and causes the internal pH to decrease. This inhibits bacterial cell metabolism and enzyme activity. Thus inhibit the growth of pathogens especially gram-negative bacteria in fish gut but acid tolerant bacteria like Lactobacillus spp. etc. remained unchanged or may even be enhanced in numbers (Zhou et al. 2009).
Dietary acidifiers have demonstrated effectiveness in enhancing the growth performance and the nutrient availabilities in various aquatic species as they lower down the pH of the digesta of the stomach and the foregut, which results in stimulating the pepsin activity, increasing protein digestibility and mineral absorption (Lückstädt 2007; Jun-sheng et al. 2006). Dietary inclusion of citric acid/formic acid enhances the bioavailability of minerals, including phosphorus, magnesium, calcium and iron in rainbow trout (Oncorhynchus mykiss) and sea bream (Pagrus major) (Jun-sheng et al. 2006; Vielma and Lall 1997). It was reported that the total bacteria per gram of faeces was significantly reduced in the fish fed with an organic acid blend and potassium diformate diets (Ng et al. 2009). Similarly, in marine shrimp propionate, butyrate and acetate salts display the highest inhibitory capacity against vibrio species (Da Saliva et al. 2013), as these acids can penetrate through the cell wall of gram-negative bacteria and release protons into the cytoplasm. Thus, the bacteria use a large amount of ATP to excrete protons in trying to maintain a balanced intracellular pH and result in the depletion of cellular energy with eventual cell death (Defoirdt et al. 2009). Dietary supplementation of acidifier increased growth, survival and reduced FCR in Arctic charr Salvelinus alpinus (Ringo 1991), rainbow trout Oncorhynchus mykiss (deWet 2005), tilapia (Ramli et al. 2005) and catfish Clarias gariepinus (Owen et al. 2006) and Kuruma shrimp Masurpenaeus japonicas (Tung et al. 2006). To our knowledge, there have been no previous reports on the acidifier (mixture of formic acid and calcium propionate in 1:1 ratio) to influence growth, nutrient utilization, survival and the humoral and cellular non-specific immunity of Cirrhinus mrigala which is an important fish species in tropical countries. Hence, the current study was carried out to assess the effect of dietary supplementation of acidifier on growth performance and haemato-biochemical parameters of Cirrhinus mrigala juvenile.
Materials and methods
Acclimation of experimental fish
Juveniles of mrigal, Cirrhinus mrigala were procured from Palghar fish farm, Panvel, Maharashtra, India. Fish were transported in 21 l polythene pack with sufficient oxygen (20 fish/pack). On reaching the wet laboratory, they were carefully transferred to a circular tank (1000 l) and were left undisturbed the whole night with aeration. In the next day, in order to ameliorate the handling stress, the fish were given a mild salt treatment followed by antibiotic treatment using oxytetracycline at the rate of 15 mgl−1 (APHA– AWWA – WEF 1998) for first 3 days. Water was exchanged every 24 h for first 2 days and later only 50% water was exchanged for every 3 days. The stock was acclimatized under aerated conditions for a period of 15 days and was fed with a practical diet containing 30% crude protein.
Experimental design, set up and fish maintenance
The experiment was conducted for a period of 60 days in the wet laboratory of the Aquaculture Division, CIFE, Mumbai, Maharastra, India. Total 12 plastic rectangular tubs (80 × 57 × 42 cm, 150 l capacity) covered with perforated lids were used for conducting the experiment. The tubs were initially washed and filled with potassium permanganate solution (4 mg l−1) and were left overnight. Then they were flushed out the very next day and were thoroughly washed with clean water. One hundred eighty acclimatized fingerlings of mrigal with initial body weight ranging from 2.87 ± 0.01 g to 3.26 ± 0.05 g were randomly distributed in the three replicates of each of four experimental groups such as control (without organic acid, OA), 0.5% OA, 1% OA and 1.5% OA using completely randomized design (CRD). Mixture of formic acid and calcium propionate in 1:1 ratio was used as organic acid source in this experiment. Feed was given at 2.5% of the body weight for 60 days, twice in day at 8:00 and 18:00 h under normal light regime (light/dark: 12/12 h). Uneaten feed and faecal matters were siphoned out daily with about 75% water exchange. The water quality parameters, i.e. temperature, pH, dissolved oxygen (DO), carbon dioxide (CO2), ammonia-nitrogen and nitrite-nitrogen were recorded every week. Dissolved oxygen (DO) and pH ranged from 6.46 to 7.12 mgL−1 and 7.4 to 8.5, respectively. The total ammonia and nitrite levels varied between 0.22–0.49 mgL−1 and 0.03–0.15 mgL−1, respectively. Water temperature varied from 27.4 to 29.2 °C and CO2 was not detected in any of the tubs. The values were within tolerable limits for carp (Jhingran 1991).
