Abstract
Nowadays, probiotics are among the most attractive food supplements for promoting animal’s growth and health condition with the aim of increasing production and lowering losses. Four hundred and eighty fish weighing 31.8 ± 2.6 g were randomly divided into four groups each in triplicates. Groups 1 to 3 were fed with commercial carp feed containing 0.7, 0.5, and 0.3 g (1.2 × 106, 0.9 × 106, and 0.56 × 106 cfu/g feed) probiotic Lactobacillus plantarum per kilogram feed for 80 days at 26 ± 1 °C, respectively. Group 4 received normal feed and was considered as control 1. Growth factors and some immunophysiological parameters were assessed on days 0, 20, 40, 60, and 80 of the trail. An overall increase in growth factors of probiotic-treated groups was seen, and final weight and condition factor of groups 1 and 2 were higher than control group (p ≤ 0.05). The highest specific growth rate and best feed conversion ratio were obtained in group 1. Red blood cell (RBC) count, hematocrit, and hemoglobin in probiotic groups were higher than control 1, but differences were significant for group 1 (p ≤ 0.05). White blood cell (WBC) count significantly increased in groups 1 and 2 compared to other groups (p < 0.05). Respiratory burst activity significantly increased in all probiotic fed groups with the highest level recorded in group 1 (p ≤ 0.05). Complement and lysozyme activities were significantly higher in group 1 followed by group 2 than both group 3 and control 1 (p ≤ 0.05). Also, serum bactericidal activity was significantly higher in group 1 than other groups (p ≤ 0.05). Serum total protein and immunoglobulin were higher in probiotic groups than control 1. No significant changes were seen in serum level of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) among all groups. After challenging fish with virulent strain of Aeromonas hydrophila, the lowest mortality was obtained in groups 1 and 2, respectively (p ≤ 0.05). These data clearly show that application of probiotic L. plantarum in carp feed can not only improve the growth variables and immunophysiological responses of fish but also increase the fish disease resistance to motile Aeromonas septicemia caused by Aeromonas hydrophila. However, these positive improvements are dose dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to rising demands for aquatic animal products and extensive expansion of aquaculture in last decades, numerous efforts have been made for improving the growth and health conditions of aquatic animals. In other words, the goals of modern aquaculture are an increase in production and optimizing profitability. Traditional strategy for disease control in aquaculture sector is usage of chemical agents, e.g., antibiotics, but this is no longer a recommended practice due to the emergence of bacterial resistance, and also concerning of environmental impacts and human food safety (Son et al. 2009). For example, in recent years, the use of antibiotics such as oxytetracycline in aquaculture has been prohibited by several countries due to serious environmental hazards (Suzer et al. 2008). So far, some successes have been achieved in using vaccines, growth promoters, and immunostimulants such as prebiotics and probiotics with a more environmental friendly approached for managing the diseases. Probiotics are beneficial microorganisms when administered in adequate amounts as natural food additives can stimulate growth, promote vitamin synthesis, help food digestion and absorption, enhance some specific and non-specific immune status, and improve stress tolerance and disease resistance leading to healthier fish with higher growth rate (Das et al. 2013). Therefore, despite the antibiotics and chemical drugs, probiotics are known as healthy and environmental friendly agents. Probiotics are relatively cheap and easily available for use in aquaculture without any stress for fish. Thus, the use of probiotics has been suggested to become an alternative way for the prevention and control of various diseases in aquaculture (Irianto and Austin 2002; Rollo et al. 2006; Aly et al. 2008; Sahu et al. 2008; Standen et al. 2013).
Functional food additives such as probiotics are relatively a new concept in aquaculture. An ideal probiotic must be able to colonize, establish, and multiply in the host. There is a general consensus that probiotics selected from autochthonous origin have a greater capability of competing with the resident microbes and becoming predominant and able to persist in the enteric environment (Nayak 2010). Unfortunately, most of commercial probiotics used in aquaculture are of non-fish origin. The safety and efficiency of these exogenous probiotics are still questionable despite some success in fish breeding (Cerezuela et al. 2012). In addition, effects of probiotics seem to be dose dependent as their applications in higher concentration do not correlate with the best results. Also, use of inadequate amount of probiotics will not be beneficial to animals (Nikoskelainen et al. 2001). Thus, it has been suggested that the use of fish intestinal autochthonous bacteria with proper dosage might be a key source in finding an ideal probiotic (Balcázar et al. 2006; Wu et al. 2012). Lactobacilli are commonly found in aquatic and known to be present in the gastrointestinal tract of aquatic animals (Balcázar et al. 2008). Lactobacillus genus is most commonly used probiotic in aquaculture because they are resistance to the environmental conditions and have long lasting shelf life and the beneficial roles of these bacterial species in the aquaculture field are well established (Wang et al. 2008).
Food represents a major cost for intensive aquaculture, and it is one of the most important factors that influence the ability of fish to attain its genetic potential for growth and maintain proper health. In other words, immunophysiological parameters and enzymatic profile can provide noteworthy information of any changes occurring in the organism as a result of overall health condition of fish similar to those of warm blooded animals (Thrall et al. 2004; Aderfemo 2007). However, little information is available concerning incorporating the probiotics into common carp (Cyprinus carpio) culture based on growth and hemato-immunological parameters (Chi et al. 2014; Gupta et al. 2014). This study was conducted to evaluate the effect of different dosages of Lactobacillus plantarum (previously isolated from fish intestine) on growth, performance, and some immunophysiological parameters and level of common carp that is one of the most important freshwater commercial fish species especially in the Asian regions.
