Abstract
The study was conducted to evaluate the effect of dietary lipid levels on growth, liver oxidative stress, and serum metabolites of juvenile hybrid snakehead (Channa argus × Channa maculata). Five isonitrogenous (crude protein 420 g kg−1) practical diets containing 58, 87, 115, 144, and 173 g kg−1 crude lipid (named L58, L87, L115, L144, and L173, respectively) were fed to triplicate groups of 30 fish (mean initial weight 24 g) for 8 weeks. The results showed that the final body weight (58.68–78.81 g), specific growth rate (1.41–1.75 % day−1), and protein efficiency ratio (1.66–2.64) increased significantly with the increasing dietary lipid levels. Liver lipid contents (71.65–101.80 g kg−1) and crude lipid (52.10–83.63 g kg−1) of whole body increased with increasing dietary lipid levels and reached the highest values in fish of L173. Fish of L173 showed lower alkaline phosphatase (23.81 King Unit gprot−1) and catalase activities (4.44 U mgprot−1) but higher malondialdehyde content (0.69 nmol mgprot−1) in liver than the other groups. Higher alanine transaminase activity (8.20 U L−1), aspartate transaminase activity (63.65 U L−1), and triglyceride (0.29 mmol L−1) in serum were observed in fish of L173 compared to the other treatments. Fish of L144 showed higher superoxide dismutase activity and glutathione peroxidase activities in liver than that of fish fed diet L58. Fish fed diet L58 showed lower total cholesterol (3.61 mmol L−1), high-density lipoprotein cholesterol (1.39 mmol L−1), and low-density lipoprotein cholesterol (0.46 mmol L−1) in serum. These results suggested that juvenile snakehead (Channa argus × Channa maculata) achieved good growth performance with dietary lipid level 173 g kg−1. Diet with 143 g kg−1 lipid was more conductive to liver health. The appropriate dietary lipid supplementation needs to be determined in further studies .
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipid as a macronutrient is principally a source of energy and essential fatty acids and provides fundamental structural components in the membranes. An appropriate amount of lipid can spare protein and reduce feed cost, which is important for carnivorous fish with limited ability to utilize carbohydrate for energy (NRC 2011). However, diets with excessive lipid might negatively affect fish growth performance (Han et al. 2014), cause an increase of fat deposition (López et al. 2009), affect fish health and welfare (Sargent et al. 2002), and reduce the commercial value of fillets (Martino et al. 2002).
The hybrid snakehead fish, Channa argus × Channa maculata, is an air-breathing carnivorous fish with important economic value and widely cultured in China, especially in Guangdong Province. The total snakehead production of China in 2013 was over 500,000 tons (Fisheries Bureau of Ministry of Agriculture 2014). At present, commercial feed of juvenile snakehead in China commonly contains about 10 % crude lipid, which appears to be not appropriate. Furthermore, current farming of snakehead still partly depends on frozen trash fish and the absence of premium complete feed becomes one of the most serious constraints for the industrial development.
As far as we know, several researches have been published concerning dietary lipid levels for a few snakehead species. Maximum growth was reported at a dietary lipid level about 60 g kg−1 for Channa striatus fingerlings (Bloch, 1793) (Boonyaratpalin 1981; Aliyu-Paiko et al. 2010) and 130 g kg−1 for Channa striata (10.0–13.8 g) (Samantaray and Mohanty 1997). Diet supplemented with 120 g kg−1 lipid was optimal for Channa argus (Zhu and Wang 2011). However, to our knowledge, no information is available about dietary lipid levels in artificial diets for Channa argus × Channa maculata. Apart from the growth performance, other secondary indices reflecting the biological functions should also be considered in dietary nutrition investigation. Liver is a solid organ and an early stress indicator in the body, carrying a complex array of functions (Antonopoulou et al. 2014; Ebrahimkhani et al. 2014). Several data obtained from different fish species showed that liver functions could be impaired by improper level of dietary lipid inclusion (Lin et al. 1990; Hevrøy et al. 2004; Li et al. 2012; Jin et al. 2013). Therefore, the objective of the present study was to evaluate the effect of dietary lipid concentrations on growth, liver oxidative stress, and serum metabolites of juvenile hybrid snakehead (Channa argus × Channa maculata).