Diet formulation and preparation
The basal diet (Table 1) was used as the control diet. 0.5% acidifier (formic acid + calcium propionate in 1:1 ratio), 1.0% acidifier (formic acid + calcium propionate in 1:1 ratio) and 1.5% acidifier (formic acid + calcium propionate in 1:1 ratio) were supplemented in the basal diet to prepare the three respective experimental diets. All the diets were isonitrogenous (35% Crude Protein) and isoenergetic (420 to 425 Kcal/100 g). Full proximate composition of diet is shown in Table 2.
Soybean meal, fish meal and groundnut oil cake were used as protein source, wheat bran, rice flour and corn flour were used as energy source, oil mix (sunflower oil + cod liver oil) was used as lipid source, vitamin and mineral mixture (PREMIX PLUS), and vitamin C were taken as feed supplement, and Butylated hydroxytoluene (BHT) was used as antioxidant/preservative for feed formulation. As per formula all the ingredients weighed, ground and mixed (except oil and additives) well and distilled water was added to make dough followed by cooking in autoclave for 20 min. Butylated hydroxytoluene (BHT) was dissolved in oil and added in the dough after cooling and mixed thoroughly. The vitamin-mineral mixture and vitamin C were then mixed followed by addition of required inclusion level of acidifier (In case of treatment diets). Pellets were prepared by pelletizer having 2 mm diameter size. Finally the pellets were air-dried (final moisture level <12%). After drying, the pellets were packed in airtight polythene bags, labelled properly and stored until further use. During feeding pellet strands were broken in to smaller size.
Growth performance and nutrient utilization
Fish from each tank were collectively weighed at every 15-day interval and accordingly feeding rate was adjusted. Fishes were starved overnight before taking the weight. Growth performance and nutrient utilization in mrigal juvenile was evaluated in terms of percent weight gain (PWG), specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER) and percent survival based on the following standard formulae:
Analysis of gut microflora activity
The gut microflora analysis was done at the end of feeding trial after 60 days. Nine fish were randomly selected from each treatment (i.e. three fish from each replicate group) and collected in sterile plastic bags. The fish were starved overnight before the sampling.
The surface of the fish was sterilized by the application of alcohol and standard aseptic procedures were used to make the biopsy. The gut was aseptically taken out from each fish. The gut of all three fishes in each replicate of a treatment was weighed equally to make 1.0 g of sample and homogenized. This was then transferred to tubes containing 9.0 ml sterile 0.85% saline. The homogenates were serially diluted to 10−6 in 9-ml volumes of sterile 0.85% saline.
Media for bacterial analysis were obtained from Hi-Media. The solid media was employed and the bacteria enumerated were as follows: total plate count agar (total bacterial count), Bacillus medium (for Bacillus count). The isolation was made by serial dilution and spread plate technique. 0.1 ml of diluted sample was plated on the appropriate enriched medium. The plates were incubated at 28° C for 24–48 h in BOD incubator. After the requited incubation period, the plates were taken out and reported as an average of duplicate plates. The plates with 30–300 colonies were taken for total bacterial count and for the remaining bacteria, the plates having colonies from lowest dilution were taken for enumeration. Counts were expressed as cfu g−1 and transformed into log10 values before statistical analysis. Numbers of colonies reported in the present study are an average of three replicate plates. Isolated colonies (at least 10 per plate) were used for identification by morphological tests (Bergy 1986; Guimaraes et al. 2006).
Sample collection
Two fish from each replicate with a total of six fish from each treatment were anaesthetized with clove oil (50 μl L−1). Blood was collected by caudal puncture using a medical syringe (no. 23), which was previously rinsed with 2.7% EDTA solution. Blood collected was then transferred immediately to the test tube containing a pinch of EDTA powder (as an anticoagulant) and shaken gently in order to prevent haemolysis of blood. The blood samples were used for determination of haemoglobin content, total erythrocyte count, total leukocyte counts and for nitroblue tetrazolium (NBT) assay. For serum, again three fishes from each replicate were anaesthetized and the blood was collected without anticoagulant and allowed to clot for 2 h, centrifuged (3000×g for 5 min) and serum was separated out and kept at −20 °C.
