Abstract
The black pygmy mussel Limnoperna securis (Lamarck 1819) is endemic to the brackish waters of New Zealand and Australia, but over the past decade, it has successfully invaded the inner Galician Rias of NW Spain. There is growing concern that L. securis will expand its range to the outer zones of the Rias, where it would pose a threat to the intensive raft culture of the indigenous mussel Mytilus galloprovincialis (Lamarck 1819). In this paper, we compare the valve-opening behaviour of the two mytilids under simulated raft conditions, i.e. full-strength seawater (35 g l−1) and a low current flow regime (2–5 cm s−1). Modes of valve-opening amplitudes that were most frequently observed in both species were in the range of 60–90 %, indicating a tendency towards full valve openness. Both species displayed circadian periodicity (τ = 24 h): maximal gaping was generally observed during periods of darkness and minimum gaping during daylight hours. The only prominent difference in behaviour between the two species was related to the degree of valve opening. The maximum recorded gape angle was 8.2° (SE = 0.9) for L. securis versus 14.8° (SE = 1.4) for M. galloprovincialis. This difference may place L. securis at a competitive disadvantage on substrates, where the two species coexist, such as over rocky shores or potentially mussel culture ropes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The black pygmy mussel Limnoperna securis (Lamarck 1819) is endemic to the brackish waters of New Zealand and Australia but has been introduced over the past several decades to Japan (Kimura et al. 1999), Spain (Garci et al. 2007) and Italy (Sabelli and Speranza 1994; Barbieri et al. 2011). L. securis colonises various substrate types and establishes high-density populations which foul submerged structures (Garci et al. 2007; Pascual et al. 2010) and pose serious threats to indigenous faunal communities (Darrigran 2002). Recently, it has been listed among the “100 worst invasive species” in the Mediterranean Sea (Streftaris and Zenetos 2006).
In Galicia (NW Spain), the presence of L. securis was first recorded in 2002 in the Ria de Vigo (Garci et al. 2007) (Fig. 1). More recent observations indicate that it has since expanded its range into the Ria de Pontevedra (Gestoso et al. 2012). The invasion is thus far confined to the inner parts of these Rias, possibly because low salinity favours the invader’s larval stages (Wilson 1969). Settled (adult) stage abundance increases with decreasing salinity (Gestoso et al. 2012).
Although apparently confined to the inner Rias, there is new information suggesting that L. securis larvae can reach the outer areas of the Rias, where intensive raft culture of the indigenous mussel Mytilus galloprovincialis (Lamarck 1819) is carried out. The evidence is based on molecular detection of L. securis larvae in the stomach contents of a copepod sampled in the outer Ria de Vigo (Guerra et al. 2013). It is possible that the copepod consumed the larvae in the inner Ria, but the investigators concluded, based on the hydrographical forcing patterns in this Ria, that the larvae was likely transported by surface waters from the Verdugo River to the outer Ria. The hypothetical expansion of L. securis’ range from the inner to the outer Rias raises a serious concern for the mussel farming industry in Galicia. If L. securis were to successfully colonize these outer areas, its larvae would likely settle onto the culture ropes, where they would not only compete for space and food, but also inevitably lead to serious farm husbandry and plant processing challenges. While the potential economic impact is difficult to assess, it is noteworthy that the culture of M. galloprovincialis in the outer Rias is carried out at a scale of 250,000 tons per year, which represents about 40 % of the European mussel production and 15 % of the world’s production (Labarta et al. 2004).
