Abstract
Aquaculture is faced with the challenges of the use of synthetic compounds as growth enhancers and the presence of several contaminants in water. These factors severely deteriorate the quality and quantity of aquaculture products. Phytochemicals play a major role by working as antioxidant agents of which curcumin has become the gold standard. Curcumin, from Curcuma longa shows a wide spectrum of biological activities which include anticancerous, antioxidant, anti-inflammatory, antibacterial, antiviral, antifungal, antidiabetic, antistress, hepatoprotective and gastroprotective effects. Curcumin in 0.5 and 1 % doses were given as feed additive to Oreochromis mossambicus for 35 days. After feeding trial, activities of digestive enzymes such as α-amylase, protease and lipase were analysed. There was a significant increase in the activities of α-amylase, protease and lipase with 0.5 and 1 % curcumin supplementation in feed. Real-time quantification of GH in brain, and IGF-1 and IGF-2 genes in muscle revealed that curcumin significantly increased the expression of these genes. This is the first study to report that curcumin supplementation at concentrations of 0.5 and 1 % in the feed improved the activities of digestive enzymes and also modulates the expression of GH in brain and growth factors such as IGF-1 and IGF-2 in muscle of O. mossambicus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is an important weapon in the global fight against malnutrition, poverty and a food source rich in essential nutrients (Tacon et al. 2010). Appropriate nutrition is one of the key factors in successful aquaculture, which promotes normal growth and provides the essential nutrients for normal physiological functions and sustaining the health of the fish (Gatlin 2002).
Curcumin, a polyphenolic compound, derived from the tuber of turmeric plant (Curcuma longa), is one of the best-studied natural compounds. Experiments by various researchers have suggested a wide range of potential therapeutic or preventive effects associated with curcumin including antioxidant, anti-cancer, anti-microbial, hepatoprotective and gastroprotective effects (Soudamini and Kuttan 1989; Elizabeth and Rao 1990; Ruby et al. 1995; Allen et al. 1998; Ramyrez-Tortosa et al. 1999; Kelloff et al. 2000; Park et al. 2000). The information on the use of curcumin is limited in aquaculture. AL-Sultan (2003) has reported that turmeric as a feed additive enhanced the overall performance in broiler chickens. Manju et al. (2008, 2009, 2012) have reported a protective effect of natural and synthetic curcumin on lipid peroxidation in the teleost, Anabas testudineus. Studies by D’Souza and Prabhu (2006) have suggested that turmeric tuber powder inhibits in vitro lipid peroxidation in Somberus sombrus.
The growth of some aquatic animal species is limited by the capacity of the digestive system to breakdown and assimilate specific nutrients (Houlihan et al. 1988). The digestive capacity of fish is influenced by the levels of digestive enzyme activities and ultimately affects growth and, to some extent, health (Shan et al. 2008). Most of the gastroprotective effects of curcumin have been based on studies conducted either in mammals or in mammalian cell lines (Ramirez-Tortosa et al. 1999). When consumed through the diet, spices produce significant stimulation on the activities of pancreatic lipase, amylase and protease (Platel and Srinivasan 2000). There is often a strong relation between the activity levels of specific digestive enzymes and dietary preference of several animals. Evidence shows that digestive enzymes may set physiological limits on growth rates in fish (Lemieux et al. 1999).
Growth hormone (GH) produced from pituitary binds to a single transmembrane receptor, the growth hormone receptor (GHR) (Perez-Sanchez et al. 2002). GH participates in almost all major physiological processes in the fish including the regulation of ionic and osmotic balance, lipid, protein and carbohydrate metabolism, skeletal and soft tissue growth, reproduction and immune function, and also GHR has extensive tissue distribution (Reinecke et al. 2005). The insulin-like growth factors (IGFs) are mitogenic peptides that regulate vertebrate growth (Humbel 1990; Jones and Clemmons 1995; Reinecke and Collet 1998). They locally stimulate cell division as an autocrine or paracrine factor. There are no studies on the role of various doses of curcumin as a feed supplement other than earlier reports from our lab. This is the first report on the dietary effect of curcumin on the activities of digestive enzymes and expression of GH and growth factor genes such as IGF-1 and IGF-2 in Oreochromis mossambicus.
