Abstract
The present study was conducted to investigate the effects of high dietary lipid levels on growth, metabolism, antioxidant capacity, and immune responses of largemouth bass. Fish (initial body weight 13.38 ± 0.11 g) were fed three isonitrogenous semi-purified diets containing 5%, 10%, and 20% lipid, respectively. The results indicated that fish fed 10% lipid diet showed significantly better final body weight, specific growth rate (SGR), protein efficiency ratio (PER), and feed conversion ratio (FCR) compared with that fed 5% lipid diet. Meanwhile, fish fed 20% lipid diet had a significantly higher viscera ratio (VR), hepatosomatic index (HSI), intraperitoneal fat ratio (IPF), and liver lipid content than those fed the other diets. Higher alanine aminotransferase (ALT) and aspartate transaminase (AST) activities, total cholesterol (TC), triglyceride (TG), free fatty acids (FFA), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) contents, and LDL-C/HDL-C value in plasma were recorded in fish fed 20% lipid diet, while higher insulin contents were obtained in fish fed 5% lipid diet. In addition, the highest carnitine palmitoyltransferase I (CPT1), AMP-activated protein kinase (AMPK), fructose-1,6-bisphosphatase (FBPase), and phosphoenolpyruvate carboxykinase (PEPCK) activities in the liver were also observed in fish fed 20% lipid diet. However, fish fed 20% lipid diet had a significantly lower superoxide dismutase (SOD) and catalase (CAT) activities and higher MDA contents in liver than those fed the other diets. The higher nitric oxide (NO) contents and inducible nitric oxide synthase (iNOS) activity in liver were recorded in fish fed 10% lipid diet. Moreover, the alkaline phosphatase (ALP), inducible nitric oxide synthase (iNOS) and lysozyme activities, and nitric oxide (NO) contents in plasma were higher in fish fed the 10% diets than the other groups. In conclusion, high dietary lipid levels could suppress growth performance and liver anti-oxidative capacity, and reduce immune responses of largemouth bass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of studies have reported health disadvantages of the high lipid diet, such as inducing obesity, metabolic syndrome, and liver damage, along with hypertriglyceridemia, hepatic insulin resistance, and steatosis (Figueiredo-Silva et al. 2012; Bargut et al. 2014; Borges et al. 2014; Bargut et al. 2015). The changes observed in farmed fish fed a high lipid diet are accompanied by an increase of ectopic lipid accumulation in liver and abdominal adipose tissue, and lead to metabolic disturbances (Du et al. 2008; Lu et al. 2014). In addition, excessive dietary fat also has negative effects on fish immunity, disease resistance, and promoting inflammatory processes (Montero et al. 2010; Tan et al. 2016). However, high levels of dietary lipid are incorporated in feeds for most teleost fish to promote growth and reduce nitrogen waste in recent years (Cho et al. 1994). In particular, in some carnivorous species, high lipid diets have been reported to promote better growth (Hillestad and Johnsen 1994; Dias et al. 1998; Zhao et al. 2016), although it was mostly due to increased fat deposition. Therefore, understanding how dietary lipid level regulates metabolism is essential for fish health culture and environmental protection.
High levels of dietary lipid were assumed to significantly affect the physiological status of fish, especially the hepatic function, through increasing hepatic lipid accumulation (Du et al. 2008; Zhao et al. 2016). Previous studies showed that the optimum dietary lipid level of Micropterus salmoides ranged from 7 to 16% containing above 13% starch (Bright et al. 2005; Chen et al. 2012), and the results were often difficult to compare. To our knowledge, the effects of dietary lipid levels on this phenomenon have not been investigated in detail and no results are available regarding M. salmoides. The largemouth bass (Micropterus salmoides) is a commercially important carnivorous species for freshwater culture given its high market value in China. How M. salmoides responds to a high dietary lipid level and to what extent this would affect fish health status remain unclear. Thus, the specific objective of this study was to comprehensively investigate the response of M. salmoides to a higher level of dietary lipid (compared with optimal lipid requirement). The results can also be useful in the formulation of diets for largemouth bass.
