Abstract
The aim of this study was to investigate the molecular mechanism behind androgen-induced sex reversal and growth increase in Nile tilapia. Three-day-old mixed-sex tilapia juveniles were treated with differential dose (0, 5, 10, 20, 30 mg/kg diet) and duration (0, 15, 30, 45, 60 days) regimes of 17α-methyltestosterone. Treatment regimes 10, 20, 30 mg/kg-30, 45, 60 days showed ~95% males, while 0 mg/kg-0 days (control) showed ~50% males (P < 0.01). Next, tilapia was cultured for 6 months in cistern and pond culture systems with 17α-methyltestosterone-treated diet (10 mg/kg) for three different durations: 0 days (control), 30 days (30-day-treated), and 180 days (throughout-treated). Fish in pond culture showed significantly higher (P < 0.05) final weight compared with tilapia in cistern culture (235.76, 156.82 g, respectively). Both 30-day-treated and throughout-treated fish showed significantly higher (P < 0.05) final weight, length, daily weight gain, specific growth rate, and muscle protein content compared with control fish. The androgen receptor β mRNA and protein expression levels in both 30-day-treated and throughout-treated fish were significantly higher (~twofolds, P < 0.05) compared with hormone-untreated control males. However, there was not much difference for all these parameters among the two hormone-treated groups. This higher growth probably results from an androgen-induced increased ‘androgen-androgen receptor’ mediated anabolic pathway. Serum testosterone concentration of 30-day-treated fish after 6 months was similar to that of control males (0.9 ng/ml). Moreover, culture of 17α-methyltestosterone-treated monosex tilapia was found beneficial than hormone-untreated mixed-sex tilapia culture for maintenance of natural fish diversity in indigenous water bodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), is widely cultured in many tropical and subtropical regions of the world and forms an important dietary protein source (Lin et al. 2008). Rapid growth rate, high tolerance to low water quality, efficient food conversion, resistance to disease, good consumer acceptance, and ease of spawning make tilapia a suitable fish for culture (El-Saidy and Gaber 2005). However, this efficiency of reproduction in tilapia has undesirable consequences as the major concern for tilapia aquaculture is precocious and excessive reproduction resulting in various sizes of small fish production (Lèveque 2002). Hence, the desirability of monosex male populations of tilapia is well sought for increased production potential and low management requirements (Pillay 1993; Beardmore et al. 2001; El-Sayed 2006; Baroiller et al. 2009). Besides, sex-specific differences in growth are significant in tilapia where males grow significantly faster, larger, and more uniform in size than females (Bwanika et al. 2007). Thus, the development of strategies for generation of monosex populations remains a critical objective for tilapia culture industry.
Sex determination in fish is a flexible process, and individuals are subject to modification by external factors (Devlin and Nagahama 2002; Baroiller et al. 2009). Here, estrogens and androgens play a critical role in gonadal sex differentiation (Ijiri et al. 2008), and exogenous sex steroids administered at the time of sex determination can strongly influence the course of sex differentiation (Devlin and Nagahama 2002). Numerous studies on fish including tilapia have shown that treatment of XX larvae with a synthetic androgen 17α-methyltestosterone (MT) can induce sex reversal toward testicular development (Yamamoto 1969; Pandian and Sheela 1995; Abucay and Mair 1997; Kitano et al. 2000; Smith and Phelps 2001; Devlin and Nagahama 2002; Bhandari et al. 2006). But, there are relatively few published studies comparing the growth performance of MT-treated monosex tilapia with mixed-sex fish, under culture, especially from the Indian perspective (Varadaraj et al. 1994; Mair et al. 1995).
Tilapia is often cultured in earthen ponds without supplemental feeding (Liti et al. 2005). Pond culture provides an opportunity to balance the use of supplementary feeding in correlation with the natural food availability. Intensive culture of tilapia in tanks has been globally expanding (El-Sayed 2006). Several factors such as savings in manpower and easier stock management are the main reasons for intensification of fish farming in concrete cisterns in many countries (El-Saidy and Gaber 2005). Tilapia has been widely introduced in the shallow and seasonal ponds of eastern region of India (Sugunan 1995). It has rapidly gained popularity among the fish farmers as a readily available source of animal protein in the diets of rural and urban dwellers belonging to the lower socioeconomic strata. However, ecologists are concerned with the potential adverse effect of this exotic species on the indigenous fish population (De Silva et al. 2004). Strikingly, its performance in open water reservoirs of India has been discouraging (Sugunan 2000). Culture of monosex tilapia may provide an effective measure to these problems. Moreover, the grow-out performance of MT-treated monosex tilapia in different culture systems must be analyzed.
Although faster growth rate of MT-treated, sex-reversed fish has been observed, the functional mechanism behind elevated fish muscle growth has remained unclear (Feist and Schreck 1996). Androgen receptor (AR) has been considered as a critical mediator for action of male sex steroid hormone in fish (Todo et al. 1999). AR can either up or down-regulate the expression of androgen target genes, and the outcome may probably depend on the interactions with specific adapters or coactivators (Hong et al. 1996; Yeh and Chang 1996). Two isoforms (α and β) of AR have been demonstrated in several fish species including tilapia (Blázquez and Piferrer 2005). However, the expression levels of ARβ during the growth of androgen-treated monosex tilapia are not well documented.
Considering these aspects, in this study, an optimum dose and duration regime of MT treatment for monosex tilapia production in India have been determined. Moreover, the grow-out performances of mixed-sex and MT-treated monosex fish in cistern and pond culture systems have been demonstrated, and the variations in ARβ expression in fish muscle tissue at both RNA and protein levels during hormone treatment have been highlighted. Thus, the preliminary steps of a molecular mechanism behind the anabolic action of male sex hormone on tilapia muscle growth have been postulated, which may be used as a platform to initiate a deeper investigation. Finally, it has also been shown that monosex tilapia culture in an open system may exert an impact conducive for the sustenance of native fish diversity in natural water bodies.
Materials and methods
Collection of fish
Just hatched juveniles of mixed-sex Nile tilapia were collected from the fish hatchery at Naihati, West Bengal; oxygen packed and transported to the laboratory. After acclimatizing to the laboratory condition, the fish were weaned to finely ground (<500–1,000 μm) artificial diet containing 30% crude protein (Tokyu, Japan). In the laboratory, they were reared in 5-liter aerated aquaria and maintained in heated (T = 27 ± 2°C) static systems. Water in all aquaria was replaced daily, and the fish were kept under similar photoperiod (14 L: 10 D) and density (10 fish/l).