The aim of the present study was to gain insight into the behaviour of settled L. securis in a marine environment. To date, this species has successfully invaded areas in the Galician Rias, where the velocity of brackish water currents can be quite elevated due to riverine discharge, attaining for instance 123 cm s−1 at the mouth of the Verdugo River (Babarro and Lassudrie 2011). L. securis has a distinct cylindrical shape, which is presumably suited to dynamic environments such as those found at river mouths. A mussel culture raft, however, is an obstacle that reduces flow rates considerably within its structure. The maximum reported velocity within a raft in the outer Rias was 30.7 cm s−1 (Camacho et al. 1995); average velocities range between 2 and 3 cm s−1 (Camacho et al. 1995; Petersen et al. 2008), similar to velocities recorded within rafts in Saldanha Bay South Africa (Boyd and Heasman 1998), where M. galloprovincialis is also farmed. Here, we test the hypothesis that the invasive mussel L. securis responds negatively to low current velocities typical of raft culture. The hypothesis was tested by acclimating the two species, L. securis and M. galloprovincialis, to a high salinity environment (35 g l−1) and then monitoring (1 Hz) the degree of their valve opening in response to various flow regimes (2–40 cm s−1). Our premise was that atypical valve activity, such as a tendency towards the closure of shell valves, is indicative of physiological stress and consequently of limited colonisation potential.
Methods
Field sampling and holding conditions
Mytilus galloprovincialis and Limnoperna securis were collected from the sheltered intertidal coastline of the inner Ría de Vigo (San Simón 42°19′31″N, 8°36′77″W), where the two species currently coexist, forming monolayer beds competing for space and food. Collection was carefully achieved by scraping the rocks to avoid damaging the byssus gland or foot. Mussels were transported to the Instituto de Investigaciones Marinas in Vigo, where they were held in four 19-L holding tanks under the same conditions as described in Babarro and Fernández-Reiriz (2010). Tanks were continuously supplied with filtered (10 μm) seawater (35 g l−1, 15 °C) supplemented with a mixture of microalgae (Tahitian Isochrysis aff. galbana, T-ISO) and sediment collected from the seafloor below the mussel culture rafts (40:60 microalgae/sediment, by weight). Particulate material load was maintained at 1.0 mg L−1 with an organic content percentage of 50 %, simulating mean food availability in the Galician Rías (Babarro et al. 2000).
Flume tank environment
Prior to each experiment, randomly selected mussels, ranging in shell length from 31 to 39 mm, were transferred from the holding tanks to a circulating flume tank containing 1,720 L of aerated seawater (35 g l−1, 15 °C) supplemented with the same food elements as described above. The custom flume tank is described in Babarro and Carrington (2013). Briefly, the working section of the tank into which the mussels were placed had dimensions of 80 cm (length), by 60 cm (width), by 40 cm (water depth). To remove large-scale turbulence, the seawater flowed through a system of collimators (PVC pipes, 2-cm diameter opening × 100 cm long) positioned upstream of the working section. Flow was generated by an axial flow pump and was measured in the vicinity of the experimental mussels to the nearest cm s−1 using a flow metre (2D-ACM Falmouth Scientific, Inc. Cataumet, MA 02534 USA).
Artificial lighting was limited to a 9-h period from 8:00 to 17:00 h. This background lighting was supplemented by natural light entering the building through large windows. While lighting intensity was not rigorously controlled, it was continuously monitored using Hobo UA-002 light loggers (Onset Computer Corporation, Massachusetts, USA), which were placed above the flume tank.
Valvometry
Valve opening was monitored using a valvometry system described in Nagai et al. (2006) and Comeau et al. (2012). The system allowed for the simultaneous monitoring of 24 individuals. A coated Hall element sensor (HW-300a, Asahi Kasei, Japan) was glued to one valve at the maximum distance from the hinge. Then a small magnet (4.8 mm diameter × 0.8 mm height) was glued to the other valve, directly below the Hall sensor. The magnet and the Hall element weigh 0.1 and 0.5 g, respectively. For comparison purposes, a small (6 mm diameter) live barnacle weighs approximately 0.12 g. The magnetic field (flux density) between the sensor and magnet was a function of the gap between the two valves. The magnetic field in the form of output voltage (μV) was acquired by strain recording devices (DC 104R, Tokyo Sokki Kenkyujo Co., Japan). Output voltage was recorded at a frequency of 1 Hz and was subsequently converted into valve opening by applying conversion algorithms specific to each sensor assembly.