Materials and methods
Experimental diets
The formulation and proximate composition of the experimental diets are given in Table 1. Five isonitrogenous (crude protein 420 g kg−1) practical diets were formulated to contain graded levels of fish oil (0, 30, 60, 90, and 120 g kg−1; named L58, L87, L115, L144, and L173, respectively). All ingredients were ground through a 320 μm mesh before final mixing and then blended with the oil. The experimental diets were prepared using a cooking extruder (TSE65S, Beijing Modern Yanggong Machinery S&T Development CO., Ltd., China) in Tongwei Fishery Science and Technology Park, then air-dried and stored at 4 °C until used.
Experimental animals
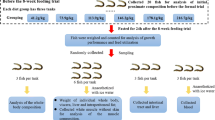
Snakehead (Channa argus × Channa maculata) used in this experiment were obtained from a commercial farm (Yongchuan, Chongqing, China). Prior to the trial, fish were acclimated and fed a commercial feed (Zhejiang Zhongda Group Feed CO., Ltd., China) for 1 week. The experimental design and procedure were approved by the Animal Care and Use of Committee of Southwest University following the requirements of the Regulations for the Administration of Affairs Concerning Experimental Animals of China (The State Science and Technology Commission 1988).
At the start of the experiment, the fish were fasted for 24 h and weighed after being anesthetized with 0.01 % MS-222 (Sigma, USA). Snakehead (mean initial weight: 24 g) were randomly allocated into 15 cylindrical plastic tanks (capacity: 177L) with screened covers for the growth trial (30 fish per tank). Each dietary treatment was randomly assigned to three tanks. Fish were fed to apparent satiation by hand three times (08:30, 12:30, and 18:00) daily for 8 weeks. During the experiment, wasted feed were collected and corresponding weight was calculated. Dead fish were weighed and taken into account in the growth performance calculation. Photoperiod was held to a constant 12-h light/12-h dark cycle. The static water in each tank was monitored daily for temperature (24.5 ± 2.5 °C), pH (7.4 ± 0.4), dissolved oxygen content (6–7 mg L−1), ammonia nitrogen content (<0.10 mg L−1), nitrite nitrogen (0.005–0.010 mg L−1), and sulfide (<0.05 mg L−1).
Sample collection and chemical analysis
At the termination of the experiment, fish were fasted for 24 h before harvest. Total numbers were counted, and mean body weight of fish was measured. Three fish per tank were anesthetized with overdose of MS-222 to assess the whole-body composition. Another six fish from each tank were randomly selected and anesthetized with 0.01 % MS-222 (Sigma, USA), and blood sample was collected from the caudal vein using a 1-mL syringe with a 27-gauge needle and allowed to clot at 4 °C. Following centrifugation (3000 rpm, 10 min) at 4 °C (Sorvall ST 16R, Thermo Fisher Scientific Inc., Germany), serum was separated, immediately frozen in liquid nitrogen and stored at −20 °C until analyzed. The bloodless fish were then dissected to obtain viscera, liver, and intraperitoneal fat for calculating morphological parameters. Pooled livers of another three fish per tank were also immediately frozen in liquid nitrogen and stored at −20 °C until used for malondialdehyde determination and enzymatic analysis.
Feed intake (FI), specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER), survival, viscera ratio (VR), hepatosomatic index (HSI), and intraperitoneal fat ratio (IPF) were measured using the following formulae: FI = feed consumption (g)/[(initial weight + final weight (weight of dead fish included))/2 × 56 days], SGR = [ln (mean final weight)–ln (mean initial weight)/56 days] × 100, FCR = total feed intake in dry basis (g)/weight gain (g), PER = total weight gain (g)/protein intake (g), survival rate (%) = (final number of fish/initial number of fish) × 100, VR = 100 × viscera weight (g)/body weight (g), HSI = 100 × hepatic weight (g)/body weight (g), IPF = 100 × intraperitoneal fat weight (g)/body weight (g).