Preparation of hormone-treated diet
Hormone-treated diets were prepared by the alcohol evaporation method (Shelton et al. 1981). Differential amount of MT (Sigma) was dissolved in 95% ethanol and then mixed with the food pellets (1 l ethanol/Kg pellets), followed by air-drying. Control pellets were prepared similarly without the addition of MT. Fresh feed were prepared weekly and stored in a cool, dry place.
Determination of optimum dose and duration regime for hormone treatment
This experiment was conducted with 3-day-old spawns (n = 3,000; mean weight 0.02 ± 0.003 g; mean length 0.98 ± 0.02 cm) in a completely randomized design with 3 replicates. Within each replicate, the fish were stocked into 20 groups with 50 individuals in each group. Among these, 4 groups were considered as control and were provided with hormone-untreated diet throughout the experimental period. The other 16 groups were given MT-treated diet for the following dose and duration regimes—5 mg/kg for 15 days, 5 mg/kg for 30 days, 5 mg/kg for 45 days, 5 mg/kg for 60 days, 10 mg/kg for 15 days, 10 mg/kg for 30 days, 10 mg/kg for 45 days, 10 mg/kg for 60 days, 20 mg/kg for 15 days, 20 mg/kg for 30 days, 20 mg/kg for 45 days, 20 mg/kg for 60 days, 30 mg/kg for 15 days, 30 mg/kg for 30 days, 30 mg/kg for 45 days, and 30 mg/kg for 60 days. After their respective periods of hormone treatment, these groups were provided with untreated control diet until sexing. Throughout the experimental period, the fish were fed twice daily at the rate of 20% body weight/day.
Sexing of fish
After 60 days, all the fish from each treatment group were anaesthetized with 2-phenoxyethanol (1 ml/l), individually weighed, measured, and sacrificed. Sex ratio following each treatment was determined by macroscopic and microscopic examinations of gonad tissue using the standard acetocarmine gonadal squash technique (Guerrero and Shelton 1974).
Grow-out performance study
A six-month grow-out trial was conducted to determine the effect of MT treatment on tilapia growth in two different culture systems. The experiment had 2 × 3 factorial design: the first factor was related to culture systems (cistern culture and pond culture systems), the second factor was related to duration of MT treatment (0 days or control, 30 days, and 180 days or throughout). The pond culture system consisted nine 0.01-ha earthen ponds, while cistern culture was carried out in 9 concrete tanks of similar size. For the entire duration of the grow-out experiment, the various water quality parameters like temperature, dissolved oxygen, pH, and total alkalinity were regularly monitored (APHA 1998) and maintained within tolerable limits for tilapia culture. During this period, water temperature in cistern culture ranged from 30.0–31.2°C, pH from 7.3–7.8, alkalinity from 122.1–143.6 mg/l, DO2 from 5.2–6.9 mg/l, while in pond culture temperature ranged from 29.0–31.7°C, pH from 7.0–8.0, alkalinity from 120.1–148.2 mg/l, and DO2 from 4.5–7.4 mg/l.
Mixed-sex juvenile Nile tilapia (mean weight 0.02 ± 0.003 g; mean length 0.98 ± 0.02 cm) was stocked randomly in 9 concrete tanks (0.01 ha) and 9 earthen ponds (0.01 ha) at a density of 20,000 fingerlings/ha. Fish in 3 concrete tanks and 3 ponds were fed with control diet throughout the entire culture period. Fish in another 3 tanks and 3 ponds were given MT-treated diet with a dose of 10 mg/kg for the first 30 days and control diet for the rest of the culture duration. Fish in the remaining 3 tanks and 3 ponds were provided with MT-treated food with a dose of 10 mg/kg for the entire 6 months of culture period. The fish were fed twice daily at a constant rate of 20% body weight/day for the first month, 10% body weight/day for the next 2 months, and 5% body weight/day for the rest of the culture period. Fish from each pond and tank were measured individually for weight and length every 4 weeks and at the end of the trial. Growth parameters like specific growth rate (SGR) and daily weight gain (DWG) were calculated at the end of the culture period as follows (Pechsiri and Yakupitiyage 2005):
Crude muscle protein content was also determined using Kjeltec system 1026 distilling unit taking equal amount (5.0 g) of dorsal muscle from 10 fish from every treatment group in cistern and pond culture systems. Secondary sexual characteristics (especially genital papilla) and macroscopic examination of gonads were used to distinguish males from females.
ELISA
Serum testosterone concentration of 10 male fish of same age from control, 30-day-treated and throughout-treated categories under pond culture system was determined using a commercial ELISA kit from Omega Diagnostics (United Kingdom) according to the protocol provided. Blood collected from the caudal vein of the fish was centrifuged to isolate the plasma. At first, 10 μl of plasma was dispensed into the microtiter well, 100 μl of testosterone HRP conjugate reagent, and 50 μl of rabbit anti-testosterone reagent were added respectively into it, mixed thoroughly, and incubated at 37°C for 90 min. After rinsing the well with distilled water, 100 μl of substrate solution was added into it, mixed, and again incubated in the dark at room temperature for 20 min. Finally, 100 μl of stop solution was added, gently mixed, and absorbance at 450 nm was read immediately within 10 min using a microplate reader.
RNA isolation
Total cellular RNA from muscle tissue of 10 males and 10 females from MT-untreated control fish and 10 phenotypic males from each of the different MT-treated dose-duration groups was isolated after the completion of all treatment duration regime (60 days), while that of 10 phenotypic males from each of the 30-day-treated and throughout-treated categories and 10 males and 10 females from MT-untreated control fish in pond culture system was isolated at the end of the 6-month culture period using an RNA isolation kit from Bangalore Genei (India) using the protocol provided by the manufacturer. Briefly, about 100 mg of the tissue was homogenized in 1 ml of denaturing solution, 1 ml of water-saturated phenol, and 200 μl of chloroform—isoamyl alcohol mix (freshly prepared in the ratio of 49:1) was added, respectively, to the homogenate, mixed and incubated in ice for 15 min. After incubation, it was centrifuged at 10,000 rpm for 20 min at 4°C and the upper aqueous layer was separated. Then 1 ml of 100% isopropanol was added to it, incubated at −20°C for 30 min, and centrifuged at 10,000 rpm for 20 min at 4°C. The pellet was resuspended in 0.3 ml of denaturation solution, 0.3 ml of 100% isopropanol was added to it, incubated at −20°C for 30 min, and centrifuged at 10,000 rpm for 20 min at 4°C. The pellet was again washed in 75% ethanol, incubated at room temperature for 10–15 min, and centrifuged at 10,000 rpm for 20 min at 4°C. Finally, the RNA pellet was dissolved in 100 μl of DEPC water, incubated for 10–15 min at 55°C, and stored at −80°C.