Materials and methods
Fish and experiment design
Four hundred and eighty common carp weighing 31.8 ± 2.6 g were obtained from a cyprinid fish farm (Ahvaz, Khuzestan, Iran), transported to laboratory tanks and allowed to be acclimatized for 2 weeks before the beginning of the experiment. Water quality parameters were as follows: temperature 26 ± 1 °C, dissolved oxygen 9 ± 1 mg/l, pH 7.7 ± 0.33, NH3 < 0.01 mg/l, NO2 < 0.1 mg/l, and salinity 700 μS/cm. The rearing tanks were equipped with a recirculation biofiltration system, and water change was undertaken at 30% per day to maintain the optimum water quality. Fish were randomly divided into four groups each in triplicates (each triplicate containing 40 fish). Groups 1 to 3 were fed with commercial extruded carp feed containing 0.7, 0.5, and 0.3 g probiotic/kg feed, respectively. Group 4 received normal feed and was considered as control 1. The fish were fed 3% of body weight per day, and the experiment was run for 80 days.
Probiotic and food preparation
Lactobacillus plantarum powder (1010 CFU/g) was used in this study. This species has been previously isolated from Persian sturgeon (Acipenser persicus) fish (Accession number KC426951). Adequate probiotic was dissolved in 200 ml water at 25 °C and then sprayed on 1 kg extruded carp feed (Faradaneh Co. Shahrekord, Iran). The surface of treated feed was then sprayed with 3% gelatin solution to stabilize the probiotic on feed granules. The control feed was coated with gelatin but without probiotic. Feeds were dried in room temperature and packed in separate plastic containers for each group and stored in cool and dry condition until used. Viable count of the probiotic was undertaken in gram feed using MRS medium at 30 °C, and so the final concentrations of the probiotic used were 1.2 × 106, 0.9 × 106, and 0.56 × 106 cfu/g feed. As there are some reports saying that the concentration probiotics in diet may decrease considerably during storage time (Sun et al. 2012), so the experimental feeds were prepared every 30 days intervals. The ingredients of the feed are given in Table 1.
Biometry and blood sample
Biometry and blood sampling were conducted on days 0, 20, 40, 60, and 80 of experiment. Weight and standard length of fish were recorded in grams and centimeters, respectively. Fifteen fish of each treatment was anesthetized by MS222, and blood samples were taken from caudal vein using heparin-embedded syringes. The only exception was samples of the day 0 which were taken from 15 fish randomly, before being divided in four groups.
Assessment of growth parameters
The specific growth rate (SGR), feed conversion ratio (FCR) and protein efficiency ratio (PER), daily weight gain (DWG) and condition factor (CF) were calculated according to the following equations:
- FCR:
-
Food intake (g) / weight gain (g)
- PER:
-
Wet weight gain (g) / total protein intake (g)
- DWG:
-
Average final weight (g) − Average initial weight (g) / experimental period (80 days)
- CF:
-
Weight (g) / [standard length (cm)]3
Survival rate
Daily mortality was recorded and the survival rate was obtained using below equation.
Hematological factors
Hemoglobin (Hob) level was determined by cianometa-hemoglobin method using Total Hemoglobin kit made by Ziest Chem Diagnostic Co (Tehran, Iran). Packed cell volume (PCV) or hematocrit (Hct) was determined by centrifuging micro hematocrit in 10,000g for 10 min according to the method described by Feldman et al. (2000). The blood sample was diluted with Natt–Herrick solution in 1 to 200 ratios, to determine total red blood cells (TRBC) and total white blood cells (TWBC) using neubauer hemocytometer chamber, and TRBC and TWBC were calculated using below formulas (Thrall et al. 2004). Differential count of leukocytes was undertaken following the method of Schaperclaus et al. (1991).
Respiratory burst assay
The respiratory burst assay was carried out using Nitro Blue Tetrazolium (NBT) by intracellular superoxide radicals (Anderson and Siwicki 1994). Briefly, 100 μl of heparinized blood from fish of each group was mixed with 100 μl of 0.2% NBT (Merck, Germany) solution for 30 min at 25 °C. After incubation, 50 μl of the mixture was added with 1 ml of N,N-diethylmethyl from amide (Sigma, USA), centrifuged at 3000g for 5 min, and the optical density of the supernatant was measured at 540 nm.
Alternative complement assay
Alternative pathway of complement was measured using rabbit red blood cells (RaRBC). RaRBCs were first placed in 1.5% Agarose (pH 7.2), containing 75 μl MgCl2 1 M and 150 μl CaCl2 1 M in 100 ml phosphate-buffered saline (PBS, 0.1 M, pH = 7.0). The RaRBC suspension was washed with PBS by centrifugation at 3000 rpm for 5 min, and the cell concentration was adjusted to 1 × 108 cell/ml. A volume of 12 ml agarose containing RaRBC was dispensed into a plate, incubated at 4 °C and holes punched (3 mm in diameter). Subsequently, each hole was filled with 20 μl of serum sample and incubated at room temperature for 48 h, after which the diameter of lysis was subsequently measured (Brata 1993).