Materials and methods
Experimental diets
Three isonitrogenous (47% crude protein) semi-purified diets were formulated to contain 5%, 10%, and 20% lipid, respectively (Table 1). Fish meal, wheat protein, cottonseed protein, and isolated soy protein (no sugar) were used as protein sources, and fish oil and soybean oil were used as the lipid source. All ingredients were ground through a 320-μm mesh before final mixing through a commercial food mixer and then blended with the oils. Pellets were prepared using a dry power press MUZL180 (Muyang Group, Jiangsu, China), then air-dried and stored at 4 °C until used.
Experimental procedures
Largemouth bass was obtained from a commercial farm (Changshou, Chongqing, China). Prior to the trial, fish were acclimated and fed a commercial feed (Guangzhou Haid Group Co., LTD, China) for 10 days. The experimental design and procedure were approved by the Animal Care and Use of Committee of Southwest University following the requirements of the Regulations for the Administration of Affairs Concerning Experimental Animals of China (The State Science and Technology Commission 1988).
At the start of the experiment, the fish were fasted for 24 h and weighed after being anesthetized with 0.1 g kg−1 MS-222 (Sigma, USA). Largemouth bass (mean initial weight 13.38 ± 0.11 g) were randomly allocated into 12 cylindrical plastic tanks (capacity 280 L) with screened covers for the growth trial (25 fish per tank). Each dietary treatment was randomly assigned to four tanks. Fish were fed to apparent satiation by hand three times (08:30, 12:30, and 18:00) daily for 56 days. The water was allowed to flow into each tank at 600 mL min−1. During the growth period, the water temperature ranged from 26.3 to 28.7 °C, ammonia-N was < 0.43 mg L−1, dissolved oxygen was above 6.5 mg L−1, and pH was around 6.9. The photoperiod was 12 L:12 D, with the light period from 08:00 to 20:00.
Sample collection
At the end of the trial, fish were fasted for 24 h before harvest. Total numbers were counted, and mean body weight of fish was measured. Three fish per tank were anesthetized with an overdose of MS-222 (Sigma, USA) to assess the whole-body composition.
Six hours after feeding, five fish from each tank were randomly selected and anesthetized with 0.1 g kg−1 MS-222 (Sigma, USA), and blood sample was collected from the caudal vein using a 1-mL syringe with a 27-gauge needle. Blood was centrifuged (3500g, 10 min) to isolate the plasma, flash frozen in liquid nitrogen, and stored at − 80 °C for further analysis. The bloodless fish were then dissected to obtain viscera and liver for calculating morphological parameters. Pooled livers of another four fish per tank were also immediately frozen in liquid nitrogen and stored at − 80 °C until analyzed.
Chemical analysis
All chemical composition analyses of diets and whole body were conducted by standard methods (AOAC 2005). Moisture was determined by oven drying to a constant weight at 105 °C in DHG-9240A (Keelrein Instrument Co., Ltd., China). Protein was determined by measuring nitrogen (N × 6.25) using the Kjeldahl method in FOSS Kjeltec 2300 (Foss Analytical Instruments Co., Ltd., Sweden); lipid by ether extraction (without acid hydrolysis) using Soxtec, liver lipid by chloroform-methanol (2:1); ash by combustion at 550 °C for 12 h in a muffle furnace (Shenyang Energy-Saving Electric Furnace Factory, China). Gross energy was analyzed using a Parr 1281 Automatic Bomb Calorimeter (Parr, Moline, IL, USA).
Plasma globulin, total cholesterol (TC), triglyceride (TG), free fatty acids (FFA), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) contents, and alanine aminotransferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) activities were assayed using automatic biochemical analyzer HITACHI 7100 (ISE) and attached kit (Sichuan Maker Biotechnology Co., Ltd., Chengdu, China). Plasma insulin was measured by radioimmunoassay (RIA) using bonito insulin as the standard and rabbit anti-bonito insulin as antiserum, according to the method described by Gutiérrez et al. (1984).
Lysozyme level in plasma was determined by turbidimetric assay according to the method described by Ellis (1990) with previously described modifications (Lin et al. 2017). One unit of lysozyme activity was defined as the amount of enzyme producing a disease in absorbance of 0.001 min−1.