RT-PCR
Reverse transcription of RNA was performed using an RT-PCR kit from Bangalore Genei (India). About 1–5 μl (~1 μg) of total RNA was taken, the volume was brought to 9 μl with DEPC-treated water, 1 μl of Oligo (dT)18 primer was added, incubated at 65°C for 10 min and at room temperature for 2 min. Then, 1 μl RNase inhibitor, 1 μl 0.1 M DTT, 4 μl RT buffer (5X), 2 μl 30 mM dNTP mix, 0.5 μl M-MulV reverse transcriptase (50U/μl), and 1 μl sterile water were added sequentially to it, mixed well, and incubated at 42°C for 1 h. Finally, it was incubated at 95°C for 2 min, spun briefly, and quickly placed on ice. Then, 3 μl of this RT product was added to 5 μl 10X PCR buffer, 1 μl 30 mM dNTP mix, 1 μl of each specific sense and antisense primer (Table 1) and 1.5 μl Taq DNA polymerase (1U/μl). Then, the total reaction volume was made to 50 μl with sterile water, and the PCR was carried out in a thermal cycler (Perkin-Elmer, Foster City, CA) for 35 cycles: 94°C for 1 min, 54°C for 1 min, and 72°C for 1.5 min. The products were electrophoresed on a 1.2% agarose gel. After gel-doc, the quantifications were performed using Quantity One Software (Bio-Rad).
Western blotting
Western blotting was performed following methods described by Sambrook and Russel (2001). In brief, total cellular protein (100 μg) from muscle tissue of the same fish, used for RNA studies, was resolved by 10% SDS-PAGE and then electro blotted to a PVDF membrane (Amersham). Blots were probed with 1:1,000 dilution of anti-AR IgG (PG-21, polyclonal rabbit antibodies raised against amino acids 1-21 of the rat AR; gifted by Prof. Prins GS, University of Illinois, Chicago) (Prins et al. 1991) and developed by the alkaline phosphatase colorimetric method. The antibody was extensively used in AR-related studies in various animals including a cichlid (Munchrath and Hofmann 2010, Pouso et al. 2010).The blots were scanned on a Bio-Rad-GS710 densitometric scanner followed by quantifications using Quantity One Software (Bio-Rad).
Study of impact of tilapia on fish diversity of ponds
Diversities (Shannon–Weaver index values) of indigenous fish species for 50 small natural ponds, naïve to tilapia, were calculated before introduction of tilapia. The ponds were partially drained, seined twice, and sampled for different indigenous fish species. After counting, the fish were again released into the ponds. Then, 25 ponds under ‘group 1’ were stocked with fifty control Nile tilapia fingerlings (2 months old), while the other 25 ponds under ‘group 2’ were stocked with same number of 10 mg/kg-30 days MT-treated Nile tilapia of same age. Shannon–Weaver index was again calculated after a period of 6 months to interpret the ecological implication of monosex tilapia culture in natural water bodies. The ponds were again drained to similar depth as during initial sampling, seined twice, and sampled. Shannon–Weaver indices (Shannon and Weaver 1949) were calculated using the following formula:
where p i , the proportion of the individual species to the total or relative abundance (n i /N); n i , the number of individuals in each species; N, the total number of all individuals; S, the number of species.
Statistical analysis
The results were reported as the mean ± standard error (SE). In results where the data were count data in percentage values (results of ideal treatment regime determination), those were transformed by angular transformation for further analysis. Here, one-way ANOVA was done taking each individual variable separately, and Duncan’s multiple range test (Duncan 1955) was applied to compare the differences among means. In grow-out performance study, all growth parameters were analyzed by two-way ANOVA with culture systems (cistern culture and pond culture) and MT treatment duration (0 days or control, 30 days, and 180 days or throughout) as main factors. The main factor effects means along with interaction means were compared with Fisher’s LSD test (at 5%) (Fisher 1935), if found significant. For comparison of serum testosterone concentration, and relative AR mRNA and protein levels, statistical analysis was performed by one-way ANOVA, and comparisons of means conducted via Duncan’s multiple range test (Duncan 1955). Finally, Shannon–Weaver indices within each group of ponds before and after tilapia introduction were compared by Student’s t test (at 5%). The statistical works were performed using SPSS 10.0 version for Windows.
Results
Determination of optimum hormone treatment regime for sex reversal
Differential proportions of males were observed among various experimental classes (Fig. 1). In all the hormone-untreated control groups, almost 50% of the population were males. Every dose vs. duration treatment combinations showed significantly higher (P < 0.01) mean percent count of male than that of control classes (Fig. 1). Again, treatment doses like 10, 20, and 30 mg/kg diet had significantly higher (P < 0.05) mean percent male counts than that of 5 mg/kg (Fig. 1). Additionally, treatment duration period of 30, 45, and 60 days yielded higher mean percent male counts than that of 15 days (Fig. 1).