Flume vibrational noise
We were initially concerned about the vibrational noise created by the flume tank engine, particularly at times when the engine was operating at high flow regimes. For this reason, the effect of vibrational noise on mussel behaviour was tested by placing eight mussels of each species into glass chambers inside the flume. These mussels were isolated from the flowing water, but were nevertheless exposed to the vibration and noise created by the flume engine. Valve opening was monitored using the valvometry system described above. The outcome indicated that opening amplitudes were similar between periods when the flume was operating at low and high velocities, suggesting that vibrational noise within the context of our experiments had no effect on valve gape behaviour.
Experimental design and statistics
Two experiments were conducted in the flume tank. The first experiment was designed to assess the mytilids’ response to sustained flow. Twelve mussels of each species were exposed to a constant velocity of 3 cm s−1, a gradual increase from 3 to 40 cm s−1, and ultimately to a sustained peak in velocity of 40 cm s−1. The rise in velocity was performed during the daytime, whereas the low (3 cm s−1) and high (40 cm s−1) sustained treatments were applied during two consecutive night-time periods, specifically from 18:00 to 8:00 (14-h periods). The entire experiment was replicated once using a new cohort of mussels.
Statistical analyses were restricted to the low and high velocity night-time treatments and therefore excluded the daytime period of gradual increase in velocity. In order to standardize the data, a relative valve-opening metric was computed as a per cent of the maximal recorded opening amplitude specific to each individual. The metric was then partitioned into ten ranges from 0 to 100 % amplitude. Per cent occurrence was calculated as the number of observations in a specified range (e.g. 0–10 % amplitude) divided by the total number of observations (Tran et al. 2010). A mixed model analysis of variance (SPSS version 20, procedure GLM) was used to test the main fixed effects (species and velocity) and their interactions on per cent occurrence at the specified ranges of valve opening. The model can be summarizes as follow:
where Occ is the per cent occurrence at a specified range of valve opening (e.g. 0–10 %), μ is overall mean of the population, Sp represents species [i = 1 (M. galloprovincialis), 2 (L. securis)], Vel is the current velocity [j = 1 (3 cm s−1), 2 (40 cm s−1)], Rep is the replicated experiment (k = 1, 2) and ε is the model error. The replicated experiment (Rep) was set as a random effect. Data were rank-transformed because variances were heterogeneous (Levene’s test).
The second experiment was designed to assess behavioural responses to a tidally driven current regime. In this experiment, current velocity was automatically controlled by a computer; the programme was set to create sinusoidal current profiles such as those generated by semi-diurnal tides. Velocity increased gradually over 3 h, and thereafter decreased over another 3 h, as it typically would during successive flood and ebb tides. Eight mussels of each species were exposed to low sinusoidal forcing (2–5 cm s−1) over 6 days; the same individuals were subsequently challenged to elevated sinusoidal forcing (2–25 cm s−1) for another 6-day period. Mussels were positioned in the flume with their incurrent siphon and mantle margin facing upstream. At the end of the experiment, the adductor muscle was severed, and small calibration wedges were manoeuvred between the two valves at the point farthest from the hinge. Wedge height was 1–6 mm. The relationships between voltage and wedge height (i.e. valve opening) were nonlinear and strong (r 2 > 0.90). Valve-opening (mm) data were converted into gape angles (θ in degrees) using the following equation (Wilson et al. 2005):
where W is the valve opening (mm) and L (mm) is the mussel’s shell length.
Periodogram analysis was used to ascertain whether significant periodic components existed in the valve-opening time series. Linear trends were removed using the ordinary least squares (OLS) method prior to performing the analysis. Fourier spectral analyses were then performed on either the residuals from the OLS trend analysis (Warner 1998) or directly on the valve-opening measures (for series where no trends were apparent). Periodogram values were calculated for each Fourier frequency, thus providing a numerical representation of the magnitude of the periodicity present in the data at each periodic cycle. The Fisher’s test and critical values tabled by Russell (1985) were applied to test the significance of each periodic cycle. The Fisher’s test required the calculation of the g value, which in turn provided the proportion of the total variance that was accounted for by each periodic component. Because circadian periodicity was of primary interest, a paired t test was used to test the null hypothesis that the g statistic for the 24-h periodic component was similar under low and high sinusoidal velocity regimes.