The activities of carnitine palmitoyltransferase I (CPT1), AMP-activated protein kinase (AMPK), phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), fructose-1,6-bisphosphatase (FBPase) and glucose-6-phosphate dehydrogenase (G6PDH), and in liver were determined by the commercial kit (Sinobest Biological Technology Co., Ltd., Shanghai, China). Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), inducible nitric oxide synthase (iNOS) activities, and malondialdehyde (MDA) and nitric oxide (NO) contents in liver or plasma were assayed by the commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The protein concentration of the enzyme extracts was determined according to Bradford (1976). All enzyme assays were performed in triplicate.
Statistical analysis
All statistical procedures were performed with the aid of the SPSS software version 22.0 for Windows. Differences between groups were evaluated by one-way ANOVA followed by Tukey’s multiple comparison. All results were presented as the mean ± standard error (SE). The level of significance was chosen at P < 0.05.
Results
Growth performance
The growth performance of largemouth bass is shown in Table 2. Fish fed 10% lipid diet showed significantly better final body weight and specific growth rate (SGR) compared with the other diets, and the lowest SGR was recorded in fish fed 5% lipid diet (P < 0.05). Feed intake (FI) of fish fed 20% lipid level was significantly lower than those fed the other diets. In addition, the protein efficiency ratio (PER) in fish fed 5% lipid diets was lower than the other groups, whereas the feed conversion ratio (FCR) showed the opposite trend (P < 0.05), and no significant differences were observed between the 10% lipid level and the 20% lipid level. However, the survival rate (SR) was not affected by dietary lipid levels (P > 0.05).
Whole-body composition
The VR, HSI and IPF values, liver lipid, the whole-body moisture, and lipid contents increased significantly with dietary lipid levels (P < 0.05, Table 3). However, the lower whole-body protein content was recorded in fish fed 20% lipid diet. No significant differences were found in the whole-body ash contents among dietary treatments (P > 0.05).
Plasma biochemistry parameters and innate immune indices
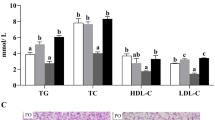
TG, TC, FFA, and LDL-C contents in plasma increased significantly with dietary lipid levels (P < 0.05, Table 4). Higher ALT and AST activities and HDL-C content in plasma were recorded in fish fed 20% lipid diet, but no significant differences were observed between the 5% lipid level and the 10% lipid level. However, lower LDL-C/HDL-C value and higher insulin content were showed in 5% lipid group (P < 0.05).
Compared with the 5% and 10% lipid, the 20% lipid group could significantly reduce NO contents, and ALP, iNOS, and lysozyme activities in plasma (P < 0.05). However, no significant differences were observed in plasma globulin among dietary treatments (P > 0.05).
Liver key metabolic enzymes
There were significant differences in hepatic metabolism enzymes in fish fed diets with different dietary lipid levels (Table 5). CPT1 and AMPK activities in liver increased significantly as dietary lipid levels increased. Moreover, higher FBPase and PEPCK activities in the liver were recorded in fish fed 20% lipid diet. However, there were no significant differences in G6PDH and G6Pase activities among dietary treatments (P > 0.05).
Liver antioxidant indices
The lowest SOD and CAT activities and higher MDA contents in the liver were observed in fish fed 20% lipid diet (P < 0.05, Table 6). Compared with the 5% and 20% lipid, the 10% lipid group could enhance significantly the liver nitric oxide (NO) content, and increase the activities of inducible nitric oxide synthase (iNOS) in liver (P < 0.05), while no significant differences were observed between the 5% lipid level and the 10% lipid level. Moreover, there was no significant difference in the glutathione peroxidase (GPx) activity among dietary treatments (P > 0.05).