Serum bactericidal activity
Bactericidal activity of sera samples was studied following procedure by Azza (2009) with slight modification. Sera samples were first diluted three times with 0.1% gelatin-veronal buffer (GVBC2) (v/v) (pH 7.5, containing 0.5 mM/ml Mg2+ and 0.15 mM/ml Ca2+). Aeromonas hydrophila (live, washed cells) was suspended in the same buffer at concentration 105 CFU/ml. The diluted sera and bacteria cells were mixed at 1:1 v/v, incubated for 90 min at 25 °C with shaker. Control group containing bacterial suspension was also included. The number of viable bacterial cells (cfu/ml) was then determined after culturing on Tripticase Soy Agar (TSA) plates at 25 °C for 24 h.
Lysozyme assay
A turbidometric assay using lyophilized Micrococcus lysodeikticus (Sigma-Aldrich) was used to determine lysozyme activity in sera samples (Sharifuzzaman and Austin 2009). A volume of 135 μl of M. lysodeikticus at concentration 0.2 mg/ml (w/v) in 0.02 M sodium phosphate buffer (SPB), pH 5.8 (Sigma-Aldrich), was added to 15 μl of serum sample. For negative control, serum was replaced with SPB. Results were expressed in units of lysozyme per milliliters of serum. A unit of lysozyme activity was defined as the amount of serum causing a reduction of 0.001/min at 450 nm at 22 °C.
Serum total protein, albumin, and globulin assays
Total protein and albumin concentrations were determined through colorimetric method at 550 nm method using commercial kits (Pars Azmoon®, Tehran, Iran) (Nayak et al. 2008; Sahoo et al. 2008). Globulin content was estimated by subtracting the albumin content from total protein content.
Assay of AST, ALT, ALP, and LDH enzymes
The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) were determined using commercial kits (Pars Azmoon®, Tehran, Iran), a colorimetric spectrophotometer at specific wave length suggested by the manufacturer (Reitman and Frankel 1957).
Disease resistance (challenge test)
Aeromonas hydrophila (AH-04) previously recovered from silver carp and approved by Institute of Aquaculture, Uni. Sterling, Scotland, were cultured for 48 h, harvested, washed three times with sterile phosphate-buffered saline (PBS) (pH = 7.4) and centrifuged at 4000g for 15 min. Tenfold serial dilutions of the cells were then prepared into sterile PBS, and total cell count was determined using a hematocytometer. The bacterial suspension was checked by Gram staining and sub-culturing on TSA prior to use for challenge test.
Two replicates of 10 healthy fish for each concentration were intraperitoneally (i.p.) injected with the bacterium at 101, 102, 103, 104, 105, 106, 107, 108, and 109 cells/fish at 25 °C after anesthetization fish by MS222 (50 mg/l), and they were kept separately in 60 l aquaria with clean water at 25 °C and well aeration for 10 days. Control groups were i.p injected with 0.1 ml/fish of sterile PBS and were kept separately. Daily mortality was recorded, and the cause of death was ascertained by re-isolating the infecting organism from kidney and liver of dead fish (Soltani and Kalbassi 2001). The LD50 of Aeromonas hydrophila (2.1 × 107 cells/fish) was calculated according to Reed and Muench (1938).
To assess the disease resistance of the treated fish, 36 fish from each group (12 fish in replace) were i.p. injected with the 200 μl bacterial suspension (2.1 × 107 CFU/ fish equal to LD50) after fish being anesthetized with MS222. Daily mortality was recorded for 14 days, and the cause of death was ascertained by re-isolating the infecting organism from kidney of dead fish. Cumulative daily mortality curve was drawn according to Misra et al. (2006).
Statistical analysis
Mean values of measured factors were statistically compared using a one-way ANOVA (P < 0.05), and differences among means were identified using the Duncan Multiple Range Test. Analyses were carried out with the IBM Statistical analysis software package (SPSS, version 22).
Results
Growth indices
Results of growth performance are shown in Figs. 1, 2, 3, 4, 5, and 6. Almost all growth factors have been positively affected by the use of probiotic in the fish feed. Daily weight gain (DWG) was higher in probiotic groups, and the difference reached to significant level in groups 1 and 2 after 40 days of probiotic feeding with the highest DWG obtained in group 1 (Fig. 1) (P < 0.05). Also, specific growth rate (SGR) was higher in probiotic groups with significant differences seen in group 1 after day 40 (Fig. 2) (P < 0.05). Feed conversion ratio (FCR) was improved in all fish groups fed with the probiotic, with the best FCR obtained in group 1 followed by group 2 (Fig. 3). However, significant difference was obtained in group 1 after day 40 of experiment compared to control 1 (P < 0.05). Protein efficacy ratio (PER) was recorded higher in the probiotic-treated groups almost during the experiment, but it was significantly higher in groups 1 and 2 at day 40 and thereafter (P < 0.05). The highest PER was obtained in group 1 (Fig. 4). Condition factor (CF) was significantly higher at days 60 and 80 in groups 1 and 2 compared to control (Fig. 5) (P < 0.05). Total biomass and final weight (FW) were different among four groups during sampling stages (Fig. 6). Final weight of groups 1 and 2 was significantly higher than control on day 40 of experiment (P < 0.05). The highest FW was obtained in group 1 (Fig. 6). No mortality was occurred during experiment in all groups.