Grow-out performance analysis
Survival of control and two MT-treated groups for both cistern and pond culture systems was around 90%. Significant interaction effects (P < 0.05) of culture systems and treatment durations were observed for growth parameters such as final weight, length, DWG, and muscle protein content (Table 2). The growth rate of MT-untreated control, 30-day-treated, and throughout-treated fish in cistern and pond culture systems is represented in Fig. 2. The weight and length of 30-day-treated and throughout-treated tilapia increased significantly (P < 0.05) compared with those of MT-untreated control fish after the first month of culture in both cistern and pond culture systems, and this trend was maintained for the entire culture duration (Fig. 2). Within cistern culture system, final weight, length, DWG, and protein content of MT-untreated control tilapia were significantly lower (P < 0.05) than both 30-day-treated and throughout-treated fish, but there was no significant difference (P > 0.05) in these growth parameters between the 30-day-treated and throughout-treated fish (Table 2, Fig. 2). Similar observations were found for final length, DWG, and protein content in pond culture system (Table 2, Fig. 2). Final weight of control fish in pond culture system was significantly lower (P < 0.05) than that in both 30-day- treated and throughout-treated fish, and the throughout-treated fish showed significantly higher (P < 0.05) weight compared with 30-day-treated tilapia (Table 2, Fig. 2). There was a significant interaction (P < 0.05) between the culture systems and treatment durations for the total fish production also. In cistern culture system, the total production obtained for MT-untreated control tilapia culture was 1184.4 ± 109.7 kg/ha, the total production for 10 mg/kg-30-day-treated fish was 3638.5 ± 31.02 kg/ha, and the total production for 10 mg/kg-throughout-treated fish was 3719.4 ± 32.7 kg/ha, while in pond culture system, total productions obtained were 1765.7 ± 71.3, 5452.4 ± 26.2 and 5564.2 ± 33.1 kg/ha for MT-untreated control tilapia, 10 mg/kg-30-day-treated fish and 10 mg/kg-throughout-treated fish, respectively. The weight and length of MT-untreated control, 30-day-treated and throughout-treated fish in pond culture system after the first month of culture increased significantly (P < 0.05) compared with those of control, 30-day-treated and throughout-treated fish in cistern culture system (Fig. 2). Moreover, final weight, length, DWG, and protein content of MT-untreated control, 30-day-treated and throughout-treated tilapia in pond culture system were significantly higher (P < 0.05) compared with those of control, 30-day-treated and throughout-treated fish in cistern culture system (Table 2, Fig. 2).
Fish in pond culture system showed significantly higher (P < 0.05) final weight (235.76 ± 18.34 g), final length (22.66 ± 0.72 cm), DWG (1.3 ± 0.2 g/day), SGR (5.17 ± 0.07%/day), and muscle protein content (15.73 ± 0.3%) compared with final weight (156.82 ± 12.09 g), final length (19.61 ± 0.55 cm), DWG (0.89 ± 0.1 g/day), SGR (4.94 ± 0.07%/day), and protein content (13.69 ± 0.6%) in fish from cistern culture system. The total production for cistern culture system was 2847.4 ± 417.3 kg/ha, and it was significantly lower (P < 0.05) than total production from pond culture system (4260.8 ± 624.4 kg/ha).
Increase in serum testosterone level
In the 30-day-treated group, there was significant increase (P < 0.05) in serum testosterone level compared with that of control males of same age up to four-month stage (Table 3). However, beyond 4 months, the 30-day MT-treated fish showed similar serum hormone concentration to that of the control males (P > 0.05). In throughout-treated fish, serum testosterone level was significantly higher (P < 0.05) compared with control male and 30 days MT-treated fish during entire culture period (Table 3).
Increase in ARβ expression
In males, the muscle growth is brought about by the androgen (An) in association with the AR (Bhasin et al. 2001). So it was necessary to check the change in AR expression, if any, with the increase in fish growth. A single band of ~2,292-base pairs (bp) could be observed with the RT-PCR and the Western blot resulted in ~84 kDa product. The ARβ expression patterns during the MT dose–response study are depicted in Fig. 3. Control females showed significantly lower (P < 0.05) level of ARβ mRNA and protein expressions compared with control males (Fig. 3). RT-PCR experiments revealed that all duration regimes under 5 mg/kg dose category and treatment duration of 15 days in all the different dose categories showed low ARβ expression levels (Fig. 3a). The treatment regime of 10 mg/kg for 30 days showed significantly higher (~2.5-fold, P < 0.05) expression of ARβ at the mRNA level compared with that of control males (Fig. 3a). But, higher treatment regimes such as 10 mg/kg-45 days, 10 mg/kg-60 days, 20 mg/kg-30 days, 20 mg/kg-45 days, and 30 mg/kg-60 days showed no significant increase (P > 0.05) in ARβ mRNA expression compared with 10 mg/kg-30 days treatment regime (Fig. 3a). However, treatment regimes such as 20 mg/kg-60 days, 30 mg/kg-30 days, and 30 mg/kg-45 days showed significantly higher (P < 0.05) ARβ mRNA expression levels than 10 mg/kg-30 days treatment regime (Fig. 3a). Of note, all the experimental OD values of ARβ mRNA expression were normalized to β-actin expression in the respective samples.
ARβ expression in the muscle tissue of MT-untreated control male, female, and MT-treated tilapia at same age during MT dose-response study. a Upper panel RT-PCR analyses of RNA from 15, 30, 45, and 60 days regime of 5, 10, 20, and 30 mg/kg MT-treated, and MT-untreated control female and control male fish muscle tissue. The course of mRNA expression under different treatment dosages has been represented through gel bands and the corresponding normalized OD values below each lane. (Only the +ve integers have been shown and fractional values have been replaced with −s). Lower panel. ARβ mRNA expression levels (normalized OD values) in different treatment categories. Means with different alphabets are significantly different (P < 0.05). b Upper panel Western blot of total muscle tissue protein from samples as mentioned in a, using anti-AR IgG. The differential expression of ARβ protein at different time points under different treatment dosages have been depicted through blot-bands and the corresponding normalized OD values below each lane. (Only the +ve integers have been shown and fractional values have been replaced with −s). Lower panel ARβ protein expression levels (normalized OD values) in different treatment categories. Means with different alphabets are significantly different (P < 0.05)
For obvious reasons, we checked whether this increased message was translated into proteins or not, since it has to be the abundance of the receptor protein which can bring about all the changes. The same pattern, as in mRNA level, was also maintained for ARβ expression at the protein level (Fig. 3b) i.e.; 10 mg/kg–30 days treatment regime led to a significant increase (~twofold, P < 0.05) in the ARβ protein abundance (Fig. 3b) compared with that of the control males (Fig. 3b). Treatment regimes such as 10 mg/kg-60 days, 20 mg/kg-45 days, and 20 mg/kg-60 days did not bring about significant increase (P > 0.05) to this expression level, indicating a level of saturation (Fig. 3b). The highest ARβ protein expression level was observed in 30 mg/kg-60 days treatment regime, and it was significantly higher (P < 0.05) compared with all other treatment regime categories (Fig. 3b). Here also, all the experimental OD values of ARβ protein expression were normalized to β-actin expression in the respective samples.