All chemical composition analyses of diets and body were conducted by standard methods (AOAC 2005). Moisture was determined by oven drying to a constant weight at 105 °C in DHG-9240A (Keelrein instrument Co., Ltd., China). Protein was determined by measuring nitrogen (N × 6.25) using the Kjeldahl method in FOSS Kjeltec 2300 (Foss Analytical Instruments Co., Ltd., Sweden); lipid by ether extraction (without acid hydrolysis) using Soxtec; ash by combustion at 550 °C for 12 h in a muffle furnace (Shenyang Energy-Saving Electric Furnace Factory, China). The gross energy content of feed was determined by direct combustion in an adiabatic calorimeter GR3500 (Changsha Instrument Factory, P. R. China). Total lipid of liver was measured following the method of Bligh and Dyer (1959). Serum enzymes and biochemical indexes including alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were assayed using automatic biochemical analyzer HITACHI 7100 (ISE) (Hitachi, Ltd., Japan) and attached kit (Sichuan Maker Biotechnology Co., Ltd., China).
Hepatic malondialdehyde (MDA) contents and enzyme activities
Samples of liver were homogenized into 10 volumes (w/v) of ice-cold, 0.85 % physiological saline at 10,000 rpm for 1 min using fluko superfine homogenizers (FLUKO Equipment Shanghai Co., Ltd. China). Homogenates were centrifuged at 4000 rpm for 10 min at 4 °C (Sorvall ST 16R, Thermo Fisher Scientific Inc., Germany). The supernatant was used to determine the malondialdehyde (MDA) contents, alkaline phosphatase (AKP; EC 3.1.3.1), catalase (CAT; EC 1.11.1.6), superoxide dismutase (SOD; EC 1.15.1.1), and glutathione peroxidase (GPx; EC 1.11.1.9) activities spectrophotometrically using diagnostic reagent kits (Nanjing Jiancheng Bioengineering Institute, China). Thiobarbituric acid reactive substances (TBARS) were determined as described by Rueda-Jasso et al. (2004). MDA undergoes a condensation reaction in the presence of thiobarbituric acid and generates a red product with a maximum absorption peak at 532 nm. The results were expressed as nmol of MDA mg−1 protein. AKP activity was assayed based on the method of Bessey et al. (1946), and one unit of AKP was defined as the quantity of enzyme producing one milligram phenol in 15 min at 37 °C. The results were expressed as King Unit of AKP mg−1 protein. CAT activity assayed at 37 °C according to Beers and Sizer (1952), and one unit of the enzyme is defined as 1 μmol of H2O2 consumed per minute. SOD activity determined at 37 °C according to Panchenko et al. (1975) and one unit of SOD was defined as the amount of protein needed to decrease the reference rate to 50 % of maximum inhibition. GPx activity was assayed at 37 °C according to Wheeler et al. (1990). One GPx unit is defined as 1 μmol of NADPH consumed per minute. The specific activities of CAT, SOD, and GPx were represented as units per mg protein. The protein concentration of the enzyme extracts was determined according to Bradford (1976). All enzyme assays were performed in triplicate.
Statistical analysis
All data were accord with normal distribution and subjected to one-way ANOVA (analysis of variance) using SPSS 22.0 for Windows (International Business Machines Corporation, Armonk, NY, USA). Differences between the means were tested by Duncan’s multiple range tests. Overall significance level = 0.05 and the results are presented as mean ± SEM (standard error of the mean) of three replicate groups.