All analyses were performed in SPSS version 20 (IBM SPSS Inc, Chicago). Statistical significance for all statistical tests was set at 0.05.
Results
Figure 2 summarizes the valve gape behaviour of the mussels during the first experiment or more specifically the mean occurrence as a function of valve-opening amplitude (ten ranges from 0 to 100 % of maximal opening amplitude). For M. galloprovincialis, mean occurrence followed a negatively skewed normal distribution; modes of opening amplitudes that were most frequently observed were in the range of 60–90 %, indicating a tendency towards full openness. This behaviour was consistent in both replicate experiments (panels a–c and g–i). In comparison, the behaviour of L. securis differed between the two replicate experiments: mean occurrences followed either a flattened (replicate 1, panels d–f) or negatively skewed (replicate 2, panels j–l) normal distribution. Nevertheless, complete valve closures or near closures were rarely recorded in the two replicate experiments. Table 1 summarizes the statistical outcome of the mixed model analysis of variance. No significant differences were detected among treatments, including between species or low and high velocity phases of the experiment. The only significant effects were linked to the variance between replicate experiments.
Per cent occurrence as a function of valve-opening amplitude (ten ranges from 0 to 100 % of maximum amplitude) for M. galloprovincialis (open bars) and L. securis (dark bars) at low velocity (LV—3 cm s−1), rising velocity (RV—3 to 30 cm s−1) and high velocity (HV—40 cm s−1). Panels are grouped according to the first (a–f) and second (g–l) replicate experiments. Error bars show mean ± standard error, n = 10 (M. galloprovincialis) and n = 12 (L. securis)
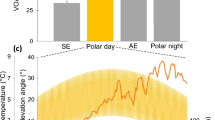
Figure 3 shows the sinusoidal period at which the flume was operating and mean gape angle as a function of time during the second experiment. Gape angle differed between species, regardless of the velocity applied. The maximum recorded angle for L. securis was 8.2° (mean of six individuals, SE = 0.9), compared to 14.8° for M. galloprovincialis (mean of eight individuals, SE = 1.4). These maximal values were significantly different from each other (Mann–Whitney, P = 0.003). There were no indications that L. securis responded negatively to the low velocity phase of the experiment, which was intended to mimic conditions within culture rafts.
Sinusoidal current velocity (top) and mean gape angle (bottom) of mussels in the flume tunnel. Means were calculated from individual mussels (n = 8 for M. galloprovincialis and n = 6 for L. securis). The time series extended from January 30 (21:00) to February 12 (18:00) 2012. Shaded areas indicate periods of darkness
With respect to rhythmicity, mussel behaviour was not synchronized to the flume current periodicity (τ = 6 h). Instead, there was a tendency for both species to exhibit maximal gape angle during periods of darkness and a minimum during daylight hours. Spectral analysis and the Fisher’s test indicated that the 24-h periodicity was dominant and highly significant (P < 0.001) for each individual. However, there were significant differences between the low and high velocity phases in terms of the proportion of variance accounted for by the 24-h periodicity. Initially, during the low velocity phase, the proportion of the variance accounted for by the 24-h periodicity averaged 23.5 % (SE = 5.2) and 12.6 % (SE = 3.4) for L. securis and M. galloprovincialis, respectively. These proportions fell during the high velocity phase, averaging only 7.1 % (SE = 5.4) and 4.7 % (SE = 1.5) for L. securis and M. galloprovincialis, respectively. These differences in circadian periodicity between the two velocity phases were significant (paired t tests applied to g values, P = 0.03 for L. securis, P = 0.02 for M. galloprovincialis) and were of similar magnitude for both species. The proportion of variance accounted for by the 24-h periodicity fell by 71.0 % (SE = 15.5) in L. securis and 54.5 % (SE = 15.1) in M. galloprovincialis (Mann–Whitney, P = 0.20). Therefore, when subjected to forceful sinusoidal currents, circadian gaping rhythmicity was significantly but equally disrupted in L. securis and M. galloprovincialis.