In grow-out experiment also, control females showed significantly lower (P < 0.05) level of ARβ mRNA and protein expressions compared with control males (Fig. 4). Here, males in both the MT-treated groups (10 mg/kg-30 days and 10 mg/kg-throughout) had significantly higher (~twofold, P < 0.05) ARβ mRNA and protein expression levels than the control males (Fig. 4). Though fish in the throughout treatment category showed significantly higher (P < 0.05) ARβ mRNA expression level than that in the 30-day-treated category, there was no significant difference (P > 0.05) in ARβ protein expression level among these two MT-treated groups (Fig. 4). The level of β-actin expression was monitored as control (Fig. 4) to normalize the OD values in the respective samples.
ARβ expression in the muscle tissue of MT-untreated control female, male, and MT-treated tilapia at same age during grow-out study. a Upper panel RT-PCR analyses demonstrating levels of ARβ mRNA, as well as β-actin mRNA (control) expression in MT-untreated control females, control males, 10 mg/kg-30 days MT-treated and 10 mg/kg-throughout MT-treated fish muscle tissues. (Only the +ve integers have been shown and fractional values have been replaced with −s). Lower panel ARβ mRNA expression levels (normalized OD values) in MT-untreated control females, control males, 10 mg/kg-30 days MT-treated and 10 mg/kg-throughout MT-treated fish. Means with different alphabets are significantly different (P < 0.05). b Upper panel Western analyses demonstrating ARβ protein, and β-actin protein (control) expression in MT-untreated control females, control males, 10 mg/kg-30 days MT-treated and 10 mg/kg-throughout MT-treated fish muscle tissues. (Only the +ve integers have been shown and fractional values have been replaced with −s). Lower panel ARβ protein expression levels (normalized OD values) in MT-untreated control females, control males, 10 mg/kg-30 days MT-treated and 10 mg/kg-throughout MT-treated fish. Means with different alphabets are significantly different (P < 0.05)
Fish diversity study
The fish diversity indices of two groups each comprising of 25 natural ponds before and after introduction of tilapia are given in Fig. 5a. The number of control tilapia in all the group 1 ponds increased greatly during the study period but that of other indigenous fishes decreased to different extent, as a result the Shannon–Weaver index value for these ponds decreased significantly (P < 0.05). But, in the other group, the number of MT-treated tilapia introduced remained the same. Here, on the contrary, the number of other indigenous fishes was either increased or stably maintained as a result of which the Shannon–Weaver index value increased, though not significantly (P > 0.05) (Fig. 5a). The individual differences in the numbers of different indigenous fish species in a single experimental set (Fig. 5b) provide a simple physical representation of this changed scenario.
a Shannon--Weaver index values for two groups of natural ponds before and after introduction of tilapia. Ponds of group 1 were stocked with control tilapia while ponds of group 2 with MT-treated tilapia. * Significant difference (P < 0.05) in diversity index between before and after introduction of tilapia in group 1 ponds. There is no significant difference (P > 0.05) in diversity index between before and after introduction of tilapia in group 2 ponds.  : Before tilapia introduction,
: Before tilapia introduction,  : after tilapia introduction. b Comparative numbers of different fish species in two ponds at the start and end of the study period. Pond 1 was stocked with control tilapia while pond 2 with MT-treated tilapia.
: after tilapia introduction. b Comparative numbers of different fish species in two ponds at the start and end of the study period. Pond 1 was stocked with control tilapia while pond 2 with MT-treated tilapia.  : Number at beginning of experiment in pond 1,
: Number at beginning of experiment in pond 1,  : number at beginning of experiment in pond 2,
: number at beginning of experiment in pond 2,  : number at end of study period in pond 1,
: number at end of study period in pond 1,  : number at the end of study period in pond 2
: number at the end of study period in pond 2
Discussion
The result of this study suggests that there is an intricate relationship between the dose and duration, and timing of hormone treatment to induce successful sex reversal in tilapia. Low hormone concentration was not good enough to cause effective sex reversal even after a prolonged treatment period, and treatments of fish with excessive androgen can also lead to reduced masculinization and paradoxical feminization (Beardmore et al. 2001, Devlin and Nagahama 2002). In this experiment, the highest percentage of males was obtained in 20 mg/kg-60 days treatment category (Fig. 1). But, 10 mg/kg-30 days MT treatment category was the lowest treatment regime among the treatment categories that showed more than 95% mean percent male counts (Fig. 1). Hence, this was considered to be effective, practical, and economically viable optimum hormone treatment regime under Indian pretext. It may be mentioned here that even previous attempts have failed to achieve 100% sex reversal in O. niloticus, in spite of using higher dose of MT for a greater time period than what has been postulated by us (Vera Cruz and Mair 1994; Ekwu and Sikoki 2001). In Esox lucius also, 100% male population were not obtained by applying high dose of MT for a prolonged period of time (Luczynski et al. 2003). On the other hand, Kamaruzzaman et al. (2009) obtained only 75% males in O. niloticus fed MT-treated diet at a concentration of 5 mg/kg for 21 days, which corroborated well with our findings. Probably, the particular time point for starting the treatment might be crucial for an optimum result and the functional activity of exogenous sex steroid causing sex reversal decreased with the full establishment of endogenous pathways for gonadal differentiation (Devlin and Nagahama 2002). However, tilapia younger than 3 days was incapable of consuming external food and thus could not be fed with hormone-treated artificial diet.