Discussion
The present findings extend the growth of fish is sensitive to the effects of a high lipid diet. Influences of dietary lipid level on growth have been evaluated in some aquaculture species with varying results. In the present study, high dietary lipid level (20%) was detrimental to fish growth, which was consistent with some previous studies conducted in meager (Chatzifotis et al. 2010), Totoaba (Rueda-Lopez et al. 2011), and giant croaker (Han et al. 2014). However, several studies have shown that an increase in dietary lipid can promote growth (Hillestad and Johnsen 1994; Dias et al. 1998; Zhao et al. 2016). The growth-promoting effect of high dietary lipids was generally correlated with the protein-sparing effect of dietary lipids (Boujard et al. 2004; López et al. 2009). Similarly, this phenomenon was also observed in largemouth bass fed diets with lipid level from 5 to 10%. In contrast, the growth of fish was not significantly affected by dietary lipid levels in other species (Peres and Oliva-Teles 1999; Wang et al. 2015). Moreover, recent studies found that high dietary lipids did reduce growth and feed utilization without a protein-sparing effect in some fishes (Akpınar et al. 2012; Sevgili et al. 2014). Therefore, the effects of dietary lipid level on fish growth are complex, which may be related to fish species, fish sizes, feed composition, and environment. Further studies on possible protein-sparing effect of lipid in diets containing limited protein levels are required.
The lipid content in both the whole body and liver was significantly increased with increasing dietary lipid level in this study. The increase of dietary lipid levels leading to higher lipid deposition has also been observed in snakehead (Zhao et al. 2016), large yellow croaker (Wang et al. 2015), and Atlantic cod (Hansen et al. 2008). Also, increased dietary lipid levels resulted in increased HSI, plasma TG, TC, FFA, HDL-c, and LDL-c levels, indicating an active endogenous lipid transport, which was consistent with results previously obtained in snakehead (Zhao et al. 2016) and large yellow croaker (Wang et al. 2015). The increased plasma FFA level in fish fed the high lipid diet may be an important source of lipid accumulation in the liver of largemouth bass. This suggested that largemouth bass mobilized adaptive mechanisms to maintain lipid homeostasis in the liver. At the same time, the activity of hepatic CPT I was significantly elevated with increasing dietary lipid level. Generally, CPT I is considered to be the main regulatory enzyme in mitochondrial fatty acid oxidation (Morash et al. 2009). This result was also confirmed in mammals (Tabarin et al. 2005) and in other fish species (Yan et al. 2015; Yuan et al. 2016) that feeding high-fat diets will increase CPT I expression compared with low-fat diets. By contrast, Lu et al. (2014) reported that the activity and expression of CPT I were significantly downregulated in fish fed the high-fat diet. As expected, in this study, high dietary lipid exhibited enhanced activation of AMPK in liver of largemouth bass. As master regulator of sensing oxidative stress, AMPK plays a key role in controlling fat metabolism. Once activated, AMPK increases fatty acid oxidation and decreases lipogenesis (Tang et al. 2013). However, previous studies reported that upregulating AMPK can attenuate lipid accumulation in rat liver (Rui et al. 2016). Activated AMPK plays a vital role in attenuating fatty acid synthesis in livers. Consequently, both CPT I and AMPK did not play its proper role in lowering hepatic lipid deposits in this study, and these results suggested that the elevation of CPT I and AMPK activities might be only a kind of compensatory response to high dietary lipid. Unfortunately, this compensatory response could not completely mitigate the hepatic lipogenesis of fish which was caused by excess intake of fat. Therefore, the mechanism of liver fat deposition needs to be further explored. Taken together, combined with fat deposition from dietary lipid, this appears to oxidize fat at rates that are lower than the lipogenesis capacity of largemouth bass, causing increases in the liver lipid content.