Materials and Methods
Experimental design
Fish, weighing 9.45 ± 0.63 g, were collected from Fisheries College, Mangalore, India, and transported to the laboratory in polythene bags filled with oxygenated water. They were reared in large stock tanks with aerated water (26–28 °C) and natural photoperiod 12L:12D for a month. During acclimatization, the fish were fed ad-libitum once daily with control feed containing fishmeal, groundnut oil cake, rice bran, tapioca flour, with adequate amount of vitamin drops (A–Z drops, ALKEM Laboratories Ltd., Mumbai), in the form of dry pellets (Table 1) (Hardy 1980). Control feed was prepared in our laboratory according to the method of Hardy (1980), Johnson (2004) and Manju et al. (2013). Its components and proximate compositions are as described in Tables 1 and 2 (Manju et al. 2013). The feed for the experimental fish was prepared by supplementing 0.5 and 1 % curcumin (by weight) to the basal feed, made into pellets and air-dried in shade. The doses 0.5 and 1 % curcumin were selected on the basis of previous study conducted in our lab (Manju et al. 2008, 2009, 2012).
At the end of acclimatization, fish were moved to aquarium tanks (0.61 × 0.30 × 0.30 m) with conditions identical to the stock tank. Three replicate tanks for each treatment were established. Tank A series constituted the control group, and B and C series 0.5 and 1 % curcumin, respectively. Fish in tank A series were fed with basal feed (control), tank B series with 0.5 % curcumin-supplemented feed and tank C series with 1 % curcumin-supplemented feed. The fish were fed on the experimental diets of 10 % body weight daily in equal proportions between 8.00 a.m.–9.00 a.m. and 4.00 p.m.–5 p.m. for a period of 35 days. Generally, all the feed provided was consumed. The unconsumed pellets were siphoned out from the tank bottom 3 h subsequent to feeding, and the water was changed every day.
Digestive enzyme assays
At the end of the treatment period, eight fish were selected at random from each triplicate group fasted overnight and anesthetized using MS-222 (Sigma). Fish were then immediately dissected, and the gut from control and treated fish were taken out, washed in PBS, homogenized and centrifuged at 8000 rpm for 5 min. The clear supernatant after centrifugation was used for enzymatic assays. Total protein of the gut was analysed by the method of Bradford (1976). The α-amylase activity was determined by the method of Bernfeld (1955). Protease and lipase activity were determined using the method of Keay et al. (1970) and the method of Winkler and Martina (1979), respectively.
RNA isolation
The isolation of total RNA was done by Trizol method. Briefly, brain and muscle tissues were isolated from the fish using sterilized scissors and surgical blades. Samples were immediately transferred into 1 mL Tri reagent (Sigma-Aldrich) and kept in −80 °C refrigerator till the processing for RNA isolation. The quality and concentration of RNA were determined by measuring absorbance at 260 and 280 nm in a Nanodrop (Thermoscientific, USA), and the RNA integrity was confirmed by agarose gel electrophoresis.