Discussion
Valve opening signals the activation of a complex nervous mechanism involving the heart and adductor muscles (Taylor 1976), resulting in the bivalve exposing itself to the environment and exercising metabolically demanding processes, such as the collection and assimilation of food particles. In the present study, our premise was that atypical valve activity is indicative of physiological stress and hence colonisation potential of the black pygmy mussel L. securis in the outer Rias where the indigenous mussel M. galloprovincialis is cultivated. We conclude that raft conditions (i.e. high salinity and low flow) have no detrimental effect on the valve gaping behaviour of L. securis. We base this conclusion on the observation that complete valve closures or near closures were rarely recorded and also on normal distribution of opening modes, which showed a tendency towards full openness at 3 cm s−1 (Fig. 2). Current velocities in the range of 2–3 cm s−1 are typical of those recorded within mussel culture rafts in NW Spain (Camacho et al. 1995; Petersen et al. 2008). The reason full openness for L. securis was more evident in the second replicate experiment may be attributable to these mussels having been acclimated longer to laboratory conditions.
Another finding of the work conducted here is that both species exhibited strong circadian rhythmicity. To the best of our knowledge, we provide the first evidence of valve gaping rhythmicity in L. securis. Rhythms were not synchronized to the tidal flow cycle (τ = 6 h) in either species, suggesting that tidal currents are not the main driving influence behind these rhythms. Instead, there was a tendency for both species to exhibit maximal gape angles during darkness periods and minimum angles during daylight hours. A lack of tidal rhythmicity and dominance of circadian rhythmicity has been previously reported for M. galloprovincialis (Gnyubkin 2010), the blue mussel Mytilus edulis (Ameyaw-Akumfi and Naylor 1987; Wilson et al. 2005; Robson et al. 2010) and more recently the green-lipped mussel Perna canaliculus (Lurman et al. 2013). Considering that bivalves possess photoreceptor cells (Ramirez et al. 2011) and that mussels respond to sudden changes in light level (Lurman et al. 2013), it is plausible that light is the main environmental cue entraining circadian rhythms in bivalves. With regard to its adaptive significance, it is generally thought that nocturnal gaping is part of a strategy to feed while minimizing the likelihood of predation, particularly when the foot is protruding from the shell during nocturnal byssus thread production (Martella 1974). In the present study, circadian gaping rhythmicity was significantly but equally disrupted in L. securis and M. galloprovincialis when they were subjected to forceful sinusoidal currents, similar to those that occur under rafts at certain locations in the Rias (Camacho et al. 1995). The implications of degraded circadian rhythms are not known, but they are likely irrelevant to raft colonisation since M. galloprovincialis also displayed degraded rhythms.
The only prominent difference between L. securis and M. galloprovincialis was related to the absolute gape angle of their valves (Fig. 3). Valve gape was consistently lower in L. securis compared to M. galloprovincialis. It is possible that L. securis responded to the high salinity (35 g l−1) in the holding tanks, although exploratory work indicated the same inter-species difference under a lower salinity environment (~20 g l−1, results not shown). Morphological features provide a more plausible explanation. The flexible ligament, which pulls the two valves apart while the adductor muscle actively holds them together, is about 25 % shorter in L. securis than in M. galloprovincialis (JMF Babarro, unpublished data). The shorter ligament in L. securis could explain the narrower shell gape. Also, compared to M. galloprovincialis, L. securis has a more cylindrical shape, a relatively narrow shell height and low external shell surface area. These shell characteristics provide insight into metabolic requirements given that gill tissues are distributed along the internal cavity of the shells. We calculated the shell surface area for our experimental mussels based on allometry relationships provided in Babarro and Lassudrie (2011). We found that while the two experimental groups (L. securis and M. galloprovincialis) had similar shell lengths (~35 mm, Mann–Whitney, P = 0.30), external shell surface area was on average 28 % lower in the L. securis group compared to the M. galloprovincialis group (Mann–Whitney, P = 0.002). Therefore, considering that gill tissues are distributed along the internal cavity of the shells, L. securis probably has a low gill area compared to M. galloprovincialis. This interpretation is supported by clearance and ingestion rates being reportedly lower in L. securis than in M. galloprovincialis (Fragoso Pérez 2012) and also consistent with growth rates being lower in L. securis than in M. galloprovincialis (Babarro and Abad 2013). Such traits are not entirely unexpected, given that L. securis is foremost an infaunal and semi-infaunal mytilid. It produces a multitude of short and weak byssus threads, creating an extensive network of filaments anchored to small particles on the soft bottom (Pearce and LaBarbera 2009). Wide gaping would presumably compromise the stability of this anchorage system or render the mytilid susceptible to sand particles falling into the internal cavity and causing tissue abrasion (Rius and McQuaid 2006; Zardi et al. 2008).