Surprisingly, the present study showed that insulin resistance, the lipid ratios (LDL-C/HDL-C), and TG increased, while plasma insulin secretion decreased with increasing dietary lipid level. Many studies have shown that increasing TG and decreasing HDL-C could cause insulin resistance (Zhang et al. 2015; Gu et al. 2019). Moreover, high FFAs could result in insulin resistance via oxidative stress pathways (Li et al. 2008; Jin et al. 2017; Gu et al. 2019). A similar result was also observed in this study, in which plasma FFAs were significantly negatively associated with plasma insulin secretion. The rise of triglyceride is accompanied by the rise of FFA level, which can inhibit the phosphorylation of IR (insulin receptor) and IRS (insulin receptor substrate) tyrosine, and the inhibition degree increases with the increase of FFA concentration. In addition, insulin enhanced lipogenesis in the liver of fish (Kao et al. 1999; Zhuo et al. 2014), in coincidence with increased TG content. It is generally known that insulin exhibits an anti-lipolytic function by inhibiting fatty acid catabolism. Thus, it is predicted that decreased insulin content will affect the FA content of the liver. However, information about insulin action on lipid metabolism in fish is scarce and contradictory (Plagnes-Juan et al. 2008; Jin et al. 2017). The underlying mechanisms remained unknown. On the other hand, gluconeogenesis potential was upregulated as indicated by an increase in the activities of gluconeogenic enzymes (PEPCK and FBPase) in the current study. Enhanced gluconeogenesis, which triggers the de novo synthesis of glucose from non-carbohydrate precursors, in combination with the glycogenolysis progress, contributes to glucose homeostasis in low starch diet of largemouth bass (≤ 8–10% digestible carbohydrate in commercial feeds). Gluconeogenesis is crucial to glucose homeostasis in vertebrates (Moon 2011). Similarly, Jiang et al. (2017) reported that the liver PEPCK activity of largemouth bass was enhanced during fasting. Generally, gluconeogenesis is induced by nutritional history in most fish species. Although glycogen was not measured specifically in the liver, we cannot exclude the possibility of a greater glycogen accumulation in the liver of largemouth bass fed the high lipid diet having at least partly contributed towards the HSI in our study, in addition to FA biosynthesis and lipid storage. Even though lipogenesis is not directly linked to glucose metabolism, this pathway may play an important role in glucose homeostasis due to the conversion of fat (in excess) into glucose. The above results revealed potential adaptive strategies of maintaining metabolic balance in largemouth bass.
Fat deposition in the liver causes the generation of reactive oxygen species (ROSs), and ROSs induce damage that impairs organelle integrity (Halliwell and Gutteridge 1999). In the current study, SOD and CAT activities decreased and MDA levels dramatically increased in fish fed a high-fat diet, which implies high ROS production. This indicated that consuming high fat causes injuries to the largemouth bass. Previous studies also have demonstrated that the largemouth bass is a fish species very sensitive to peroxidation (Yun et al. 2013). Moreover, the increased plasma ALT and AST levels of the high-fat group indicated that oxidative stress could induce hepatic dysfunction and hepatotoxicity in largemouth bass, which was further proved by histopathological examination which showed obvious injury in the liver tissue (unpublished). The liver plays a central role in metabolic homeostasis and coordinates body metabolism in response to various dietary lipid level, and plasma transaminase has been proposed as an important index for the diagnosis of liver function and damage (Pohl et al. 2001; Pérez-Jiménez et al. 2017). However, little is known about how liver of fish responds to changes in dietary lipid intake. Thus, the correlation between dietary lipid level and liver function of fish deserves to be studied further.
Although the interplay between nutrition and immune system is well recognized, basic and applied research on the interactions between diet and health in fish is lagging behind the mammalian studies (Martin and Król 2017). Interest in nutrition immune of fish has grown dramatically in recent years, and the links between diet and fish health will almost certainly become a major focus in the next few years. Some studies reported that deficient or excessive dietary lipid contents could suppress innate immune indexes in carnivorous fish (Zhao et al. 2016; Mu et al. 2018). Similarly, in turbot, a high-fat diet could also impair health by downregulating the immune ability (Jia et al. 2017). Generally, research into the impacts of dietary nutrition level on fish immunity has recently gained momentum by combining feeding trials with large-scale analyses of immunization indicators (Wang et al. 2015). Nowadays, both lysozyme and ALP activities have been shown as the biomarker of immune defense mechanisms (Zhao et al. 2016; Lin et al. 2017). Our study indicated that plasma ALP and lysozyme activities were significantly affected by the dietary lipid level, suggesting that high dietary lipid level causes stress in fish and significantly reduced immunity. Different immune responses to dietary levels have been reported in other fish species (Montero et al. 2010; Tan et al. 2016). In addition, as two key immunoregulatory factors in human and fish (Sakai et al. 1996; Öner et al. 2008), both iNOS and NO levels were also obviously affected by the dietary lipid level in this study. Low iNOS and NO levels were observed in largemouth bass fed high lipid levels, which furtherly showed higher lipid level may have detrimental effects on hepatic immune function, weakening fish health. Based on these findings, it can be assumed that excess lipid intake will aggravate lipid metabolic disorder, and consequently lead to reducing immune ability of largemouth bass. Understanding of the mechanisms that underpin the links between nutrition and immune is waiting for further studies.