Haematological and haemato-biochemical parameters
Haemoglobin content
The haemoglobin content of blood was analysed by estimating cyanmethaemoglobin using Drabkins Fluid (Qualigens). Five millilitres of Drabkins working solution was added to 20 μl of blood in a clean and dry test tube. The absorbance was measured using a spectrophotometer (MERCK, Nicolet, evolution 100) at a wavelength of 540 nm. The final concentration was calculated by comparing with the standard cyanmethaemoglobin (Qualigens, India).
where OD (T) = absorbance of test and OD (S) = absorbance of stand.
Total erythrocyte and leukocyte count
RBC and WBC diluting fluids were used for taking total erythrocytes count and total leukocyte counts, respectively. It was done by mixing 20 μl of blood with 3980 μl of corresponding diluting fluid in a clean test tube and was shaken well to suspend the cells uniformly in the solution. A small drop of this mixture was charged to Neubauer’s counting chamber of haemocytometer and counting was carried out.
Serum total protein, albumin, globulin and AG ratio
Serum total protein and albumin were estimated by biuret and bromocresol green (BCG) dye binding method, respectively (Reinhold 1953; Doumas et al. 1971) using the kit Qualigens Diagnostics. The absorbance was measured against a blank in a spectrophotometer at 630 nm. Globulin level was calculated by subtracting the albumin values from the total serum protein. A/G ratio was calculated by dividing albumin values by globulin values.
Respiratory burst activity
The respiratory burst activity of the phagocytes was measured by nitroblue tetrazolium assay following the method of Secombes (Secombes 1990) modified by Stasiack and Baumann (Stasiack and Bauman 1996). Fifty microlitres of blood were placed into the wells of ‘U’-bottom microlitre plates and incubated at 37 °C for 1 h to allow adhesion of cells. Then the supernatant was removed and the wells were washed three times with phosphate-buffered saline (PBS). After washing, 50 μl of 0.2% NBT was added and incubated for a further 1 h. The cells were then fixed with 100% methanol for 2–3 min and washed three times with 30% methanol. The plates were air-dried and 60 μl of 2 N potassium hydroxide and 70 μl of dimethyl sulphoxide were added to each well. The OD was recorded in an ELISA reader at 540 nm.
Serum lysozyme activity
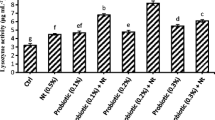
Serum samples were diluted with phosphate buffer (pH 7.4) to final concentration of 0.33 mg per ml. In a suitable cuvette, 3 ml of Micrococcus luteus suspension in phosphate buffer (A450 = 0.5–0.7) was taken, to which 50 μl of diluted serum sample was added. The content of the cuvette was mixed well for 15 s and reading was taken in a spectrophotometer at 450 nm exactly after 60 s of addition of serum sample. This absorbance was compared with standard lysozyme of known activity following the same procedure as above. The activity was expressed as U/min/mg protein.
Challenge study with Aeromonas Hydrophila
After 60 days of feeding, 12 fish per group were challenged with virulent Aeromonas hydrophila 018 obtained from the Aquatic Animal Health and Management Division, Central Institute of Fisheries Education (CIFE), Mumbai. Initially, the A. hydrophila were grown on nutrient broth for 24 h at 30 °C in a BOD incubator and harvested by centrifuging the culture broth at 6000 rpm for 10 min at 4 °C. The cells were then washed three times in sterile phosphate-buffered saline (PBS), pH 7.2 and final concentration was adjusted to 108 cfu ml−1. The fish in each experimental group were intraperitoneally injected with 0.2 ml (2 × 107 cfu ml−1) of bacterial suspension. Mortality was observed for 15 days. Tissues were removed from the dead fish for bacteriological culture to confirm A. hydrophila as the cause of death.
Relative percent survival (RPS) in different treatment groups were calculated as follows:
blood was collected for analysis of haematological parameters, haemato-biochemical parameters, NBT and lysozyme activity in post-challenged fish.
Statistical analysis
Mean values of all parameters were subjected to one-way ANOVA to study the treatment effects and Duncan’s multiple range tests were used to determine the significant differences among means. Comparisons were made at 5% probability level. All the data were analysed using statistical package SPSS (Version 16).