cDNA synthesis and real-time PCR (RT-PCR) analysis of GH, IGF-1 and IGF-2 genes in Oreochromis mossambicus
One microgram of RNA from each of the samples was used to prepare cDNA in 10 μL nuclease-free water. Briefly, 1 μg of RNA was added to 10 μL PCR mix (Applied Biosystem, USA). The RNAs were converted into cDNA using commercially available cDNA Synthesis Kit (Applied Biosystem, USA). RT reactions were done with LC 480 RT-PCR (Roche, USA). The primers (Table 3) for the specified genes were dissolved in nuclease-free water according to the specification in the IDT (Integrated DNA Technologies, USA) primer chart sheet. Real-time PCR was carried out in a 20-μL system of which different composition were mixed as follows: 10 μL SYBR, 1 μL reverse, 1 μL forward primers and 2 μL cDNA. Template (cDNA) was diluted to 1:9 ratio (2 μL cDNA and 18 μL nuclease-free water). The expression level of three genes IGF-1 (insulin-like growth factor 1), IGF-2 (insulin-like growth factor 2) and GH (growth hormone) was analysed with β-actin as the internal control. All the readings were performed in triplicate with appropriate non-template control (NTC). Further, the specificity of primer and homogeneity of the PCR product were confirmed by melt curve analysis post-amplification. For the three genes, the melt curve analysis revealed a single peak. The relative expression level of gene was calculated by 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analyses
The data were statistically analysed by one-way analysis of variance (ANOVA). The significant difference among mean was determined by Duncan’s multiple range test (Duncan 1955) at the level p ≤ 0.05. All statistical analyses were performed using the SPSS version 16 statistical package.
Results
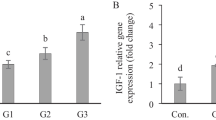
The 0.5 and 1 % doses of curcumin increase the activity of α-amylase, protease and lipase enzymes in the gut (Table 4). The activities of α-amylase, protease and lipase were higher in 0.5 and 1 % curcumin-fed fish compared to control (p ≤ 0.05). 0.5 % and 1 % curcumin-fed fish showed significant increase in IGF-1 (Fig. 1) and IGF-2 (Fig. 2) genes in muscle and GH (Fig. 3) gene expression in brain. Supplementation of curcumin at different concentrations in the feed increased enzyme activities and the expression of GH, IGF-1 and IGF-2 genes in O. mossambicus.
Discussion
Various strategies have been derived and adapted to enhance the quality and quantity of aquaculture products. A growing interest has emerged in using plants containing numerous bioactive phytoconstituents in finfish feed to promote growth (Chakraborty et al. 2014). The present study was designed to analyse the effects of dietary polyphenolic phytochemical curcumin on the activities of digestive enzymes and relative expression of GH in brain, and IGF-1 and IGF-2 genes in muscle in teleost tilapia (O. mossambicus). The results show that the activities of α-amylase, protease and lipase enzymes were higher in 0.5 and 1 % curcumin-fed fish when compared to control. The capacity of an animal to obtain nutrients from a particular food source is largely determined by the profile and activity of its digestive enzymes (Furne et al. 2005). The results denote that the increase in the production of digestive enzymes and digestive capacity are correlated. α-amylase, protease and lipase are the major enzymes which take part in the intestinal digestion process. These enzymes are targets of several substrate forms, mainly carbohydrate, protein and lipids, respectively. Studies in albino rats showed that 0.5 % curcumin has also improved intestinal lipase activity (Platel and Srinivasan 2000). Hu et al. (2003), Wang and Wu (2007) have shown that curcumin enhanced growth performance and decreased feed conversion ratio of grass carps (Ctenopharyngodon idells) and large yellow croaker (Pseudosciaene crocea). Further, Cui et al. (2013) proved the beneficial dietary effects of curcumin on growth performance, antioxidant ability and resistance to infection by S. iniae. Long-term dietary supplementation of curcumin was proven to have a protective effect in A. testudineus (Manju et al. 2013). Quillaja saponin-supplemented diet in Cyprinus carpio was shown to increase the activity of the enzymes amylase and trypsin, suggesting stimulation of protein and carbohydrate digestion in the intestine (Francis et al. 2002). Fish intestine plays an important role in the digestive and absorptive functions of the alimentary tract, thereby showing a significant effect on fish nutrition and growth (El-Bakery and El-Gammal 2010). Increase in the activity of enzymes suggests stimulation of protein digestion in the intestine and thereby contributing to growth-promoting effects of curcumin. Phytochemicals and their metabolic products may provide health benefits as selective growth factors and fermentation substrates for beneficial gastrointestinal bacteria, while acting as selective inhibitors of deleterious intestinal bacteria, thereby exerting prebiotic-like effects in fish (Zheng et al. 2009; Harikrishnan et al. 2011). Curcumin is known to have a large number of biologically active compounds such as triterpenoids, alkaloids, reducing sugars that may exhibit immunomodulatory properties and act as a prebiotic (Kurhekar 2013). Curcumin may also have acted as a prebiotic that enhanced the balance of positive and negative intestinal flora, increased intestinal digestion and absorption, thereby ultimately leading to stimulation of general health and growth increase in fish.