Regardless of the reason for the inter-species differences in valve opening, a wide valve gape may offer a competitive advantage to M. galloprovincialis where the two species compete in nature, such as over rocky shores or potentially mussel ropes. Byssus is secreted by the extension of a secretory organ, the foot, when it explores the surrounding substrate. The size of the foot has been reported to be significantly larger for M. galloprovincialis than for L. securis (Babarro and Lassudrie 2011), suggesting that wide gaping may be needed to accommodate a large and extensible foot. Such features increase the mobility of M. galloprovincialis (Brazee and Carrington 2006; Shinen and Morgan 2009; Babarro and Carrington 2011), allowing it to escape bottom layers in mixed beds. In the inner Ria de Vigo, for example, the indigenous M. galloprovincialis colonises the upper portions of beds, thereby smothering the invasive L. securis and introducing a physical interference competition (Babarro and Abad 2013). Nicastro et al. (2012) have also reported that the extent of valve gaping in intertidal mussels plays a role in microhabitat reorganization. Our suggestion that wide gaping offers a competitive advantage to M. galloprovincialis is consistent with this species being a highly successful invader in its own right. Although M. galloprovincialis is cultivated as food for humans in Galicia, it has successfully invaded many other regions worldwide, where it is sometimes considered a nuisance species (Branch and Steffani 2004; Bownes and McQuaid 2006).
In summary, L. securis and M. galloprovincialis behaved similarly under laboratory conditions intended to mimic those found under mussel rafts. Modes of opening amplitudes that were most frequently observed were in the range of 60–90 %, indicating a tendency towards full openness. Also, the two species displayed similar circadian periodicity: they tended to exhibit maximal gaping during periods of darkness and minimal gaping during daylight hours. The only prominent difference recorded between the two species was related to the degree of their valve opening, with M. galloprovincialis consistently exhibiting a wider valve opening than L. securis. This wider valve gape may offer M. galloprovincialis a competitive advantage on substrates where the two species coexist, such as over rocky shores or potentially mussel culture ropes.