In conclusion, the high dietary lipid level could suppress the growth performance of largemouth bass, and increase the lipid deposition in the liver. Meanwhile, it induced the immune response reflected by downregulating the activation of enzymes in largemouth bass. In addition, intrahepatic adaptations of largemouth bass in response to high lipid intake were to induce mitochondrial FA oxidative (CPT1 and AMPK) and simultaneously increase gluconeogenesis (PEPCK and FBPase) to deal with excessive lipid intake. More specifically, consuming excessive fat increases plasma lipid levels, decreases oxidative status, and also inhibits insulin sensitivity. The present findings would be not only helpful to feed management in largemouth bass farming, but also provide new mechanistic targets by which high lipid could exert their harmful metabolic effects. Therefore, additional studies should further pay attention to the relationships between dietary lipid and health status of largemouth bass in order to facilitate the dietary lipid utilization.
References
Akpınar Z, Sevgili H, Demir A, Özgen T, Emre Y, Eroldoğan OT (2012) Effects of dietary lipid levels on growth, nutrient utilization, and nitrogen and carbon balances in shi drum (Umbrina cirrosa L.). Aquac Int 20:131–143
Association of Official Analytical Chemists (AOAC) (2005) Official Methods of Analysis, 18th ed. Association of Official Analytical Chemists, Gaithersburg
Bargut TCL, Frantz EDC, Mandarim-de-Lacerda CA, Aguila MB (2014) Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 49:431–444
Bargut TCL, Mandarim-de-Lacerda CA, Aguila MB (2015) A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice. J Nutr Biochem 26:960–969
Borges P, Valente LMP, Véron V, Dias K, Panserat S, Médale F (2014) High dietary lipid level is associated with persistent hyperglycaemia and downregulation of muscle akt-mTOR pathway in senegalese sole (Solea senegalensis). PLoS One 9(7):e102196
Boujard T, Gélineau A, Covès D, Corraze G, Dutto G, Gasset E, Kaushik S (2004) Regulation of feed intake, growth, nutrient and energy utilisation in European sea bass (Dicentrarchus labrax) fed high fat diets. Aquaculture 231:529–545
Bradford MM (1976) A refined and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
Bright LA, Coyle SD, Tidwell JH (2005) Effect of dietary lipid level and protein energy ratio on growth and body composition of largemouth bass Micropterus salmoides. J World Aquacult Soc 36(1):129–134
Chatzifotis S, Panagiotidou M, Papaioannou N, Pavlidis M, Nengas I, Mylonas CC (2010) Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 307:65–70
Chen NS, Xiao WW, Liang QL, Zhou HY, Ma XL, Zhao M (2012) Effects of dietary lipid to protein ratios on growth performance, body composition and non-specific immunity of largemouth bass (Micropterus salmoides). J Fish China 36(8):1270–1280 [in Chinese with English abstract]
Cho CY, Hynes JD, Wood KR, Yoshida HK (1994) Development of high-nutrient-dense, low-pollution diets and prediction of aquaculture wastes using biological approaches. Aquaculture 124:293–305
Dias J, Alvarez MJ, Diez A, Arzel J, Corraze G, Bautista JM, Kaushik SJ (1998) Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass (Dicentrarchus labrax). Aquaculture 161:169–186
Du ZY, Clouet P, Huang LM, Degrace P, Zheng WH, He JG, Tian LX, Liu YJ (2008) Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): mechanism related to hepatic fatty acid oxidation. Aquac Nutr 14:77–92
Ellis AE (1990) Lysozyme assays. In: Stolen, J.S., Fletcher, T.C., Anderson, D.P.,Robertsen, B.S., Van Muiswinkel, W.B. (Eds.), Techniques in Fish Immunology. SOS Publications, Fair Haven, pp. 101–103
Figueiredo-Silva AC, Panserat S, Kaushik S, Geurden I, Polakof S (2012) High levels of dietary fat impair glucose homeostasis in rainbow trout. J Exp Biol 215:169–178