Results
Growth
The growth performance of snakehead is shown in Table 2. The survival ranged from 92.22 to 98.89 % and showed no significant differences among all groups (P > 0.05). The final body weight, SGR, and PER increased significantly with the increasing dietary lipid levels (P < 0.05), whereas FI and FCR showed a contrary trend (P < 0.05).
Morphological parameters and body composition
The morphological parameters and body composition of snakehead are presented in Table 3. VR and IPF were significantly affected by the dietary lipid levels and were higher of L173 group than those of other groups (P < 0.05). HSI of L144 and L173 groups were significantly higher than that of L58 group (P < 0.05). Liver lipid contents and crude lipid of whole body increased with the increase of dietary lipid levels and achieved the highest level in fish fed diet L173, whereas moisture and crude protein decreased with the increase of the dietary lipid levels (P < 0.05). No significant differences were observed in crude ash among all groups (P > 0.05).
Liver malondialdehyde (MDA) contents and enzyme activities
Liver MDA contents and enzyme activities were significantly affected by the dietary lipid levels (P < 0.05) (Table 4). AKP activity significantly decreased with the increasing dietary lipid levels (P < 0.05). Fish fed diet L173 showed lower CAT activity, but higher MDA contents compared to the other treatments (P < 0.05), while no significant differences were observed in CAT activity and MDA contents of L58, L87, L115, and L144 groups, respectively. Fish fed diet L144 showed higher SOD activity than that of fish fed diet L58 (P < 0.05). GPx activities of L144 and L173 groups were higher than those of the other groups (P < 0.05).
Serum enzymes activities and serum lipid metabolism indexes
The serum enzymes and biochemical indexes of snakehead are presented in Table 5. The ALT activity of group L173 was higher than those of other groups (P < 0.05). The AST activities increased with the dietary lipid levels and achieved the highest value in fish fed diet L173 (P < 0.05). TC of fish fed diet L58 were significantly lower than those of L115, L144, and L173 groups (P < 0.05). TG increased with increasing dietary lipid levels and achieved the highest activities in fish fed diet L173 (P < 0.05). HDL-C and LDL-C of fish fed diet L173 and diet L144, respectively, were significantly higher than that of fish fed diet L58 (P < 0.05), but HDL-C/TC and LDL-C/TC showed no differences among all groups.
Discussion
In the present study, survival was over 90 % in all dietary treatments, and increasing dietary lipid levels improved the growth performance of snakehead. Best growth performance was observed at the highest lipid level, suggesting that 173 g kg−1 lipid could be effectively utilized by snakehead. Similarly, maximum growth was observed at a dietary lipid level of 180 g kg−1 in Channa striatus with an initial weight of 30 g (Ghaedi et al. 2016). However, some previous studies showed relatively lower lipid levels in snakehead feed, such as 60 g kg−1 lipid for Channa striatus fingerling (Boonyaratpalin 1981; Aliyu-Paiko et al. 2010), 130 g kg−1 lipid for Channa striata (10.0–13.8 g) (Samantaray and Mohanty 1997) and 120 g kg−1 lipid for Channa argus (Initial weight approximately 11.0 g) (Zhu and Wang 2011). The discrepancy might be resulted from the varietal difference with distinct sizes. On the other hand, this study suggests that diet with lipid level over 173 g kg−1 is acceptable for better growth of snakehead. Further study would be needed to investigate the higher dietary lipid supplementation of snakehead feed.
The decreasing FCR indicates that the increasing lipid level provided fish more energy for fish growth and snakehead could utilize high-energy feed well. High-energy feed reduced the feed consumption of fish, which is the reason for lower FI in groups with higher lipid levels. Besides, PER significantly increased with elevated lipid levels and remained constant in treatments containing 144 and 173 g kg−1 lipid. This suggests that lipid has the effect of sparing dietary proteins within certain range and a further increase does not have any further advantages (Samantaray and Mohanty 1997; NRC 2011).