MT treatment was reported to have no effect on survival of tilapia (Vera Cruz and Mair 1994). In this study also, similar survival rates were observed in MT-treated and MT-untreated fish. A few studies have demonstrated the enhanced growth of monosex male Nile tilapia populations under experimental conditions (Mair et al. 1995, Dan and Little 2000, Little et al. 2003). Similar values of individual growth for monosex tilapia, as in our study, were also observed by Diana et al. (1991, 1994). Moreover, significant increase in growth was recorded in MT-treated Oreochromis spilurus (Ridha and Lone 1990) and Oreochromis mossambicus (Macintosh et al. 1985; Kuwaye et al. 1993; Sparks et al. 2003) compared with the untreated fish reared under similar conditions. Several studies are in agreement that testosterone produces muscle hypertrophy by increasing muscle protein synthesis (Bhasin et al. 2001). The higher serum testosterone concentration up to a particular time period, increased growth performance, and greater muscle protein content of the MT-treated fish can be appreciated considering our study. Failure of throughout-treated categories to elicit a much greater impact on the growth performance over the 30-day-treated groups may be attributed to the achievement of an optimum saturation level of hormone activity after a particular titer of testosterone is reached. However, many workers have suggested that any improved growth of androgen-treated tilapia is more related to the superior growth of males than the more classical anabolic response related to enhanced protein synthesis and increase in muscle mass (Phelps and Popma 2000).
Again, the increased growth of fish in pond culture system compared with that in cistern culture system could have been facilitated by the additional availability of relatively energy-rich natural food materials in ponds that might confer an energetic advantage for increased growth (Dan and Little 2000; El-Sayed 2002; Bwanika et al. 2007). Moreover, the significantly higher (P < 0.05) total production from pond culture system may be transmitted as greater economic advantage to the fish farmers.
In our study, there was a significant difference (P < 0.05) in the serum testosterone level in 30-day MT-treated and control males up to the 4-month stage but beyond that the difference got abolished. Such lack of difference in the serum testosterone level may make 10 mg/kg-30 days MT-treated tilapia safe for consumption. However, concentration of other androgens must also be considered while determining the safety of human consumption of MT-treated tilapia.
Androgens play an important role in male sexual differentiation and development (Devlin and Nagahama 2002). In tilapia, MT can induce sex reversal by suppressing the expression of all steroidogenic enzymes by down-regulation of the respective genes (Bhandari et al. 2006). However, the molecular basis of the anabolic effect of testosterone in fish is not clearly known. Some workers have proposed that physiological concentrations of testosterone directly stimulate growth hormone (GH) mRNA production at the pituitary level and MT treatment stimulates GH/IGF-I axis (Huggard et al. 1996; Shepherd et al. 1997; Riley et al. 2002). On the other hand, several workers have associated the functional effect of androgens with increased expression of the AR in the target organs (Hanke et al. 2001; Ma et al. 2005).
The cognate receptors need to be present for the sex steroids to elicit their functional effect (Ijiri et al. 2008). Levels of AR have been shown to correlate with the cellular response to androgen, suggesting that upregulation of AR protein may lead to enhanced cellular sensitivity to the hormone (Diamond and Barrack 1984; Takane et al. 1991; McPhaul et al. 1993; Dai et al. 1996). Here, the increased hormone (An) level is modulating the cellular system to enhance the sensitivity toward it. It is not inconceivable that the significantly higher (P < 0.05) growth pattern of MT-treated tilapia may be due to the increased androgen-androgen receptor complex (An-AR) formation and subsequent downstream interactions, i.e., binding of the An-AR to specific DNA recognition sites [including a putative HRE within the ARβ cDNA itself (GenBank Accession No. AB045212), leading to ARβ auto-upregulation] (Fig. 6) and modification of transcription by up or down-regulation of the expression of androgen target genes (Dai and Burnstein 1996), in the muscle tissue, compared to that of control fishes. Thus, this growth phenomenon may be partly attributed to the increased androgen titer, prevalent up to a particular age, of the 10 mg/kg-30 days MT-treated monosex fish population. In this case, An-AR is probably upregulating the transcription of several other growth promoting genes either directly or through upregulation and induction of other transcription factors (TFs). One may not also rule out that An-AR is either upregulating AR transcription alone or in association with other upregulated TFs (Fig. 6). It is possible that, may be, an auto-upregulation circuit is coming into play in this event. However, presence of some other factor(s) contributing toward enhancing the stability of the mRNA itself cannot also be ruled out, and further study is required in this regard. Moreover, it was observed during the optimum treatment determination study that although significantly higher (P < 0.01) percentage of males were found in all MT treatment regimes than MT-untreated controls, but that did not lead to an increased growth in cases of 5 mg/kg-15, 30, 45, 60 days (7.8 ± 0.08 g) and 10, 20, 30 mg/kg-15 days (7.73 ± 0.1 g) MT-treated fish compared to control tilapia (7.68 ± 0.2 g) after 60 days. Probably the level of testosterone in those fish was not enough to stimulate the muscle tissue to undergo the subsequent events.
Putative model of androgen-induced, AR-mediated growth response in muscle tissue. A positive feed-back loop for AR synthesis has been depicted. The statements within the parentheses represent the observations of the present study. The dotted lines indicate to the pathways yet to be confirmed. See text for details
The phenomenon of sex reversal and the molecular interplay of genes responsible for it project great ecological implications. The results from the species diversity indices clearly indicate that introduction of tilapia in a native ecosystem may lead to unintended consequences. But, it has been transplanted to and stocked in waters of many countries of the world. The persistence and coexistence of species in a habitat depend on the availability of a minimum food patch size (P*) and resource concentration (R*). The exclusive niche for one species depends on the P* and R* of all the species present in that ecosystem (Ritchie and Olff 1999). Being omnivore, tilapia shares a common food niche with almost every indigenous fish species of India, thus reducing their exclusive niche. Hence, the competitive success of indigenous fish population for coexistence with tilapia mainly depends on the ability to breed and propagate. But, in this regard also, the huge auto-stocking propensities of tilapia out-compete the indigenous fish populations that are prone to breeding failure. The monosex population of the fish cannot breed to increase in number and alter the ecological balance of the water body. Hence, the natural diversity of the system can still be maintained even after the introduction of tilapia. However, further study is required for comprehensive analysis of the ecological impact of MT-treated tilapia introduction in natural aquatic systems.
At the end, it may be concluded that 10 mg/kg-30 days MT treatment category has been considered optimum MT treatment regime for monosex tilapia production in India. Significant increase in growth, and ARβ mRNA and protein expression levels have been observed in 10 mg/kg-30 days MT-treated tilapia compared to MT-untreated control fish. An androgen-induced increased ‘androgen-androgen receptor’ mediated anabolic pathway may be postulated for such increased growth in MT-treated tilapia. Culture of MT-treated monosex tilapia has been found beneficial than MT-untreated mixed-sex tilapia culture for maintenance of natural fish diversity in indigenous water bodies. Thus, the upregulation of AR leading to growth increase in androgen-treated monosex tilapia is of immense eco-socioeconomic importance. Culture of MT-treated monosex tilapia in earthen ponds in India may be advocated, and large-scale field trials with such fish are needed before they are marketed.