Hematological factors
TRBC count was increased in group 1 and 2, but it was significantly higher in group 1 compared to control in the second half of the experiment (P < 0.05) (Fig. 7).
Hematocrit (Hct) was significantly higher in group 1 on days 40, 60, and 80 of the trail compare to other groups (P < 0.05). Similar increase in Hct level was observed in group 2 on days 60 and 80, but the difference was not significant compared with control 1 (P > 0.05) (Fig. 8).
As shown in Fig. 9, hemoglobin (Hb) level in groups 1 and 2 was significantly higher than control fish after day 40 of feeding (P < 0.05). In addition, Hb level in group 1 was significantly higher than group 2 on day 40 of feeding (P < 0.05).
The results of TWBC count and differential leukocyte count are shown in Table 2. The TWBC count was higher in all fish groups fed with the probiotic compared to control fish. When comparing with control group, groups 1 and 2 showed a significant increase after day 40 of experiment with the highest TWBC count recorded in group 1 on day 80 of feeding (P < 0.05). Results revealed an increase in neutrophil count in probiotic-treated groups which started from day 40 of experiment with a significant difference observed in groups 1 and 2 on days 60 compared to control 1 (P < 0.05). No significant difference was seen in the volume of other leukocyte count among the experimental groups (P > 0.05).
Immunological factors
Respiratory burst activity
The level of respiratory burst activity was higher in groups 1 and 2 on days 20 and 40 of the experiment. This increment was persistent until the end of experiment, and the highest activity was recorded in group 1 at day 80 (Fig. 10).
Alternative complement activity
The alternative complement activity was significantly higher in groups 1 and 2 compared with control (P < 0.05), and the highest activity was measured in group 1 on day 80 of feeding. No significant difference was seen between groups 1 and 2 except on day 80 of feeding (Fig. 11).
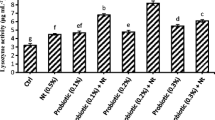
Serum lysozyme activity
Serum lysozyme activity was higher in all probiotic-treated groups, with the highest activity obtained in group 1 followed by group 2. This activity in group 1 was significantly higher than control after 40 days of feeding compared to control (P < 0.05) (Fig. 12).
Serum bactericidal activity
As shown in Fig. 13, the number of viable bacterial count was lower in all fish fed with probiotic compared to control 1. This difference was only significant between group 1 and control (P < 0.05). No significant difference was seen among groups 1, 2, and 3 (P > 0.05) (Fig. 13).
Serum proteins and enzymes
Results of sera proteins and enzymes are given in Table 3. Serum total protein was increased in groups 1 and 2 compared to other groups, but it was only significantly different in group 1 on days 60 and 80 of feeding. Also, total globulin was higher in groups 1 and 2 compared to other groups, with a significant difference seen in group 1 (P < 0.05). Although a mild increase was obtained in ALP and LDH levels in groups 1 and 2, no significant changes were observed in serum levels ALP, LDH, AST, and ALT during the experiment (P > 0.05).
Disease resistance
Results of cumulative mortalities are shown in Fig. 14. The lowest mortality was occurred in group 1 followed by group 2, with significant difference obtained groups 1 and 2 compared with control group (P < 0.05). No significant difference was seen in mortality percentage between groups 1 and 2 (P > 0.05). Also, the cumulative mortality in group 3 was insignificantly lower than control fish (P > 0.05). In addition, no significant difference was seen between groups 3 and 2 (Fig. 14).
Comparison of cumulative mortality among four different experimental groups during 14 days after bacterial challenge. Significant differences between groups are shown with different English letters (P < 0.05). G1 = 0.7 g/kg L. plantarum, G2 = 0.5 g/kg L. plantarum, G3 = 0.3 g/kg L. plantarum, G4 = control group
Discussion
With the increasing intensification and commercialization of aquaculture production, fish health and growth efficiency have become important issues in aquaculture industry. The widespread use of broad-spectrum chemotherapeutics to combat health-related problems and growth enhancement has led to the development and spread of drug-resistant pathogens, environmental hazards, and food security problems (Das et al. 2013). Nowadays, traditional methods in controlling diseases in aquaculture are not satisfied anymore. Probiotics are safe alternatives to traditional chemotherapy to improve overall health condition and growth performance (Balcázar et al. 2006; Nayak 2010).