In the present study, highest liver lipid content in fish fed diet containing the highest lipid (173 g kg−1) suggests that high lipid diet could induce high lipid deposition in the liver, which could be a main reason for highest HSI. Low AKP in liver of fish fed 173 g kg−1 lipid indicates that this enzyme might be released into the blood stream, since the liver, bile ducts or gallbladder system possibly were not functioning properly or were blocked (Brain and Kay 1927; Kaplan and Righetti 1970). The enzymes ALT and AST are also two important indicators of hepatocellular injury (Boone et al. 2005). Significantly, higher ALT and AST activities in serum when fish were fed diet containing highest lipid indicate that the liver cells might be damaged to a certain extent (Jeon et al. 2013). Diet containing high lipid could possibly increase the burden of liver and cause liver dysfunction of snakehead.
Reactive oxygen species (ROS) could affect cellular integrity by causing oxidation reaction of fatty acids (Flood et al. 1996; Rueda-Jasso et al. 2004). As one of secondary oxidation products (El-Aal 2012), MDA levels in the liver of snakehead remained at relevantly equal levels when fish were fed diets containing 58–144 g kg−1 lipid. However, higher MDA content in fish fed the diet containing 173 g kg−1 lipid implies that high dietary lipid level could impose a peroxidation burden on the snakehead to increase the risk of production of ROS. SOD, CAT, and GPx have been used as effective biomarkers to evaluate the effects of dietary lipid on fish species in many studies (Mourente et al. 2002; Zhang et al. 2009; Jin et al. 2013). The upward trend of CAT, SOD, and GPx suggests that the antioxidant defense of snakehead was enhanced to protect the liver cells from deleterious effects of endogenous ROS. On the other hand, the decreasing CAT and SOD activities in fish fed diet containing 173 g kg−1 lipid suggested that the balance between generation and removal of ROS would be in danger of breaking (Halliwell 2015) and oxidative stress would be induced if fish was fed diet containing higher lipid levels.
Up to now, no comparable information is available for the effect of dietary lipid on the blood characteristics of snakehead. In this study, TG significantly increased with increasing dietary lipid, which was similar to the report for Takifugu rubripes (Kikuchi et al. 2009). TC increased significantly with increasing dietary lipid levels, but reached a steady level in fish fed the diet with lipid over 115 g kg−1, which suggests that dietary lipid might have limited effect on serum TC of snakehead. Similarly, in Solea solea (Bonvini et al. 2015) and Argyrosomus regius (Chatzifotis et al. 2010), both TG and TC were not affected by the dietary lipid. In addition, HDL-C and LDL-C were slightly influenced by the dietary lipid. The unchanged HDL-C/TC and LDL-C/TC seemed independent of the dietary lipid.
In this study, the positive correlation of whole-body lipid content and IPF with dietary lipid levels indicated excess lipid deposition in visceral cavity and tissues. The decreasing trend of moisture content with an increment of dietary lipid in the whole body in our study was in agreement with those previously reported in some carnivorous species (Pei et al. 2004; Kim et al. 2012). However, several researchers reported that no significant difference was observed in the value of whole-body moisture among treatments with different lipid levels (Ahmad 2008; Aliyu-Paiko et al. 2010). In addition, low crude protein of fish fed diet containing 173 g kg−1 lipid might be partly in relation to the dilution effect of lipid (Page and Andrews 1973). Although good growth performance was observed at high lipid levels, excess fat deposition could potentially adversely affect marketability and shelf life of fish (Cowey 1993; Ghanawi et al. 2011).
In conclusion, the results of this trial suggest that juvenile snakehead (Channa argus × Channa maculata) is capable of achieving good growth performance with dietary lipid level ranging from 58 to 173 g kg−1 and the appropriate dietary lipid level needs to be determined in further studies, while diet with lipid level over 144 g kg−1 may have a risk of introducing the oxidative stress in fish liver. To be safe, diet including 144 g kg−1 lipid is recommended considering the liver health of fish.