Abbreviations
- MT:
-
17α-methyltestosterone
- AR:
-
Androgen receptor
- DWG:
-
Daily weight gain
- SGR:
-
Specific growth rate
- An:
-
Androgen
- HRE:
-
Hormone responsive element
- RT:
-
Reverse transcription
- GH:
-
Growth hormone
- IGF-I:
-
Insulin-like growth factor-I
- TF:
-
Transcription factor
References
Abucay JS, Mair GC (1997) Hormonal sex reversal of tilapias: implications of hormone treatment application in closed water systems. Aquacult Res 28:841–845
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA (American Public Health Association), Washington, USA
Baroiller JF, D’Cotta H, Bezault E, Wessels S, Hoerstgen-Schwark G (2009) Tilapia sex determination: where temperature and genetics meet. Compar Biochem Physiol A 153:30–38
Beardmore JA, Mair GC, Lewis RI (2001) Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197:283–301
Bhandari RK, Nakamura M, Kobayashi T, Nagahama Y (2006) Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Nile tilapia (Oreochromis niloticus). Gen Comp Endocrinol 145:20–24
Bhasin S, Woodhouse L, Storer TW (2001) Proof of the effect of testosterone on skeletal muscle. J Endocrinol 170:27–38
Blázquez M, Piferrer F (2005) Sea bass (Dicentrarchus labrax) androgen receptor: cDNA cloning, tissue-specific expression, and mRNA levels during early development and sex differentiation. Mol Cell Endocrinol 237:37–48
Bwanika GN, Murie DJ, Chapman LJ (2007) Comparative age and growth of Nile tilapia (Oreochromis niloticus L.) in lakes Nabugabo and Wamala, Uganda. Hydrobiologia 589:287–301
Dai JL, Burnstein KL (1996) Two androgen response elements in the androgen receptor coding region are required for cell-specific up-regulation of receptor messenger RNA. Mol Endocrinol 10:1582–1594
Dai JL, Maiorino CA, Gkonos PJ, Burnstein KL (1996) Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids 61:531–539
Dan NC, Little DC (2000) The culture performance of monosex and mixed-sex new-season and overwintered fry in three strains of Nile tilapia (Oreochromis niloticus) in northern Vietnam. Aquaculture 184:221–231
De Silva SS, Subasinghe RPD, Bartley M, Lowther A (2004) Tilapias as alien aquatics in Asia and the Pacific: a review. FAO Fisheries Technical Paper, No. 453. FAO, Rome
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Diamond DA, Barrack ER (1984) The relationship of androgen receptor levels to androgen responsiveness in the Dunning R3327 rat prostate tumor sublines. J Urol 132:821–827
Diana JS, Lin CK, Schneeberger PJ (1991) Relationships among nutrient inputs, water nutrient concentrations, primary production, and yield of Oreochromis niloticus in ponds. Aquaculture 92:323–341
Diana JS, Lin CK, Jaiyen K (1994) Supplemental feeding of tilapia in fertilized ponds. J World Aquacult Soc 25:497–506
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Ekwu AO, Sikoki FD (2001) Comparison of hormonally induced sex direction in two strains of Oreochromis niloticus (Trewavas). J Aquat Sci 16:147–149
El-Saidy DMSD, Gaber MMA (2005) Effect of dietary protein levels and feeding rates on growth performance, production traits and body composition of Nile tilapia, Oreochromis niloticus (L.) cultured in concrete tanks. Aquacult Res 36:163–171
El-Sayed A-FM (2002) Effects of stocking density and feeding levels on growth and feed efficiency of Nile tilapia (Oreochromis niloticus L.) fry. Aquacult Res 33:621–626
El-Sayed A-FM (2006) Tilapia culture. CABI publishing. CAB Internnational, Wallingford
Feist G, Schreck CB (1996) Brain-pituitary-gonadal axis during early development and sexual differentiation in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 102:394–409
Fisher RA (1935) The design of experiments. Oliver and Boyd, London
Guerrero RD, Shelton WL (1974) An aceto-carmine squash method for sexing juvenile fishes. Progress Fish Cult 36:56
Hanke H, Lenz C, Hess B, Spindler KD, Weidemann W (2001) Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall. Circulation 103:1382–1385
Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR (1996) GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA 93:4948–4952
Huggard D, Khakoo Z, Kassam G, Habibi HR (1996) Effect of testosterone on growth hormone gene expression in the goldfish pituitary. Can J Physiol Pharmacol 74:1039–1046
Ijiri S, Kaneko H, Kobayashi T, Wang D-S, Sakai F, Paul-Prasanth B, Nakamura M, Nagahama Y (2008) Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol Reprod 78:333–341
Kamaruzzaman N, Nguyen NH, Hamzah A, Ponzoni RW (2009) Growth performance of mixed sex, hormonally sex reversed and progeny of YY male tilapia of the GIFT strain, Oreochromis niloticus. Aquacult Res 40:720–728
Kitano T, Takamune K, Nagahama Y, Abe SI (2000) Aromatase inhibitor and 17α-methyltestosterone cause sex-reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus). Mol Reprod Dev 56:1–5
Kuwaye TT, Okimoto DK, Shimoda SK, Howerton RD, Lin H-R, Pang PKT, Grau EG (1993) Effect of 17α-methyltestosterone on the growth of the euryhaline tilapia, Oreochromis mossambicus, in fresh water and in seawater. Aquaculture 113:136–152
Lèveque C (2002) Out of Africa: the success story of tilapias. Environ Biol Fish 64:461–464
Lin Y-H, Lin S-M, Shiau S-Y (2008) Dietary manganese requirements of juvenile tilapia, Oreochromis nilotis x O. aureus. Aquaculture 284:207–210
Liti DM, Fulanda B, Munguti JM, Straif M, Waidbacher H, Winkler G (2005) Effects of open-pond density and caged biomass of Nile tilapia (Oreochromis niloticus L.) on growth, feed utilization, economic returns and water quality in fertilized ponds. Aquacult Res 36:1535–1543