References
Ameyaw-Akumfi C, Naylor E (1987) Temporal patterns of shell-gape in Mytilus edulis. Mar Biol 95:237–242
Babarro JMF, Abad MJ (2013) Co-existence of two mytilid species in a heterogeneous environment: mortality, growth and strength of shell and byssus attachment. Mar Ecol Prog Ser 476:115–128
Babarro JMF, Carrington E (2011) Byssus secretion of Mytilus galloprovincialis: effect of site at macro- and micro-geographical scales within Ría de Vigo (NWSpain). Mar Ecol Prog Ser 435:125–140
Babarro JMF, Carrington E (2013) Attachment strength of the mussel Mytilus galloprovincialis: effect of habitat and body size. J Exp Mar Biol Ecol 443:188–196
Babarro JMF, Fernández-Reiriz MJ (2010) Secretion of byssal threads in Mytilus galloprovincialis: quantitative and qualitative values after spawning stress. J Comp Physiol B 180:95–104
Babarro JMF, Lassudrie M (2011) Ecophysiological responses of invasive and indigenous mytilids in the Ría de Vigo (NW Spain). Aquat Living Resour 24:303–315
Babarro JMF, Fernández-Reiriz MJ, Labarta U (2000) Feeding behaviour of seed mussel Mytilus galloprovincialis: environmental parameters and seed origin. J Shellfish Res 145:204–213
Barbieri M, Maltagliati F, Di Giuseppe G, Cossu P, Lardicci C, Castelli A (2011) New records of the pygmy mussel Xenostrobus securis (Bivalvia: Mytilidae) in brackish-water biotopes of the western Mediterranean provide evidence of its invasive potential. Mar Biodivers Rec 4:e48
Bownes SJ, McQuaid CD (2006) Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J Exp Mar Biol Ecol 338:140–151
Boyd AJ, Heasman KG (1998) Shellfish mariculture in the Benguela system: water flow patterns within a mussel farm in Saldanha bay, South Africa. J Shellfish Res 17:25–32
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215
Brazee SL, Carrington E (2006) Interspecific comparison of the mechanical properties of mussel byssus. Biol Bull (Woods Hole) 211:263–274
Camacho AP, Labarta U, Beiras R (1995) Growth of mussels (Mytilus edulis galloprovincialis) on cultivation rafts: influence of seed source, cultivation site and phytoplankton availability. Aquaculture 138:349–362
Comeau LA, Mayrand E, Mallet A (2012) Winter quiescence and spring awakening of the Eastern oyster Crassostrea virginica at its northernmost distribution limit. Mar Biol (N Y) 159:2269–2279
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Fragoso Pérez MR (2012) PhD Thesis. Bioinvasión del mitílido Xenostrobus securis en las rías gallegas. Análisis fisiológico y potencial reproductivo bajo variables condiciones ambientales. Universidad de Santiago de Compostela, Santiago de Compostela
Garci ME, Trigo JE, Pascual S, González AF, Rocha F, Guerra A (2007) Xenostrobus securis (Lamarck, 1819) (Mollusca: Bivalvia): first report of an introduced species in Galician waters. Aquac Int 15:19–24
Gestoso I, Olabarria C, Arenas F (2012) The invasive mussel Xenostrobus securis along the Galician Rias Baixas (NW of Spain): status of invasion. Cah Biol Mar 53:391–396
Gnyubkin VF (2010) The circadian rhythms of valve movements in the mussel Mytilus galloprovincialis. Russ J Mar Biol 36:419–428
Guerra Á, Pascual S, Garci ME, Roura Á, Mucientes G, González ÁF (2013) The black-pygmy mussel Limnoperna securis in Galician Rias (north-eastern Atlantic): new records and first evidence of larval stages predation by copepods. Mar Biodivers Rec 6:e15
Kimura T, Masaaki T, Yasuhiro S (1999) Limnoperna fortunei kikuchii Habe, 1981 (Bivalvia: Mytilidae) is a synonym of Xenostrobus securis (Lamarck, 1918): introduction into Japan from Australia and/or New Zealand. Jpn J Malacol 58:101–107
Labarta U, Fernández-Reiriz MJ, Pérez-Camacho A, Pérez Corbacho E (2004) Bateeiros, mar, mejillón. Una perspectiva bioeconómica. Una perspectiva bioeconómica, Santiago de Compostela
Lurman GJ, Hitlon Z, Ragg NLC (2013) Energetics of byssus attachment and feeding in the green-lipped mussel Perna canaliculus. Biol Bull (Woods Hole) 224:79–88