References
Ahmad MH (2008) Response of African catfish, Clarias gariepinus, to different dietary protein and lipid levels in practical diets. J World Aquac Soc 39(4):541–548
Aliyu-Paiko M, Hashim R, Chong ASC, Yogarajah L, El-Sayed AFM (2010) Influence of different sources and levels of dietary protein and lipid on the growth, feed efficiency, muscle composition and fatty acid profile of snakehead Channa striatus (Bloch, 1793) fingerling. Aquac Res 41:1365–1376
Antonopoulou E, Kousidou E, Tserga E, Feidantsis K, Chatzifotis S (2014) Dietary lipid levels in meagre (Argyrosomus regius): effects on biochemical and molecular indicators of liver. Aquaculture 428–429:265–271
AOAC (2005) Official methods of analysis, 18th edn. Association of Official Analytical Chemists International, Gaithersburg
Beers RF, Sizer IW (1952) Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem 164:321–329
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bonvini E, Parma L, Mandrioli L, Sirri R, Brachelente C, Mongile F, Gatta PP, Bonaldo A (2015) Feeding common sole (Solea solea) juveniles with increasing dietary lipid levels affects growth, feed utilization and gut health. Aquaculture 449:87–93
Boone L, Meyer D, Cusick P, Ennulat D, Bolliger AP, Everds N, Meador V, Elliott G, Honor D, Bounous D, Jordan H (2005) Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet Clin Pathol 34:182–188
Boonyaratpalin M (1981) Lipid requirements of snakehead fingerling. Progress report of the regional project RAS/76/003, Network of Aquaculture Centres in Asia, Bangkok, Thailand, p 30
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brain RT, Kay HD (1927) Kidney phosphatase. II: the enzyme in disease. Biochem J 21:1104–1108
Chatzifotis S, Panagiotidou M, Papaioannou N, Pavlidis M, Nengas I, Mylonas CC (2010) Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 307:65–70
Cowey CB (1993) Some effects of nutrition on flesh quality of cultured fish. In: Kaushik SJ, Luquet P (eds) Fish nutrition in practice, proceedings of the IV international symposium fish nutrition and feeding, vol 61. Les Colloques INRA, Paris, pp 227–236
Ebrahimkhani MR, Neiman JAS, Raredon MSB, Hughes DJ, Griffith LG (2014) Bioreactor technologies to support liver function in vitro. Adv Drug Deliver Rev 69:132–157
El-Aal HAHMA (2012) Lipid peroxidation end-products as a key of oxidative stress: effect of antioxidant on their production and transfer of free radicals. In: Catala A (ed) Lipid peroxidation. INTECH Open Access Publisher, Vienna, pp 64–88
Fisheries Bureau of Ministry of Agriculture (2014) China fishery statistical yearbook. China Agriculture Press, Beijing
Flood LP, Carvan MJ III, Jaeger L, Busbee DL, Gatlin DM III, Neill WH (1996) Reduction in hepatic microsomal P-450 and related catalytic activity in farm-raised red drum. J Aquat Anim Health 8:13–21
Ghaedi A, Kabir MA, Hashim R (2016) Effect of lipid levels on the reproductive performance of Snakehead murrel, Channa striatus. Aquac Res 47:983–991
Ghanawi J, Roy L, Davis DA, Saoud IP (2011) Effects of dietary lipid levels on growth performance of marbled spinefoot rabbitfish Siganus rivulatus. Aquaculture 310:395–400
Halliwell B (2015) Free radicals and other reactive species in disease. eLS, 1–9
Han T, Li X, Wang JT, Hu SX, Jiang YD, Zhong XD (2014) Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 434:145–150
Hevrøy EM, Sandnes K, Hemre GI (2004) Growth, feed utilization, appetite and health in Atlantic salmon (Salmo salar L.) fed a new type of high lipid fish meal, Sea Grain, processed from various pelagic marine fish species. Aquaculture 235:371–392
Jeon CY, Roberts CK, Crespi CM, Zhang ZF (2013) Elevated liver enzymes in individuals with undiagnosed diabetes in the US. J Diabetes Complicat 27:333–339