Results
Effects of different dietary acidifier levels on the growth performance and feed utilization are shown in Table 3. Compared to the control treatment, fish fed on 1% acidifier supplemented diet displayed (P < 0.05) improved percent body weight gain, specific growth rate (SGR), protein efficiency ratio (PER) and feed conversion ratio (FCR) followed by fish fed on 1.5% acidifier supplemented diet, and the lowest growth performance and nutrient utilization was recorded in the fish of control group. No mortality was observed in fish of any experimental group during the experiment (Tables 4 and 5).
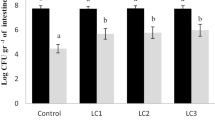
Intestinal microbiota analyses of Cirrhinus mrigala juvenile fed on different levels of acidifier are shown in Table 6. At the beginning of the trial, total heterotrophic bacteria (THB) and Bacillus levels in the intestine of Cirrhinus mrigala juveniles were 6.21 and 2.96 log10 cfu g−1, respectively. At the end of the trial, fish fed on 1.5% acidifier displayed a significant increase of total heterotrophic bacterial and Bacillus levels (P < 0.05), compared to the initial levels and the control group.
The effects of the different levels of acidifier on the innate immune responses of Cirrhinus mrigala juvenile during pre-challenge and post-challenge periods are shown in Table 5. The lysozyme activity of serum of pre-challenged fish was significantly affected by dietary supplementation of 1.5% OA (P < 0.05) as compared to fish of control group. Post-challenged fish also displayed the same pattern as found in the pre-challenged fish. No significant differences in respiratory burst activity (OD 540) were observed between the treatments (P > 0.05) during pre-challenge and post-challenge study.
Haematological and haemato-biochemical parameters of Cirrhinus mrigala juvenile fed on diets supplemented with different levels of acidifier are shown in Table 4. Red blood cell (RBC) and white blood cell (WBC) increased significantly in the fish fed diet supplemented with 1 and 1.5% acidifier compared to fish of control and 0.5% OA group (P < 0.05) during both pre-challenge and post-challenge periods. In the pre-challenge study, there was no significant difference observed for haemoglobin level in the different treatment groups, however. 1% OA group shows higher value for haemoglobin content. Platelets level in 1% acidifier fed group showed significantly increased pattern (P < 0.05) as compared to the other group. During pre-challenge and post-challenge study, haemato-biochemical parameters such as total protein, albumin, globulin and albumin-globulin ratio in fish of different treatments did not show any clear trend. However, during pre-challenge study, fish of 0.5% supplemented group showed significantly higher total protein and albumin levels than fish of control group but globulin and albumin-globulin ratio did not vary significantly among fish of any treatment. During post-challenge study, fish of 1% OA group showed significantly higher total protein and globulin levels than fish of control group. Though there was no significant difference of albumin level and albumin-globulin ratio between fish of control and 1.5% OA groups, fish of 0.5% and 1% OA groups showed significantly lower values.
Post-challenge mortality
The relative percent survival of fish of the different experimental groups during post-challenge period is illustrated in Fig. 1. Juveniles fed on the control diet showed the highest mortality rate (80%) compared with the organic acid fed groups, which showed a reduction in the mortality rate from 60 to 25% in the groups treated with 05, 1 and 1.5% acidifier supplemented in the diet.