Effects of dietary probiotics supplementation on host health may depend on various factors including the dose and type of probiotic and duration of feeding (Jiang et al. 2013; Nikoskelainen et al. 2001). Higher level of probiotics does not correlate with the best results in terms of growth and other physiological status. Overdosing can lead to undesirable side effects and cause unnecessary costs, whereas the low dose may impose a cost without acquiring satisfying results. Thus, it is important to demonstrate the appropriate dose of a probiotic to be established before its application in intensive aquaculture (Mocanu et al. 2010). Although lactobacillus bacteria are among the most resistant bacterial species to the environment conditions and have long-lasting shelf life (Wang et al. 2008), the amount of the probiotic may decrease 5–10% over 30 days of storage (Sun et al. 2012). Although a 5–10% decrease in probiotic level is not considerable, we prepared the probiotic supplemented in feed every 30 days to ensure its maximum level in the diet. It may seem difficult to prepare fresh probiotic supplemented feed in practice, but the use of vacuumed polypropylene packages and storing the prepared feed in cool and dry place can easily prolong the shelf life of probiotics (Lara-Flores 2011). Moreover, coating fish food particles with gelatin and oil and recently microencapsulation of probiotics with polysaccharides and proteins has been carried out to protect them from harsh conditions such as high temperature, pH, or salinity of water environment and gastrointestinal passage (Corona-Hernandez et al. 2013). Finally, endogenous probiotics isolated from local aquatic environments are more compatible with water environment condition; thus, the use of endogenous probiotics is preferable to other types (Lara-Flores 2011).
In the present study, we investigated effects of three different doses of L. plantarum on growth and hemato-immunological parameters of common carp fingerling for a period of 80 days. According to the results of this study, the use of L. plantarum caused an overall positive increment in growth performance and hemato-immunological factors of common carp. The highest growth and feed utilization were recorded in group 1 that was fed with 0.7 g probiotic/kg food. All growth indices were significantly higher in group 1 compared to control fish. The second growth efficacy was observed in group 2 (0.5 g probiotic/kg food). Final weight, CF, and DWG in group 2 were significantly higher than control fish. The improvement in most growth factors was correlated with both probiotic dosage and duration of administration, and significant differences in growth indices were obtained mostly in the second half of the experiment. FCR was reduced in all treated groups with the lowest FCR (about 1.3) seen in fish fed with 0.7 g/kg compared to control fish (FCR = 1.7). The almost 0.4 difference in FCR provided by the probiotic is quite an ideal factor once calculating the cost-effective of fish production in a mass production condition. Also, protein efficacy ratio (PER) was recorded to be higher in all probiotic-treated groups almost during the experiment with the best efficiency obtained in fish fed with probiotic at 0.7 g/kg feed. A higher levels of CF and FW in fish fed with probiotic supplemented feed means fish gained more energy and nutrition from their diet compared to ordinary fish feed. In addition, increase of CF to a certain amount is desirable in common carp culture because slightly wider carps in dimensions are of greater value in the market. The reason why fish were fed higher dosage of the probiotic may in part be due to some functions of the probiotic, i.e., improving in feed utilization by synthesis of some growth factors such as vitamins, co-factors, fatty acids, and amino acids and augment of digestive activity of target animal which increase nutrient absorption (Balcázar et al. 2006; Ghosh et al. 2004; Mohapatra et al. 2012; Suzer et al. 2008; Venkat et al. 2004). Probiotics also can change the gut epithelium architecture and improve nutrient absorption by providing more absorptive surface areas (Pirarat et al. 2011). Similar improvements in growth performance have been reported in different fish species such as grouper, E. coioides (Son et al. 2009), tilapia, O. niloticus (Iman et al. 2013), C. catla (Parthasarathy and Ravi 2011), Labeo rohita (Ghosh et al. 2004; Giri et al. 2013), and in crustacean models such as P. indicus (Uma et al. 1999) and L. vannamei (Kongnum and Hongpattarakere 2012) after feeding diet enriched with L. plantarum. Also, similar improvements in growth performance were seen in common carp (Yanbo and Zirong 2006; Gupta et al. 2014), tilapia, Tilapia nilotica (Aly et al. 2008), sea bream, Sparus aurata (Suzer et al. 2008), cat fish, Clarius gariepinus (Al-Dohail et al. 2009), trout, O. mykiss (Andani et al. 2012), snapper, Lutjanus peru (Reyes-Becerril et al. 2012), and freshwater prawn, M. rosenbergii (Venkat et al. 2004) by administration of other Lactobacillus sp. as the feed supplementation. However, there are some data reporting no significant benefit on using probiotics as growth promoters in some aquatic organisms (Merrifield et al. 2010; Shelby et al. 2006; Shelby et al. 2007; Reyes-Becerril et al. 2012). It is seems that different factors such as type of probiotic and its dosage, fish species and age, duration of probiotic intake, and rearing conditions are important factors influencing the outcome of a probiotic in aquaculture sector (Ramos et al. 2013). It is also important to know that the safety of candidate probiotic is crucial. In this study, we did observe no sign of stress or mortality among treatments during 80 days of trail (Das et al. 2013).
Liver enzymes are used as markers of health and physiologic conditions. Higher levels of liver enzymes are normally seen in younger animals, fast growing animals, and during pregnancy. In addition, damage to the liver cells can significantly raise level of liver enzyme activity in serum (Marzouk et al. 2008; Berk and Korenblat 2016). In the present, no significant difference was observed in levels of liver enzymes compared to control 1. Thus, application of L. plantarum in carp at 1.2 × 106 cfu/g feed showed no negative effect on some biochemical parameters such as the mentioned enzymes (Marzouk et al. 2008; Reyes-Becerril et al. 2012).