Our lab is continuing studies on the long-term effects of curcumin on fish growth. Fish fed with 0.5 and 1 % curcumin showed significant increase in GH in brain, and IGF-1 and IGF-2 gene expression in muscle. The action of GH includes direct and indirect effects on soma. Direct effects are based on the steps that boost protein synthesis such as RNA synthesis, amino acid uptake, and indirect effect occurs through the release of two IGFs such as IGF-1 and IGF-2 (Humbel 1990). In several species of fish, blood levels of IGF-1 or tissue levels of its mRNA positively correlate with dietary ration, protein content and total body growth rate (Reinecke et al. 2005). Fish growth is positively correlated with muscle growth and is controlled by extrinsic regulators such as IGF-1 and IGF-2 and myostatin which are involved in fish myogenesis and have a role in muscle growth. Moreover, the gene expression of IGF-1 and IGF-2 are dependent on the feeding regime (Gabillard et al. 2006). Hence, curcumin may enhance nutrient utilization and stimulate GH expression with subsequent increase in IGF-1 and IGF-2 expression which thereby suggests a physiological mechanism for growth. Polyphenolic phytochemicals have been reported to promote DNA, RNA and protein synthesis, stimulate GH and IGF-1 production and function and other anabolic effects in fish, resulting in growth increase (Lee et al. 2005; Turan and Akyurt 2005a, b; Cek et al. 2007; Fernandez-Navarro et al. 2008, Goda 2008; Citarasu 2010). Curcumin has also shown to act directly on cultured muscle precursor cells to stimulate both cell proliferation and differentiation under appropriate conditions (Srimal and Dhawan 1973). Curcumin may upregulate the expression of the growth-related factors (IGF-1 and IGF-2) through IGF-signalling pathway.
In the present scenario, according to FAO projections of UN (2014), it is estimated that in order to maintain the current level of per capita consumption, the global aquaculture production needs to reach 80 million tonnes by 2050. The current strategy in aquaculture is to increase the quality and quantity of the aquaculture products. The search is now on for practices which enhance growth and provide safe and quality products. Currently, aquaculture production is dwindling due to the public concerns about aquaculture practices and use of GM organisms. As a solution to this, curcumin a natural product from Curcuma longa provides to be a safe and viable alternative. Further studies are required to understand the molecular mechanism of action of curcumin in fish.