Jin Y, Tian LX, Zheng SL, Xie SW, Yang HJ, Liang GY, Liu YJ (2013) Dietary lipid requirement on non-specific immune responses in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 34:1202–1208
Kaplan MM, Righetti A (1970) Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Investig 49:508–516
Kikuchi K, Furuta T, Iwata N, Onuki K, Noguchi T (2009) Effect of dietary lipid levels on the growth, feed utilization, body composition and blood characteristics of tiger puffer Takifugu rubripes. Aquaculture 298:111–117
Kim KD, Lim SG, Kang YJ, Kim KW, Son MH (2012) Effects of dietary protein and lipid levels on growth ad body composition of juvenile far eastern catfish Silurus asotus. Asian Australas J Anim 25(3):369–374
Li XF, Liu WB, Lu KL, Xu WN, Wang Y (2012) Dietary carbohydrate/lipid ratios affect stress, oxidative status and non-specific immune responses of fingerling blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunol 33(2):316–323
Lin D, Mao YQ, Cai FS (1990) Nutritional lipid liver disease of grass carp (Ctenopharyngodon idella). Chin J Oceanol Limnol 8:363–373
López LM, Durazo E, Viana MT, Drawbridge M, Bureau DP (2009) Effect of dietary lipid levels on performance, body composition and fatty acid profile of juvenile white seabass, Atractoscion nobilis. Aquaculture 289:101–105
Martino RC, Cyrino JP, Portz L, Trugo LC (2002) Effect of dietary lipid level on nutritional performance of the surubim, Pseudoplatystoma coruscans. Aquaculture 209:209–218
Mourente G, Diaz-Salvago E, Bell JG, Tocher DR (2002) Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: attenuation by dietary vitamin E. Aquaculture 214:343–361
NRC (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington
Page JW, Andrews JW (1973) Interactions of dietary levels of protein and energy on channel catfish. J Nutr 103:1339–1346
Panchenko LF, Brusov OS, Gerasimov AM, Loktaeva TD (1975) Intramitochondrial localization and release of rat liver superoxide dismutase. FEBS Lett 55:84–87
Pei Z, Xie S, Lei W, Zhu X, Yang Y (2004) Comparative study on the effect of dietary lipid level on growth and feed utilization for gibel carp (Carassius auratus gibelio) and Chinese longsnout catfish (Leiocassis longirostris Günther). Aquac Nutr 10:209–216
Rueda-Jasso R, Conceicao LEC, Dias J, De Coen W, Gomes E, Rees JF, Soares F, Dinis MT, Sorgeloos P (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433
Samantaray K, Mohanty SS (1997) Interactions of dietary levels of protein and energy on fingerling snakehead, Channa striata. Aquaculture 156:241–249
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 181–257
Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184:193–199
Zhang H, Mu ZB, Xu LM, Xu GF, Liu M, Shan AS (2009) Dietary lipid level induced antioxidant response in Manchurian trout, Brachymystax lenok (Pallas) larvae. Lipids 44:643–654
Zhu XH, Wang GQ (2011) Effect of dietary energy and protein levels on growth, feed utilization and body composition of snakehead Channa argus. Feed Ind 32(2):15–18
Acknowledgments
The authors would like to thank Zhang X. X. and He J. for taking care of the snakehead. Special thanks to Xu T. and Chen W. Y. for helping with the chemical analysis. This research was supported by funds from Chongqing Ecological Fishery Technology System 2015-91, China and Tongwei Co., Ltd., China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, PF., Li, FJ., Chen, XR. et al. Dietary lipid concentrations influence growth, liver oxidative stress, and serum metabolites of juvenile hybrid snakehead (Channa argus × Channa maculata). Aquacult Int 24, 1353–1364 (2016). https://doi.org/10.1007/s10499-016-9993-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-9993-0