According to our results, the use of probiotic L. plantarum caused a positive hematopoietic effect, as TRBC count, TWBC, and HCT were higher in groups 1 and 2 compared to control 1. Hematocrit is the ration of the red blood cells volume to the total volume of blood which can be affected by the numbers of cells. An increase in the value of hematocrit after 2 months of feeding indicated that the safety of the probiotic and its efficacy in improving the health status as a reduction in hematocrit level indicate that fish are not eating or are suffering from some infections (Aly et al. 2008). From the literature review available, the pattern of hematological values obtained in our study is generally similar to that of other species of fish (Harikrishnan et al. 2003; Tripathi et al. 2004; Kumar et al. 2006; Aly et al. 2008; Abd El-Rhman et al. 2009; Mocanu et al. 2010; Reyes-Becerril et al. 2012; Renuka et al. 2014; Rajikkannu et al. 2015). Also, increase in WBC shows a positive stimulating effect of immunocompetent cells (leukocyte populations) made by the probiotic. Such a positive effect can be due to the valuable nutritional ingredients, e.g., vitamin B complex provided by the probiotics. Obviously, enhancement in proliferation of leukocyte population cells can result in improving of the animal immune status, as values of lyszoyme, complement activity, and respiratory burst were increased in the treated fish with the probiotic. This was also supported when the treated fish were challenged with virulent strain of Aeromonas hydrophila and significantly higher levels of survivals were observed in the fish fed with the probiotic compared to control 1. Use of B. coagulans and B. licheniformis in common carp fed for up to 80 days also could enhance some immune responses, i.e., respiratory burst and lysozyme levels (Chi et al. 2014; Gupta et al. 2014).
Serum proteins (albumins and globulins) play a significant role in immune response, and the level of the serum total protein can be attributed as a reflection of innate immunity (Wiegertjes et al. 1996). Our findings showed an increasing trend in total serum protein content of groups 1 and 2 during experiment and the differences were significant after day 60. Almost the same trend was observed for globulin of probiotic groups, but serum albumin level did not show significant change in any of groups during experimental period. This could be a result of the stimulation of gut-associated lymphoid tissue by the probiotic bacteria and increase in lymphocyte cell activity. Different results have been reported by other researchers (e.g. Nayak et al. 2007; Reyes-Becerril et al. 2012; Khattab et al. 2005; Mohammadian et al. 2016) that can be due to the differences in fish species, type of probiotic, dosage of administration, and water quality conditions. Various factors such as lysozyme, complement component, immunoglobulins, and antimicrobial peptides can affect serum bactericidal level in fish. In present study, there was a good correlation between bactericidal activity and levels of above factors in carp especially in fish fed with higher dosage of the probiotic (1.2 × 106 cfu/g feed). Similar results are reported by Mohammadian et al. (2016) using different fish species and combined probiotics. Aeromonas hydrophila is one of the most common pathogens in warm water fish species causing hemorrhagic septicemia (Austin and Austin 2012). According to our results after challenging fish with Aeromonas hydrophila, the lowest mortality occurred in fish fed with higher dosage of the probiotic (group 1) followed by group 2. Such higher survival in fish fed higher dosage of the probiotic is clearly correlated with the findings of the immunological factors.
In conclusion, the use of L. plantarum, as probiotic with an autochthonous source, could use a significant positive effect on growth factors and improved some hemato-immunological parameters of common carp. Also, a higher resistance in fish was seen when were challenged with Aeromonas hydrophila. According to our findings, the effects of this probiotic are time and dose dependent, as the best positive effects were obtained in fish fed higher dosage of the probiotic. Therefore, application of autochthonous L. plantarum as probiotic can be beneficial in overall health condition of fish and production efficacy with no notable side effects. However, histological assay is essential for further investigation of potential adverse effect.
References
Abd El-Rhman AM, Khattab YA, Shalaby AM (2009) Micrococcus Luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 27(2):175–180
Aderfemo OK (2007) Haematological profile of Clarias gariepinus (Burchell, 1822) exposed to lead. Turkish J Fish Aquat Sci 7:163–169
Al-Dohail MA, Hashim R, Aliyu-Paiko M (2009) Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac Res 40:1642–1652
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25(1):128–136
Andani HRR, Tukmechi A, Meshkini S, Sheikhzadeh N (2012) Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 28(5):728–734
Anderson DP, Siwicki AK (1994) Duration of protection against Aeromonas salmonicida in brook trout immunostimulated with glucan or chitosan by injection and immersion. Prog Fish Cult 56:258–261
Austin B, Austin DA (2012) Bacterial fish pathogens: disease of farmed and wild fish. Springer, Dordrecht
Azza MM (2009) Antagonism of Aeromonas hydrophila by propolis and its effect on the performance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27(3):454–459