References
Allen PC, Danforth HD, Augustine PC (1998) Dietary modulation of avian coccidiosis. Int J Parasitol 28:1131–1140
AL-Sultan SI (2003) The effect of Curcuma longa (turmeric) on overall performance of broiler chickens. Int J Poult Sci 5:351–353
Bernfeld P (1955) Amylases α and β. Methods Enzymol 1:49–158
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cek S, Turan F, Atik E (2007) The effects of gokshura, Tribulus terrestris on sex differentiation of guppy, Poecilia reticulata. Pak J Biol Sci 10:718–725
Chakraborty SB, Horn P, Hancz C (2014) Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev Aquac 6:1–19
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18:403–414
Cui H, Liu B, Ge X, XiE J, Xu P, Miao L, Sun S, Liao Y, Chen R, Ren M, Zhou Q, Pan L (2013) Effects of dietary curcumin on growth performance, biochemical parameters, HSP70 gene expression and resistance to Streptococcus iniae of juvenile Gift Tilapia, Oreochromis niloticus. Isr J Aquac 66:986–996
D’Souza HP, Prabhu HR (2006) In vitro inhibition of lipid peroxidation in fish by turmeric (Curcuma longa). Indian J Clin Biochem 21(2):138–141
Duncan DB (1955) Multiple range and multiple F test. Bio metrics 11:1–42
El-Bakary NER, El-Gammal HL (2010) Comparative histological, histochemical and ultrastructural studies on the proximal intestine of flathead grey mullet (Mugil cephalus) and sea bream (Sparus aurata). World Appl Sci J 8:477–485
Elizabeth K, Rao MNA (1990) Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–240
FAO/WHO (2014). State of world fisheries and aquaculture. FAO Fisheries and Aquaculture Department, Rome
Fernandez-Navarro M, Peragon J, Amores V, De La Higuera M, Lupianez JA (2008) Maslinic acid added to the diet increases growth and protein-turnover rates in the white muscle of rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C 147:158–167
Francis G, Kerem Z, Makkar HPS, Becker K (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88:587–605
Furne M, Hidalgo MC, Lopez A, Garcia-Gallego M, Morales AE, Domezain A, Domezaine J, Sanz A (2005) Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250:391–398
Gabillard JC, Kamangar BB, Montserrat N (2006) Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J Endocrinol 191:15–24
Gatlin DM III (2002) Nutrition and fish health. In: Halver JE, Hardy RW (eds) Fish nutrition. Academic Press, New York, pp 671–702
Goda AMA-S (2008) Effect of dietary Ginseng herb (Ginsana G115) supplementation on growth, feed utilization, and hematological indices of Nile Tilapia, Oreochromis niloticus L., fingerlings. J World Aquac Soc 39:205–214
Hardy R (1980) Fish feed formulation. Lectures presented at the FAO/UNDP Training course in fish feed technology, University of Washington, Seattle, 1980, pp 233–240
Harikrishnan R, Balasundaram C, Heo M-S (2011) Diet enriched with mushroom Phellinus linteus extract enhances the growth, innate immune response, and disease resistance of kelp grouper, Epinephelus bruneus against vibriosis. Fish Shellfish Immunol 30:128–134
Houlihan DF, Hall SJ, Gray C, Noble BS (1988) Growth rates and protein turn over in Atlantic cod, Gadus morhu. Can J Fish Aquat Sci 45:961–964
Hu ZZ, Yang JF, Tan ZJ, Hao JL (2003) Effect of curcumin on the growth and activity of digestive enzyme in grass carps (Ctenopharyngodon idells). Cereal Feed Ind 11:29–30
Humbel RE (1990) Insulin-like growth factors 1 and 2. Eur J Biochem 190:445–462
Johnson C (2004) Influence of certain plant extract on growth and metabolism of teleosts (Anabas testudineus and Labeo rohita) and a mammal (Rattus norvegicus). PhD thesis, University of Kerala, Thiruvananthapuram, Kerala, India
Jones JI, Clemmons DR (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34
Keay L, Moser PW, Wildi BS (1970) Proteases of the genus Bacillus II Alkaline proteases. Biotechnol Bioeng 12:213–249
Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW (2000) Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 130:467S–471S
Kurhekar J (2013) Curcuma longa and Allium sativum as prebiotics. Bionano Front 6:327–329
Lee KJ, Dabrowski K, Sandoval M, Miller MJS (2005) Activity guided fractionation of phytochemicals of maca meal, their antioxidant activities and effects on growth, feed utilization, and survival in rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture 244:293–301