Martella T (1974) Some factors influencing byssus thread production in Mytilus edulis (mollusca: Bivalvia) Linnaeus, 1758. Water Air Soil Pollut 3:171–177
Nagai K, Honjo T, Go J, Yamashita H, Seok Jin O (2006) Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture 255:395–401
Nicastro KR, Zardi GI, McQuaid CD, Pearson GA, Serrão EA (2012) Love thy neighbour: group properties of gaping behaviour in mussel aggregations. PLoS One 7:e47382
Pascual S, Villalba A, Abollo E, Garci M, González AF, Nombela M, Posada D, Guerra A (2010) The mussel Xenostrobus securis: a well-established alien invader in the Ria de Vigo (Spain, NE Atlantic). Biol Invasions 12:2091–2103
Pearce T, LaBarbera M (2009) Biomechanics of byssal threads outside the Mytilidae: Atrina rigida and Ctenoides mitis. J Exp Biol 212:1449–1454
Petersen JK, Nielsen TG, van Duren L, Maar M (2008) Depletion of plankton in a raft culture of Mytilus galloprovincialis in Ria de Vigo, NW Spain. I. Phytoplankton. Aquat Biol 4:113–125
Ramirez MD, Speiser DI, Pankey MS, Oakley TH (2011) Understanding the dermal light sense in the context of integrative photoreceptor cell biology. Vis Neurosci 28:265–279
Rius M, McQuaid C (2006) Wave action and competitive interaction between the invasive mussel Mytilus galloprovincialis and the indigenous Perna perna in South Africa. Mar Biol 150:69–78
Robson A, Garcia de Leaniz C, Wilson R, Halsey L (2010) Effect of anthropogenic feeding regimes on activity rhythms of laboratory mussels exposed to natural light. Hydrobiologia 655:197–204
Russell RJH (1985) Significance tables for the results of fast Fourier transforms. Br J Math Stat Psychol 38:116–119
Sabelli B, Speranza S (1994) Rinvenimento di Xenostrobus sp. (Bivalvia: Mytilidae) nella laguna di Venezia. Boll Malacol 29:311–318
Shinen JS, Morgan SG (2009) Mechanisms of invasion resistance: competition among intertidal mussels promotes establishment of invasive species and displacement of native species. Mar Ecol Prog Ser 383:187–197
Streftaris N, Zenetos A (2006) Alien marine species in the Mediterranean—the 100 ‘Worst Invasives’ and their impact. Mediterr Mar Sci 7:87–118
Taylor A (1976) The cardiac responses to shell opening and closure in the bivalve Arctica islandica (L.). J Exp Biol 64:751–759
Tran D, Haberkorn H, Soudant P, Ciret P, Massabuau J-C (2010) Behavioral responses of Crassostrea gigas exposed to the harmful algae Alexandrium minutum. Aquaculture 98:338–345
Warner RM (1998) Spectral analysis of time-series data. Guilford, New York
Wilson BR (1969) Survival and reproduction of the mussel Xenostrobus securis (Lamarck) (Mollusca; Bivalvia; Mytilidae) in a Western Australian estuary. Pt. II: reproduction, growth and longevity. J Nat Hist 3:93–120
Wilson R, Reuter P, Wahl M (2005) Muscling in on mussels: new insights into bivalve behaviour using vertebrate remote-sensing technology. Mar Biol 147:1165–1172
Zardi GI, Nicastro KR, McQuaid CD, Erlandsson J (2008) Sand and wave induced mortality in invasive (Mytilus galloprovincialis) and indigenous (Perna perna) mussels. Mar Biol 153:853–858
Acknowledgments
We acknowledge gratefully the guidance of D. Tran and J-C. Massabuau in the early stages of this investigation. Thanks are also extended to E. Silva Caride for her able technical assistance in the field and laboratory. C. Carver kindly agreed to read an early draft of this paper and offered very helpful comments. The Department of Fisheries and Oceans provided in-kind assistance. This study was funded by the project AGL2010-16464 (Ministerio de Ciencia e Innovación, Spanish Government).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comeau, L.A., Babarro, J.M.F. Narrow valve gaping in the invasive mussel Limnoperna securis: implications for competition with the indigenous mussel Mytilus galloprovincialis in NW Spain. Aquacult Int 22, 1215–1227 (2014). https://doi.org/10.1007/s10499-013-9742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9742-6