Balcázar JL, Id B, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114:173–186
Balcázar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Muzquiz JL, Girones O (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278:188–191
Berk P, Korenblat K (2016) Approach to the patient with jaundice or abnormal liver tests. In: Goldman L, Schafer AI (eds) Goldman-Cecil Medicine, 25th edn. Elsevier Saunders, Philadelphia chapter 147
Brata O (1993) Veterinary clinical immunology laboratory, vol Vol 2. Bar- Lab Inc, Blacksburg, pp 24–25 Section 3
Cerezuela R, Guardiola FA, Meseguer J, Esteban M (2012) Increases in immune parameters by inulin and Bacillus subtilis dietary administration to gilthead seabream (Sparus aurata L.) did not correlate with disease resistance to Photobacterium damselae. Fish Shellfish Immunol 32(6):1032–1040
Chi C, Jiang B, Yu XB, Liu TQ, Xia L, Wang GX (2014) Effects of three strains of intestinal autochthonous bacteria and their extracellular products on the immune response and disease resistance of common carp, Cyprinus carpio. Fish Shellfish Immunol 36(1):9–18
Corona-Hernandez RI, Álvarez-Parrilla E, Lizardi-Mendoza J, Islas-Rubio AR, Rosa L, Wall-Medrano A (2013) Structural stability and viability of microencapsulated probiotic bacteria: a review. Compr Rev Food Sci Food Saf 12(6):614–628
Das A, Nakhro K, Chowdhury S, Kamilya D (2013) Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol 35(5):1547–1553
Feldman BF, Zinkl JG, Jain NC (2000) Schalm’s Veterinary hematology, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1120–1124
Ghosh K, Sen SK, Ray AK (2004) Supplementation of lactobacillus acidophilus in compound diets for Labeo rohita fingerlings. Indian J Fish 51(4):521–526
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34(2):660–666
Gupta A, Gupta P, Dhawan A (2014) Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immunol 41(2):113–119
Harikrishnan R, Rani MN, Balasundaram C (2003) Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 221:41–50
Iman MKA, Wafaa TA, Elham SA, Mohammad MNA, Kawther E, Osama MS, Gamal AI, Zeinab IS, Hoda SE (2013) Evaluation of Lactobacillus plantarum as a probiotic in aquaculture: emphasis on growth performance and innate immunity. J Appl Sci Res 9(1):572–582
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642
Jiang HF, Liu XL, Chang YQ, Liu MT, Wang GX (2013) Effects of dietary supplementation of probiotic Shewanella colwelliana WA64, Shewanella olleyana WA65 on the innate immunity and disease resistance of abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol 35(1):86–91
Khattab YA, Shalaby AM, Abdel-Rhman A (2005) Use of probiotic bacteria as growth promoters, antibacterial and their effects on physiological parameters of Oreochromis niloticus. In: Proceedings of international symposium on Nile Tilapia in aquaculture, 7: 156–165
Kongnum K, Hongpattarakere T (2012) Effect of Lactobacillus plantarum isolated from digestive tract of wild shrimp on growth and survival of white shrimp (Litopenaeus vannamei) challenged with Vibrio harveyi. Fish Shellfish Immunol 32(1):170–177
Kumar R, Mukherjee SC, Prasad KP, Pal AK (2006) Evaluation of Bacillus subtilis as a probiotic to Indian major carp, Labeo rohita. Aquac Res 37:1215–1221
Lara-Flores M (2011) The use of probiotic in aquaculture: an overview. Int Res J Microbiol 2(12):471–478
Marzouk MS, Moustafa MM, Mohamed NM (2008) Evaluation of Immunomodulatory Effects of Some Probiotics on Cultured Oreochromis niloticus. 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt
Merrifield DL, Dimitroglou A, Bradley G, Baker RTM, Davies SJ (2010) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquac Nutr 16:504–510
Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of multiple injections of beta-glucan on non-specific immune response and disease resistance in Labeo rohita fingerlings. Fish Shellfish Immunol 20(3):305–319
Mocanu M, Cristea V, Dediu L, Bocioc E, Grecu RI, Ion S, Vasilean I (2010) The effect of probiotic diet on growth and hematology parameters of rainbow trout (Onchorhynchus mykiss, Walbaum 1792). Lucrari Stiintifice-Sera Zootheniie 59:258–263
Mohammadian T, Alishahi M, Tabandeh MR, Ghorbanpoor M, Gharibi D, Tollabi M, Rohanizade S (2016) Probiotic effects of Lactobacillus plantarum and L. delbrueckii ssp. bulguricus on some immune-related parameters in Barbus grypus. Aquac Int 24(1):225–242
Mohapatra S, Chakraborty T, Prusty AK, Das P, Paniprasad K, Mohanta KN (2012) Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effects on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquac Nutr 18(1):1–11
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14
Nayak SK, Swain P, Mukherjee SC (2007) Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (ham). Fish Shellfish Immunol 23(4):892–896
Nayak SK, Swain P, Nanda PK, Dash S, Shukla S, Meher PK, Maiti NK (2008) Effect of endotoxin on the immunity of Indian major carp, Labeo rohita. Fish Shellfish Immunol 24(4):394–399
Nikoskelainen S, Salminene S, Bylund G, Ouwehand AC (2001) Characterization of the properties of human- and dairy-derived probiotics for prevention of infectious diseases in fish. Appl Environ Microbiol 67:2430–2435
Parthasarathy R, Ravi D (2011) Probiotic bacteria as growth promoter and biocontrol agent against Aeromonas hydrophila in Catla catla (Hamilton, 1822). Indian J Fish 58(3):87–93