Lemieux H, Blier P, Dutil JD (1999) Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol Biochem 20:293–303
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 25:402–408
Manju M, Sherin TG, Rajeesha KN, Sreejith P, Rajasekharan KN, Oommen OV (2008) Curcumin and its derivatives prevent hepatocyte lipid peroxidation in Anabas testudineus. J Fish Biol 73:1701–1713
Manju M, Sherin TG, Rajasekharan KN, Oommen OV (2009) Curcumin analogue inhibits lipid peroxidation in a freshwater teleost, Anabas testudineus (Bloch): an in vitro and in vivo study. Fish Physiol Biochem 35:413–420
Manju M, Akbarsha MA, Oommen OV (2012) In vivo protective effect of dietary curcumin in fish Anabas testudineus (Bloch). Fish Physiol Biochem 38:309–318
Manju M, Vijayasree AS, Akbarsha MA, Oommen OV (2013) Protective effect of dietary curcumin in Anabas testudineus (Bloch) with a special note on DNA fragmentation assay on hepatocytes and micronucleus assay on erythrocytes in vivo. Fish Physiol Biochem 39:1323–1330
Park EJ, Jeon CH, Ko G, Kim J, Sohn DH (2000) Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol 52:437–440
Perez-Sanchez J, Calduch-Giner JA, Mingarro M, Vega-Rubin de Celis S, Gomez-Requeni P, Saera-Vila A, Astola A, Valdivia MM (2002) Overview of fish growth hormone family. New insights in genomic organization and heterogeneity of growth hormone receptors. Fish Physiol Biochem 27:243–258
Platel K, Srinivasan K (2000) Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung 44(1):42–46
Ramyrez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baro L, Ramirez-Tortosa CL (1999) Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis 147:371–378
Reinecke M, Collet C (1998) The phylogeny of the insulin-like growth factors. Int Rev Cytol 183:1–94
Reinecke M, Bjornsson BT, DickhoV WW, McCormick SD, Navarro I, Power DM, Gutierrez J (2005) Growth hormone and insulin-like growth factors in fish: where we are and where to go. Gen Comp Endocrinol 142:20–24
Ruby JA, Kuttan G, Dinesh Babu KV, Rajasekharan KN, Kuttan R (1995) Anti-tumour and free radical scavenging activity of synthetic curcuminoids. Int J Pharm 131:1–7
Shan X, Xiao Z, Huang W, Dou S (2008) Effects of photoperiod on growth, mortality and digestive enzymes in miiuy croaker larvae and juveniles. Aquaculture 281:70–76
Soudamini KK, Kuttan R (1989) Inhibition of chemical carcinogenesis by curcumin. J Ethnopharmacol 27:227–233
Srimal RC, Dhawan BN (1973) Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 25:447–452
Tacon AGJ, Metian M, De Silva SS (2010) Climate change, food security and aquaculture: policy implications for ensuring the continued green growth and sustainable development of a much needed aquatic food sector. In: Subasinghe RP, Arthur JR, Bartley DM, De Silva SS, Halwart M, Hishamunda N, Mohan CV, Sororgeloos P (eds) Proceedings of the Global Conference on Aquaculture, Phuket, Thailand
Turan F, Akyurt I (2005a) Effects of androstenedione, a phytoandrogen, on growth and body composition in the African catfish Clarias gariepinus. Isr J Aquac 57:62–66
Turan F, Akyurt I (2005b) Effects of red clover extract on growth performance and body composition of African catfish Clarias gariepinus. Fish Sci 71:618–620
Wang JB, Wu TX (2007) Effect of curcumin on the feed in large yellow croaker (Pseudosciaene crocea). Reserv Fish 6:105–106
Winkler UK, Martina S (1979) Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol 138(3):663–670
Zheng ZL, Tan JYW, Liu HY, Zhou XH, Xiang X, Wang KY (2009) Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 292:214–218
Acknowledgments
The authors would like to thank Central University of Kerala, Kerala State Biodiversity Board, Kerala State Council for Science Technology and Environment, Thiruvananthapuram, for Junior Research Fellowship. All the animal procedures used in this study were approved by Central University of Kerala Ethical Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we do not have any conflict of interests in the matter stated in this paper. All the authors approve on the presented material of this paper.
Rights and permissions
About this article
Cite this article
Midhun, S.J., Arun, D., Edatt, L. et al. Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, Tilapia (Oreochromis mossambicus) by dietary curcumin. Aquacult Int 24, 1277–1286 (2016). https://doi.org/10.1007/s10499-016-9984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-9984-1