Discussion
In the present study, supplementation of acidifier i.e. formic acid and calcium propionate mixture in the ratio of 1:1 has shown significant improvement in growth performance in terms of percent body weight gain, FCR, SGR, PER and percent survival in Cirrhinus mrigala juveniles and consequently immune response too. Organic acid supplementation in diet improved percent weight gain, SGR, PER and FCR in fish in comparison to fish fed diet without supplementation of organic acids. This result was supported by the observations of De Wet (2005), he fed rainbow trout fingerlings to five experimental diets, which consisted of a control diet, three diets containing 0.5, 1 and 1.5% of an organic acid blend (Bolifor FA 2400 S Aqua, KEMIRA GrowHow®, Sweden) and a diet containing an antibiotic growth promoter (AGP). The result showed the improvement in growth with increasing level of organic acid inclusion with most significant result in fish of 1 and 1.5% OA fed group as compared to the control. Similar kind of observation was also reported by Ramli et al. 2005 who tested potassium diformate (potassium salt of formic acid; Formi®) as a growth promoter in tilapia raised in a grow-out pond in Indonesia. To support this, Ringø (1992) also attributed the significant improvement (P < 0.05) in the growth performance of Arctic charr fed 1% sodium acetate supplemented diet and reared in brackish water. Furthermore, findings in salmonids revealed that supplementation of 1.4% potassium diformate (a potassium salt of formic acid) with fishmeal enriched diet showed higher SGR and lower FCR against the negative control in Atlantic salmon (Christiansen and Lückstädt 2008). In a more recent trial, Petkam et al. 2008 determined the effects of acid/salt blend (containing of calcium formate, calcium propionate, calcium lactate, calcium phosphate and citric acid) at different dietary levels (0.5, 1.0 and 1.5%) on the growth performance of tilapia. Despite a lack of statistically significant data for LWG and FCR, the blend at 1.5% dietary level resulted in a numerical increase in LWG of 11% versus negative control. Thus, organic acid salts and blends may be useful for grow-out tilapia culture (Lückstädt 2008). Baruah et al. (2007) investigated the synergistic effects of citric acid and phytase on nutrient digestibility and growth performance in Indian major carp, Labeo rohita against the low (25%) and high (35%) protein diets. The result indicated that citric acid in both diets significantly increased LWG and SGR and reduced FCR in carp juveniles. Zhou et al. 2008 observed an increase of 11.8% in the weight gain of hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) fed diets with 3 g kg−1 potassium diformate (PDF) and an 11% increase in body weight of Nile tilapia (O. niloticus L.) fed a proprietary organic acid blend 15 g kg−1 (Petkam et al. 2008). However, in a recent trial, Owen et al. (2006) found that sodium butyrate when as a feed additive added at 0.2% to two diets differing in their major protein source (fishmeal or defatted soya) of tropical catfish (Clarias gariepinus) showed slightly higher weight gain, SGR and a concomitant reduction in FCR in fishmeal-based diet as compared with the control diet, while fish fed defatted soya-based diet supplemented with 0.2% Na-butyrate showed no improvement.
Hossain et al. (2007) reported that the use of 1% each of citric acid in the diet of sea bream (Pagrus major) resulted significantly better LWGs and FCRs in fish in comparison to negative control, whereas, fish of malic acid and lactic acid supplemented groups did not exhibit improved growth performance. Various factors, such as species and the physiological age of the experimental fish, the type and level of organic acids, the diet composition and the culture conditions may all influence the manifestation of the potential growth-promoting effects of dietary organic acids in aquaculture (Ng et al. 2009).
There is very little information available on normal values of blood cell count in various species of fishes as it is subjected to changes with seasons (Fange et al. 1976). Fish blood contains small number of progenitors of blood cells, produced by lymphomyeloid tissues which, when appropriately stimulated, develop into early stages of blood cells called blast cells, which may occur in the circulating blood in great numbers. Leukocyte count is considered as an indicator of the health status of fish because of its role in non-specific or innate immunity (Roberts 1978) and leukocytes play an important role in the immune response of fish during inflammation (Secombes 1996). In the present study, total leukocyte counts and platelets were reported to be significantly higher in fish 1.5% OA group as compared to control. Roberts (1978) stated that in stress condition the WBC count increases.
An increasing trend of NBT value was observed with increasing level of dietary organic acid blend. Although, the enzymatic potential of these cells to mount an innate response through the oxidative pathway was detected. Monocytes and macrophages are activated CpG-S oligodeoxy nucleotide (ODN) to secrete a wide variety of proinflammatory cytokines (Chace et al. 1997; Sparwasser et al. 1997), some of which activate natural killer cells to secrete interferons and have increased lytic activity for tumour cells (Ballas et al. 1996). Fish serum lysozyme is believed to be of leucocyte origin (Lie et al. 1989). It has been found that lysozyme activity increases concomitantly with leukocyte numbers (Fletcher and White 1973) and antibody titre (Vladimrov 1968). Similarly, the groups that had the maximum lysozyme activity in our study also had the maximum leukocyte count. Lysozyme plays an important role in innate immunity by lysis of bacterial cell wall and thus stimulates the phagocytosis of bacteria (Ellis 1990). Balca’zar et al. (2007) observed a correlation between the colonization ability of indigenous lactic acid bacteria and the non-specific humoral response, such as an alternative complementary pathway activity and lysozyme activity in brown trout. Similarly, lysozyme is an important component in the immune system of fish. It is bactericidal by hydrolysing bacterial cell wall peptidoglycans resulting in bacteriolysis. It is also known to act as opsonin (Magnadottir 2006). In the present study, enhanced serum lysozyme activity (P < 0.05) was recorded in all the treatment groups during pre- and post-challenge study.