Little DC, Bhujel RC, Pham TA (2003) Advanced nursing of mixed-sex and mono-sex tilapia (Oreochromis niloticus) fry, and its impact on subsequent growth in fertilized ponds. Aquaculture 221:265–276
Luczynski MJ, Demska-Zakes K, Dabrowski K, Luczynski M (2003) Masculinization of gynogenetic northern pike juveniles using 17α-methyltestosterone. North Am J Aquacult 65:255–259
Ma R, Wu SZ, Lin QS, Jiang SS (2005) Regulation of androgen receptor mRNA expression by testosterone in cultured vascular smooth muscle cells. Acad J First Med College PLA 25:298–300
Macintosh DJ, Varghese TJ, Satyanarayana GPR (1985) Hormonal sex reversal of wild-spawned tilapia in India. J Fish Biol 26:87–94
Mair GC, Abucay JS, Beardmore JA, Skibinski DOF (1995) Growth performance trials of genetically male tilapia (GMT) derived from YY-males in Oreochromis niloticus L.: on station comparisons with mixed sex and sex reversed male populations. Aquaculture 137:313–322
McPhaul MJ, Deslypere JP, Allman DR, Gerard RD (1993) The adenovirus-mediated delivery of a reporter gene permits the assessment of androgen receptor function in genital skin fibroblast cultures. Stimulation of Gs and inhibition of G(o). J Biol Chem 268:26063–26066
Munchrath LA, Hofmann HA (2010) Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. J Comp Neurol 518:3302–3326
Pandian TJ, Sheela SG (1995) Hormonal induction of sex reversal in fish. Aquaculture 138:1–22
Pechsiri J, Yakupitiyage A (2005) A comparative study of growth and feed utilization efficiency of sex-reversed diploid and triploid Nile tilapia, Oreochromis niloticus L. Aquacult Res 36:45–51
Phelps RP, Popma TJ (2000) Sex reversal of tilapia. In: Costa-Pierce BA, Rakocy JE (eds) Tilapia aquaculture in the Americas, vol 2. The World Aquaculture Society, Baton Rouge, pp 34–59
Pillay TVR (1993) Aquaculture: principles and practices. Blackwell, Oxford
Pouso P, Quintana L, Bolatto C, C-Silva A (2010) Brain androgen receptor expression correlates with seasonal changes in the behaviour of a weakly electric fish, Brachyhypopomus gauderio. Horm Behav 58:729–736
Prins GS, Birch L, Greene GL (1991) Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology 129:3187–3199
Ridha MT, Lone KP (1990) Effect of oral administration of different levels of 17 α-methyltestosterone on the sex reversal, growth and food conversion efficiency of the tilapia Oreochromis spilurus (Günther) in brackish water. Aquacult Res 21:391–397
Riley LG, Hirano T, Grau EG (2002) Disparate effects of gonadal steroid hormones on plasma and liver mRNA levels of insulin-like growth factor-I and vitellogenin in the tilapia, Oreochromis mossambicus. Fish Physiol Biochem 26:223–230
Ritchie ME, Olff H (1999) Spatial scaling laws yield a synthetic theory of biodiversity. Nature 400:557–560
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Shelton WL, Guerrero RD, Macias JL (1981) Factors affecting androgen sex reversal of Tilapia aurea. Aquaculture 25:59–65
Shepherd BS, Ron B, Burch A, Sparks RT, Richman NH III, Shimoda SK, Stetson MH, Lim C, Grau EG (1997) Effects of salinity, dietary level of protein and 17α-methyltestosterone on growth hormone (GH) and prolactin (tPRL177 and tPRL188) levels in the tilapia, Oreochromis mossambicus. Fish Physiol Biochem 17:279–288
Smith ES, Phelps RP (2001) Impact of feed storage conditions on growth and efficacy of sex reversal of Nile tilapia. North Am J Aquacult 63:242–245
Sparks RT, Shepherd BS, Ron B, Richman NH III, Riley LG, Iwama GK, Hirano T, Grau EG (2003) Effects of environmental salinity and 17α-methyltestosterone on growth and oxygen consumption in the tilapia, Oreochromis mossambicus. Compar Biochem Physiol B 136:657–665
Sugunan VV (1995) Reservoir fisheries of India. FAO Fisheries Technical Paper, No. 345. FAO, Rome
Sugunan VV (2000) Ecology and fishery management of reservoirs in India. Hydrobiologia 430:121–147
Takane KK, Husmann DA, McPhaul MJ, Wilson JD (1991) Androgen receptor levels in the rat penis are controlled differently in distinctive cell types. Endocrinology 128:2234–2238
Todo T, Ikeuchi T, Kobayashi T, Nagahama Y (1999) Fish androgen receptor: cDNA cloning, steroid activation of transcription in transfected mammalian cells, and tissue mRNA levels. Biochem Biophys Res Commun 254:378–383
Varadaraj K, Kumari SS, Pandian TJ (1994) Comparison of conditions for hormonal sex reversal of Mozambique tilapias. Progress Fish Cult 56:81–90
Vera Cruz EM, Mair GC (1994) Conditions for effective androgen sex reversal in Oreochromis niloticus (L). Aquaculture 122:237–248
Yamamoto T (1969) Sex differentiation. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 3. Academic Press, New York, pp 117–175
Yeh S, Chang C (1996) Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA 93:5517–5521
Acknowledgments
This work was financed by the University Grants Commission (Minor Research Project, No.F. PSW-032/07-08). We thank Prof. Prins GS, University of Illinois, Chicago, and all our colleagues for their kind gifts of several reagents, chemicals, and antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, S.B., Banerjee, S. & Chatterjee, S. Increased androgen receptor expression in muscle tissue contributing to growth increase in androgen-treated Nile tilapia. Aquacult Int 19, 1119–1137 (2011). https://doi.org/10.1007/s10499-011-9428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-011-9428-x


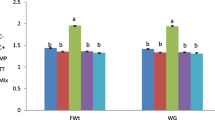



 : 45 days,
: 45 days, 
 : Control,
: Control,  : 30-day-treated,
: 30-day-treated,  : throughout-treated
: throughout-treated