Gu ZX, Mu H, Shen HH, Deng KY, Liu D, Yang MX, Zhang Y, Zhang WB, Mai KS (2019) High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp Biochem Physiol B 231:34–41
Gutiérrez J, Carrillo M, Zanuy S, Planas J (1984) Daily rhythms of insulin and glucose levels in the plasma of sea bass Dicentrarchus labrax after experimental feeding. Gen Comp Endocrinol 55:393–397
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Han T, Li XY, Wang JT, Hu SX, Jiang YD, Zhong XD (2014) Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 434:145–150
Hansen JØ, Berge GM, Hillestad M, Krogdahl Å, Galloway TF, Holm H, Holm J, Ruyter B (2008) Apparent digestion and apparent retention of lipid and fatty acids in Atlantic cod (Gadus morhua) fed increasing dietary lipid levels. Aquaculture 284:159–166
Hillestad M, Johnsen F (1994) High-energy/low-protein diets for Atlantic salmon: effects on growth, nutrient retention and slaughter quality. Aquaculture 124:109–116
Jia Y, Jing Q, Niu H, Huang B (2017) Ameliorative effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish Shellfish Immunol 67:634–642
Jiang DL, Wu YB, Huang D, Ren X, Wang Y (2017) Effects of nutritional history on stress response in gibel carp (Carassius auratus gibelio) and largemouth bass (Micropterus salmoides). Comp Biochem Phys B 210:9–17
Jin JY, Zhu XM, Han D, Yang YX, Liu HK, Xie SQ (2017) Different regulation of insulin on glucose and lipid metabolism in 2 strains of gibel carp. Gen Comp Endocrinol 246:363–371
Kao Y, Youson JA, Holmes JH, Al-Mahrouki A, Sheridan MA (1999) Effects of insulin on lipid metabolism of larvae and metamorphosing landlocked sea lamprey (Petromyzon marinus). Gen Comp Endocrinol 114:405–414
Li C, Ford ES, Meng YX, Mok dad AH, Reaven GM (2008) Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol 7(4):1–9
Lin SM, Jiang Y, Chen YJ, Luo L, Doolgindachbapornc S, Yuangsoic B (2017) Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol 70:40–47
López LM, Durazo E, Viana MT, Drawbridge M, Bureau DP (2009) Effect of dietary lipid levels on performance, body composition and fatty acid profile of juvenile white seabass, Atractoscion nobilis. Aquaculture 289:101–105
Lu KL, Xu WN, Wang LN, Zhang DD, Zhang CN, Liu WB (2014) Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS One 99(3):e93135
Martin SAM, Król E (2017) Nutrigenomics and immune function in fish: new insights from omics technologies. Dev Comp Immunol 75:86–98
Montero D, Mathlouthi F, Tort L, Afonso J, Torrecillas S, Fernández-Vaquero A, Negrin D, Izquierdo MS (2010) Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Sparus aurata. Fish Shellfish Immunol 29:1073–1081
Moon TW (2011) Adaptation, constraint and the function of the gluconeogenic pathway. Can J Zool 66:1059–1068
Morash AJ, Bureau DP, McClelland GB (2009) Effects of dietary fatty acid composition on the regulation of carnitine palmitoyltransferase (CPT) I in rainbow trout (Oncorhynchus mykiss). Comp Biochem Phys B152:85–93
Mu H, Shen HH, Liu JH, Xie FL, Zhang WB, Mai KS (2018) High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol 77:465–473
Öner M, Atli G, Canli M (2008) Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ Toxicol Chem 27:360–366
Peres H, Oliva-Teles A (1999) Effect of dietary lipid level on growth performance and feed utilization by European sea bass juveniles (Dicentrarchus labrax). Aquaculture 179:325–334
Pérez-Jiménez A, Abellán E, Arizcun M, Cardenete G, Morales AE, Hidalgo MC (2017) Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish. Comp Biochem Phys A 203:17–23
Plagnes-Juan E, Lansard M, Seiliez I, Médale F, Corraze G, Kaushik S, ... Skiba-Cassy S (2008) Insulin regulates the expression of several metabolism-related genes in the liver and primary hepatocytes of rainbow trout (Oncorhynchus mykiss). J Exp Biol 211: 2510–2518
Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T (2001) Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol 96:3142–3146
Rueda-Lopez S, Lazo JP, Correa RG, Viana MT (2011) Effect of dietary protein and energy levels on growth, survival and body composition of juvenile Totoaba macdonaldi. Aquaculture 319:385–390
Rui BB, Chen H, Jang L, Li Z, Yang JM, Xu WP, Wei W (2016) Melatonin upregulates the activity of AMPK and attenuates lipid accumulation in alcohol-induced rats. Alcohol Alcohol 51(1):11–19
Sakai M, Sawada T, Nishimura T, Nagatsu I (1996) Expression of nitric oxide synthase in the mouse and human nasal mucosa. Acta Histochem Cytoc 29:177–179
Sevgili H, Kurtoğlu A, Oikawa M, Öztürk E, Dedebali N, Emre N, Pak F (2014) High dietary lipids elevate carbon loss without sparing protein in adequate protein-fed juvenile turbot (Psetta maxima). Aquac Int 22(2):797–810
Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ et al (2005) Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 54:3510–3516
Tan P, Dong X, Mai K, Xu W, Ai Q (2016) Vegetable oil induced inflammatory response by altering TLR-NF-κB signalling, macrophages infiltration and polarization in adipose tissue of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol 59:398–405
Tang CC, Huang HP, Lee YJ, Tang YH, Wang CJ (2013) Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem Toxicol 62:786–796
The State Science and Technology Commission (1988) Regulations for the administration of affairs concerning experimental animals. The State Science and Technology Commission, Beijing, China.
Wang X, Li Y, Hou C, Gao Y, Wang Y (2015) Physiological and molecular changes in large yellow croaker (Pseudosciaena crocea R.) with high-fat diet-induced fatty liver disease. Aquac Res 46:472–482
Yan J, Liao K, Wang T, Mai K, Xu W, Ai Q (2015) Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthys crocea) by regulating lipoprotein receptors, fatty acid uptake and triacylglycerol synthesis and catabolism at the transcriptional level. PLoS One 10(6):e0129937
Yuan XC, Liang XF, Liu LW, Fang JG, Li J, Li AX, Cai W, Xue M, Wang J, Wang QC (2016) Fat deposition pattern and mechanism in response to dietary lipid levels in grass carp, Ctenopharyngodon idellus. Fish Physiol Biochem 42:1557–1569
Yun B, Xue M, Wang J, Fan Z, Wu XF, Zheng YH, Qin YC (2013) Effects of lipid sources and lipid peroxidation on feed intake, growth, and tissue fatty acid compositions of largemouth bass (Micropterus salmoides). Aquacult Int 21:97–110
Zhang LY, Chen SY, Deng AW, Liu XY, Liang Y, Shao XF et al (2015) Association between lipid ratios and insulin resistance in a Chinese population. PLoS One 10(1):e0116110
Zhao PF, Li FJ, Chen XR, Chen YJ, Lin SM, Zhang L, Li Y (2016) Effect of dietary lipid level on growth, liver function and serum biochemical indexes of snakehead (Channa argus × Channa maculata). Aquacult Int 241:353–1364
Zhuo MQ, Luo Z, Wu K, Zhu QL, Zheng JL, Zhang LH, Chen QL (2014) Regulation of insulin on lipid metabolism in freshly isolated hepatocytes from yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Phys B 177–178:21–28
Acknowledgments
We thank H. Zhao and C.M. Shi for their help during the experiment. Thanks are also due to Y. Jiang and M.Q. Song for helping with the chemical analysis.
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 31672659), the Science and Technology Council of Chongqing, China (No.cstc2017shms-xdny80012), and the Chongqing Ecological Fishery Technology System (2018-2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, YL., Guo, JL., Tang, RJ. et al. High dietary lipid level alters the growth, hepatic metabolism enzyme, and anti-oxidative capacity in juvenile largemouth bass Micropterus salmoides. Fish Physiol Biochem 46, 125–134 (2020). https://doi.org/10.1007/s10695-019-00705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00705-7