In the present study, serum proteins, albumins, globulins and albumin-globulin ratio were analysed. Proteins are the most important compounds in serum and its concentration is considered as a basic index for fish health status (Alexander et al. 2011; Misra et al. 2006). Increases in the serum protein, albumin and globulin levels are thought to be associated with a stronger immune response of fishes (Wiegertjes et al. 1996). Serum proteins are divided into two major groups, albumin and globulins. Among the serum proteins, albumin and globulin are the major proteins which play a significant role in the immune response (Alexander et al. 2011).
Supplementation of acidifier i.e. formic acid and calcium propionate mixture in the ratio of 1:1 has shown significant improvement in micro gut flora activity in juvenile of mrigal. To support this observation, Zhou et al. (2008) reported that dietary potassium diformate stimulated the colonization of some gut bacteria and inhibited the growth of others in hybrid tilapia. In contrast, Owen et al. (2006) reported that sodium butyrate supplementation did not significantly affect the gut microflora composition of Clarias gariepinus (Burchell) even though there was a tendency for a relative increase in the proportion of gram-positive bacteria. Thus eubiotic effect of organic acid blend on the proliferation of indigenous probionts may be the third reason for improving the growth performance because this gram-positive bacterium plays a vital role in fermentation of certain non-digestible carbohydrates and increases the availability of nutrients (Denev et al. 2009).
In post-challenged study with administration of Aeromonas hydrophilla infection, highest survival was obtained in 1.5% OA fed group which is in agreement with the finding of Ramli et al. (2005) who reported that the addition of potassium diformate in the diet of hybrid tilapia significantly increased the survival rates of fish after challenge with V. anguillarum and the effects were dose-dependent. In our study, relative percentage of survival after 15-day post-challenge with A. hydrophila in the diet was increased in the fish fed on the 1.5% OA supplemented diet, followed by the other 1 and 0.5% OA supplements (Fig. 1). An explanation for this may be that, gram-positive bacteria have high intracellular potassium concentrations, which provide a counteracting effect for the acid anions of the dissociated organic acids (Russell and Diez-Gonzales 1998). Conversely, it can acidify the cytoplasm of gram-negative bacteria, such as A. hydrophila and V. anguillarum, resulting in eventual cell death.
Hence, this study corroborates the functionality of acidifier at the level of 1.5% in the diet of Cirrhinus mrigala juvenile which positively affects growth performance and nutrient utilization, innate immunity, beneficial intestinal microbiota and some haematological parameters. We suggest that a dietary supplementation of acidifier (formic acid and calcium propionate at 1:1 ratio) at 1.5% may be a suitable alternative of AGP or prophylaxis use of antibiotics in aquaculture to obtain an economical and sustainable culture practice of carp species. This prima facie report may be useful for the nutritionists to recast their feed formulation for different species.