Pirarat N, Pinpimai K, Endo M, Katagiri T, Ponpornpisit A, Chansue N, Maita M (2011) Modulation of intestinal morphology and immunity in Nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res Vet Sci 91(3):92–97
Rajikkannu M, Natarajan N, Santhanam P, Deivasigamani B, Ilamathi J, Janani S (2015) Effect of probiotics on the haematological parameters of Indian major carp (Labeo rohita). Int j fish aquat stud 2(5):105–109
Ramos MA, Weber B, Gonçalves JF, Santos GA, Rema P, Ozorio ROA (2013) Dietary probiotic supplementation modulated gut microbiota and improved growth of juvenile rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 166:302–307
Reed LV, Muench H (1938) A simple method of estimating fifty percent end points. Am J Hyg 27:493–497
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacitic and glutamic pyruvic transaminases. Am J Clin Path 28:56–63
Renuka KP, Venkateshwarlu M, Naik AR (2014) Effect of probiotic (Lactobacillus acidophilus) on Haematological parameters of Catla catla (Hamilton). Int J Curr Microbiol App Sci 3(8):326–335
Reyes-Becerril M, Ascencio-Valle F, Macias ME, Maldonado M, Rojas M, Esteba MA (2012) Effects of marine silages enriched with Lactobacillus sakei 5-4 on haematoimmunological and growth response in Pacific red snapper (Lutjanus peru) exposed to Aeromonas veronii. Fish Shellfish Immunol 33(4):984–992
Rollo A, Sulpizio R, Nardi M, Silvi S, Orpianesi C, Caggiano M, Cresci A, Carnevali O (2006) Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiol Biochem 32(2):167–177
Sahoo P, Mahapatra KD, Saha JN, Barat A, Sahoo M, Mohanty BR, Gjerde B, Odegård J, Rye M, Salte R (2008) Family association between immune parameters and resistance to Aeromonas hydrophila infection in the Indian major carp, Labeo rohita. Fish Shellfish Immunol 25(1):163–169
Sahu MK, Swarnakumar N, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Indian J Microbiol 48:299–308
Schaperclaus W, Kulow H, Schreckenbach K (1991) Hematological and serological technique. In: Kothekar VS (ed) Fish disease. Oxonian Press, New Delhi, pp 71–108
Sharifuzzaman S, Austin B (2009) Influence of probiotic feeding duration on disease resistance and immune parameters in rainbow trout. Fish Shellfish Immunol 27(3):440–445
Shelby RA, Lim C, Yildirim-Aksoy M, Delaney MA (2006) Effects of probiotic supplements on disease resistance and immune response of young Nile tilapia. Oreochromis niloticus. J Appl Aquaculture 18:22–34
Shelby RA, Lim C, Yildirim-Aksoy M, Klesius PH (2007) Effects of probiotic bacteria as dietary supplements on growth and disease resistance in young channel catfish, Ictalurus punctatus (Rafinesque). J Appl Aquaculture 19:81–91
Soltani M, Kalbassi MR (2001) Protection of Persian sturgeon (Acipenser persicus) fingerling against Aeromonas hydrophila septicemia using three different antigens. Bull Eur Ass Fish Pathol 21(6):235
Son VM, Chang CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol 26(5):691–698
Standen BT, Rawling MD, Davies SJ, Castex M, Foey A, Gioacchini G, Carnevali O, Merrifield DL (2013) Probiotic Pediococcus acidilactici modulates both localised intestinal- and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol 35(4):1097–1104
Sun YZ, Yang HL, Ma RL, Song K, Li JS (2012) Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquac Nutr 18(3):281–289
Suzer C, Coban D, Kamaci HO, Saka S, Firat K, Otgucuoglu O, Kucuksari H (2008) Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture 280:140–145
Thrall MA, Weiser G, Allison R, Campbell TW (2004) Veterinary haematology and clinical chemistry. Wiley- Blackwell, Hoboken, pp 277–289
Tripathi NK, Latimer KS, Burnley VV (2004) Hematologic reference intervals for koi (Cyprinus carpio), including blood cell morphology, cytochemistry, and ultrastructure. Vet Clin Path 33(2):74–83
Uma A, Abraham TJ, Sundararaj V (1999) Effect of a probiotic bacterium, Lactobacillus plantarum on disease resistance of Penaeus indicus larvae. Indian J Fish 46(4):367–373
Venkat HK, Narottam PS, Kamal KJ (2004) Effect of feeding lactobacillus-based probiotics on the gut microflora, growth and survival of postlarvae of Macrobrachium rosenbergii (de man). Aquac Res 35:501–507
Wang YB, Li JR, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4
Wiegertjes GF, Stet RM, Parmentier HK, van Muiswinkel WB (1996) Immunogenetics of disease resistance in fish: a comparative approach. Dev Comp Immunol 20(6):365–381
Wu S, Wang G, Angert ER, Wang W, Li W, Zou H (2012) Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS One 7:e30440
Yanbo W, Zirong X (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 127:283–292
Acknowledgements
This work was funded by a Grant from Research Council of University of Tehran and Center of Excellence of Aquatic Animal Health, University of Tehran.
We would like to thank Faradaneh Company for supporting and providing us the common carp food.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors confirm that there is no conflict of interest in the publication of this paper.
Rights and permissions
About this article
Cite this article
Soltani, M., Abdy, E., Alishahi, M. et al. Growth performance, immune-physiological variables and disease resistance of common carp (Cyprinus carpio) orally subjected to different concentrations of Lactobacillus plantarum . Aquacult Int 25, 1913–1933 (2017). https://doi.org/10.1007/s10499-017-0164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0164-8