References
Alexander C, Sahu NP, Pal AK, Akhtar MS (2011) Haemato-immunological and stress responses of Labeo rohita (Hamilton) fingerlings: effect of rearing temperature and dietary gelatinized carbohydrate. J Anim Physiol Anim Nutr 95:653–663
APHA– AWWA – WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. (Clesceri LS, Greenberg AE, Eaton AD. Ed.). American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC
Balca’zar J, De Blas I, Ruiz-Zarzuela I, Vendrell D, Girone’s O, Muzquiz J (2007) Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol Med Microbiol 51:185–193
Ballas ZK, Rasmuseen WL, Krieg AM (1996) Induction of natural killer activity in murine and human cells by CpG motifs in oligodeoxy nucleotides and bacterial DNA. J Immunol 157:1840–1850
Baruah K, Sahu NP, Pal AK, Jain KK, Debnath D, Mukherjee SC (2007) Dietary microbial phytase and citric acid synergistically enhances nutrient digestibility and growth performance of Labeo rohita (Hamilton) juveniles at sub-optimal protein level. Aquac Res 38:109–120
Bergy DH (1986) Bergey’s manual of systemic bacteriology. Vol. 2 (Holt JH, Sneath PH, Krieg NR. eds) pp. 636. Lipponcott Williams and Wilkins, ISBN 0683078933
Booth IR, Stratford M (2003) Acidulants and low pH. In: Russell NJ, Gould GW (eds) Food Preservatives. Kluwer Academic/Plenum Publishers, New York, pp 25–47
Chace HJ, Hooker NA, Mildestein KL, Krieg AM, Cowdery JS (1997) Bacterial DNA-induced NK cell IFN-g production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol 84:185–193
Christiansen R, Lückstädt C (2008) Effects of different dosages of potassium diformate in fishmeal on the performance of Atlantic salmon Salmo salar. Abstract CD-Rom, World Aquacult. Soc. 19–23 may 2008, Busan, Korea
Da Saliva BC, Vieira FN, Mourino JP, Ferrira GS, Seiffert WQ (2013) Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture:107–110
De Wet L (2005) Can organic acids effectively replace antibiotic growth promotants in diets for rainbow trout Oncorhynchus mykiss raised under sub-optimal water temperatures? Abstract CD-Rom, J. World Aquacult. Soc. 9–13 may 2005; Bali, Indonesia
Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P (2009) Short-chain fatty acids and poly-b-hydroxyalkanoates: (new) biocontrol agents for a sustainable animal production. Biotechnol Adv 27:680–685
Denev S, Staykov Y, Moutafchieva R, Beev G (2009) Rev. microbial ecology of gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. In Aquacult Res 1:1–29
deWet L (2005) Organic acids as performance enhancers. Aqua Feeds: Formulation and Beyond 2:12–14
Doumas BT, Watson W, Biggs HG (1971) Albumin standards and measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 101–103
Fange R, Lundblad G, Lind J (1976) Lysozyme and chitinase in blood and lymphomyeloid tissues of marine fish. Mar Biol 36:277–282
Fletcher TC, White A (1973) Lysozyme activity in the plaice (Pleuronectes platessa L). Experientia 29:1283–1295
Guimaraes TM, Moriel DG, Machado IP, Fadel Picheth CMT, Bonfim TMB (2006) Isolation and characterization of Saccharomyces cerevisiae strain of winery interest. Brazilian J Pharm Sci 42:119–126
Halver JE (1976) The nutritional requirements of cultivated warmwater and coldwater fish species. Paper No: 31. FAO Technical conference on Aquaculture, Kyoto, 26 May–2 June, p 9
Hossain MA, Pandey A, Satoh S (2007) Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish Sci 73:1309–1317
Jhingran VG (1991) Fish culture in fresh water pond. Fish and fisheries of India (ed. V. G. Jhingran), III edn. Hindustan Publishing Corporation, New Delhi, p 276
Jun-sheng L, Jian-lin L, Ting-ting W (2006) Ontogeny of protease, amylase and lipase in the alimentary tract of hybrid juvenile tilapia Oreochromis niloticus X Oreochromis aureus. Fish Physiol Biotech 32:295–303
Lie Ø, Evensen Ø, Sørensen A, Frøysadal E (1989) Study on lysozyme activity in some fish species. Dis Aquat Org 6:1–5
Lückstädt C (2007) Effect of organic acid containing additives in worldwide aquaculture: sustainable production the non-antibiotic way. In: Lückstädt C (ed) Acidifiers in animal nutrition: a guide for feed preservation and acidification to promote animal performance. Nottingham University Press, Nottingham, pp 71–79
Lückstädt C (2008) Dietary organic acids as feed additive for tilapia (Oreochromis niloticus) culture. Program and Abstracts, 6th conference of the Society of Ichthyology, 13–15 March 2008, Munich, Germany. p. 34–35
Magnadottir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Misra S, Sahu NP, Pal AK, Xavier B, Kumar S, Mukherjee SC (2006) Pre- and postchallenge immuno-haematological changes in Labeo rohita juveniles fed gelatinised or non-gelatinised carbohydrate with n-3 PUFA. Fish Shellfish Immunol 21:346–356
Ng WK, Koh CB, Sudesh K, Siti-Zahrah A (2009) Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac Res 40:1490–1500
NRC (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington, DC
Owen MAG, Waines P, Bradley G, Davies S (2006) The effect of dietary supplementation of sodium butyrate on the growth and microflora of Clarias gariepinus (Burchell 1822). In: proceedings of the XII international symposium fish nutrition and feeding; 28 May–1 June 2006. p. 149
Petkam R, Lückstädt C, Nittayachit P, Sadao S, Encarnacao P (2008) Evaluation of a dietary organic acid blend on tilapia Oreochromis niloticus growth performance. Abstract CD-rom, world Aquacult Soc 19–23 may 2008, Busan, Korea
Ramli N, Heindl U, Sunanto S (2005) Effect of potassium-diformate on growth performance of tilapia challenged with Vibrio anguillarum. Abstract CD-rom J. World Aquac. Soc. 9–13 May 2005; Bali, Indonesia
Reinhold JG (1953) Manual determination of serum total protein, albumin and globulin fractions by biuret method. In: Reiner M (ed) Standard method of clinical chemistry. Academic Press, New York, p 88
Ringo E (1991) Effects of dietary lactate and propionate on growth and digesta in Artic charr, Salvelinus alpinus (L.) Aquaculture 96:321–333
Ringø E (1992) Effects of dietary formate and acetate on growth and lipid digestibility in Arctic charr, Salvelinus alpinus (L.). Fiskeridirektoratets Skrifter. Serie Ernaering 5:17–24
Roberts RJ (1978) The pathophysiology and systemic pathology of teleosts. In: Roberts RJ (ed) Fish pathology. Bailliere Tindal, London, pp 55–91
Russell JB, Diez-Gonzales F (1998) The effects of fermentation acids on bacterial growth. Adv Microb Physiol 39:205–234
Secombes CJ (1990) Isolation of salmonid macrophage and analysis of their killing activity. In: Stolen JSTC, Fletcher DP, Anderson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publication, Fair Haven, pp 137–152
Secombes CJ (1996) The nonspecific immune system: cellular defences. In: Iwama G, Nakanishi T (eds) The fish immune system, organism, pathogen and environment. Academic Press, Toronto, pp 63–103
Sparwasser T, Miethe T, Lipford G, Erdmann A, Hawker H, Heeg K (1997) Macrophage sense pathogens via DNA motifs: induction of tumor necrosis factor-a-mediated shock. Eur J Immunol 27:1671–1179
Stasiack AS, Bauman CP (1996) Neutrophil activity as a potent indicator concomitant analysis. Fish Shellfish Immunol 37:539
Tung HT, Koshio S, Teshima S, Ishikawa M, Ren T, Phuong NDT (2006) Effects of heat-killed lactobacillus plantarum on Kuruma shrimp Masurpenaeus japonicas juveniles. XII international symposium fish Nutrition and Feeding, May 28—June 1, 2006. Book of abstracts. p. 46
Vielma J, Lall S (1997) Dietary formic acid enhances apparent digestiblity of minerals in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Nutr 3:265–278
Vladimrov VL (1968) Immunity in fish. Bull Off Int Epizoot 69:1365–1372
Wiegertjes GF, Stet RJM, Parmentier HK, Muiswinkle WB (1996) Immunogenetics and disease resistance in fish: a comparative approach. Dev Comp Immunol 20:365–381
Williams MJ, Bell JD, Gupta MV, Dey M, Ahmed M, Prein M, Child S, Gardiner PR, Brummet R, Jamu D (2000) Responsible aquaculture can aid food problems. Nature 406:673
Zhou Z, Liu Y, Wang P, He S, Yao B, Gao X, Wang X (2008) The effects of dietary potassium diformate on growth performance, feed conversion and intestinal microbiota of hybrid tilapia. Abstract, XIII international symposium on fish nutrition and feeding, Florianopolis, Brazil
Zhou Z, Liu Y, He S, Shi P, Gao X, Yao B, Ringo E (2009) Effects of dietary potassium diformate (KDF) on growth diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus x O. aureus). Aquaculture 291:89–94
Acknowledgements
The authors are grateful to Dr. W.S. Lakra, Director, Central Institute of Fisheries Education, Mumbai, for providing facilities for carrying out the work. The first author is grateful to the Indian Council of Agricultural Research, New Delhi for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P., Jain, K.K., Sardar, P. et al. Dietary supplementation of acidifier: effect on growth performance and haemato-biochemical parameters in the diet of Cirrhinus mrigala juvenile. Aquacult Int 25, 2101–2116 (2017). https://doi.org/10.1007/s10499-017-0176-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0176-4