Abstract
Sex change was induced in Epinephelus marginatus juveniles using a nonsteroidal aromatase inhibitor (AI), a synthetic androgen (17α-methyltestosterone; MT), and a combination of both (MT + AI) in a 90-day experiment. A detailed remodeling of the gonads, the plasma level of gonadal steroids, and immunostaining of pituitary follicle-stimulating hormone (FSH), luteinizing hormone (LH), and somatolactin (SL) cells were analyzed. Sex inversion reached the final spermatogenesis stages using MT, while AI triggered spermatogenesis, but reaching only the spermatid stage. Estradiol (E2) levels did not change in fish treated with AI but decreased throughout the experimental period in animals treated with MT and MT + AI. Testosterone (T) levels increased in animals treated with MT during the first 60 days (and combined with AI in the first 30 days), decreasing in all experimental groups at 90 days, while AI-treated animals had increased plasma 11-ketotestosterone (11-KT) levels after 90 days. In control fish, FSH- and SL-producing cells (ir-FSH and ir-SL) were restricted to pars intermedia (PI) of the adenohypophysis. Pituitary ir-FSH cells were decreased at the end of the experimental period in all treatments compared with the CT animals. LH-producing cells (ir-LH) were present in proximal pars distalis (PPD) and pars intermedia (PI) of adenohypophysis and did not change after the experimental period. The decreased number of ir-FSH cells at the end of the experiment in all treatments could be related to the negative feedback loop triggered by the increase in natural and/or synthetic androgens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hermaphroditism and sex change are regulated at the gonadal level in individuals with both male and female germ cells in the gonadal compartment, which can be stimulated to proliferate and differentiate under different hormonal conditions (Devlin and Nagahama 2002). The maintenance of the female sex is related to 17β-estradiol (E2) levels, and the decrease of E2 levels can interrupt vitellogenesis and oocyte maturation, as well as allow male development in protogynous hermaphrodite teleosts (Guiguen et al. 2010; Garcia et al. 2013; Wu et al. 2017). These processes are closely linked to the ratio between testosterone (T) and E2 levels, as E2 is produced from the aromatization of T through the action of P450 aromatase (Lubzens et al. 2010). In protogynous hermaphrodite teleosts, at the beginning of the sex change process, the oocytes begin to degenerate, followed by a fall in plasma levels of estrogen, and consequently, an androgens increase that induces testis development and semen production (Bhandari et al. 2003), events that are followed by changes in the levels of pituitary gonadotropin (Kobayashi et al. 2010; Garcia et al. 2013). Gonadal steroidogenesis is modulated by the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), as well as glycoproteic hormones, which are involved in regulating gametogenesis in fish (Lubzens et al. 2010).
The use of aromatase inhibitors (AI) or synthetic androgens in early or late stages of gonadal development triggers masculinization and can even activate sex differentiation (complete and functional sex change) in some fish and reptile species, specifically hermaphrodite teleosts (Garcia et al. 2013; Wu et al. 2017). The knowledge of these processes enabled the use of AI and synthetic androgens, such as methyltestosterone (MT), in the induction of sex change in protogynous hermaphrodite fish belonging to the Serranidae family (Bhandari et al. 2003, 2004; Sarter et al. 2006; Sanches et al. 2009; Kobayashi et al. 2010; Garcia et al. 2013; Wu et al. 2017).
The serranids comprise 544 species distributed in 75 genera (Eschmeyer and Fong 2018), among them the dusky grouper, Epinephelus marginatus (Serranidae, Epinephelinae) (Lowe 1834), a threatened species (Rodrigues-Filho et al. 2009) included in the IUCN red list as vulnerable (IUCN 2019). E. marginatus has a high market value, occurs on both sides of the Atlantic Ocean (Vaini et al. 2019), and is widely distributed in the southeastern coast of Brazil (Barreiros 1998), Mediterranean Sea, and Southern Africa (Heemstra and Randall 1993).
The first sexual maturation of E. marginatus females occurs when the individual reaches about 49 cm (at ~ 6 years old) (Reñones et al. 2010), and sex change occurs naturally, from 10 years old, with length and the weight greater than 83 cm and 9 kg, respectively (Spedicato et al. 1995; Andrade et al. 2003). Due to the critical conservation status of E. marginatus, many studies have been carried out to improve the biological knowledge of the species in many areas, including reproduction (Marino et al. 2000, 2002, 2003), sex change (Sarter et al. 2006; Sanches et al. 2009; Garcia et al. 2013), nutrition (Araújo et al. 2018), larviculture (Russo et al. 2009; Cunha et al. 2013; Mello et al. 2018), and gamete cryopreservation (Cabrita et al. 2009; Sanches et al. 2009; Garcia et al. 2013). However, due to the great size of adult males, it is often unfeasible to maintain broodstock in captivity. Bhandari et al. (2004) and Sarter et al. (2006) succeeded to induce sex change in juvenile E. marginatus using aromatase inhibitor (AI) and 17α-methyltestosterone (MT) implants, respectively, but the morphophysiological events that triggered sex change were not fully understood.
In the present study, we tested the hypothesis that AI and MT, alone or combined, stimulate sex change in E. marginatus juveniles. Plasma levels of gonadal steroids were measured, parallel with the remodeling of the gonads during the whole process of sex change. The immunostaining of pituitary gonadotropin (FSH and LH)- and somatolactin (SL)-producing cells were carried out following the sex change process, additionally with the stereological analyses of gonadotropin producing cells.
Materials and methods
Experimental conditions
The experiment was performed at the Fisheries Institute (Ubatuba, SP, Brazil). Wild juveniles weighing from 250 to 500 g were captured and distributed in 12 floating cages (2 × 2 × 2 each) at sea. Animals were anesthetized by immersion in a 0.1 g/L benzocaine solution, then individually labeled with electronic chips (transponders, TROVAN®) and divided into four experimental groups with four animals in each cage (triplicate; 12 per group): (1) Control (CT) animals were injected intramuscularly (i.m.) with 1 mL of fish oil as vehicle; (2) Aromatase inhibitor (AI) animals were injected intramuscularly (i.m.) with a dose of 100 mg/kg of Letrozole (Venturepharm, China); (3) 17α-Methyltestosterone (MT) (synthetic androgen, Sigma-Aldrich, USA) animals were injected intramuscularly (i.m.) with a dose of 15 mg/kg; (4) Combined treatment (AI + MT) animals were injected intramuscularly (i.m.) with the same dose of AI and half dose of MT (7.5 mg kg−1) per injection. In all treatments, the vehicle used was fish oil (1 mL/animal).
The experiment was performed for 90 days. Animals received three injections at 0, 30, and 60 days. After 30 and 60 days, three animals from each experimental group were sampled, and after 90 days, five animals were sampled from each experimental group. Peripheral blood was collected by caudal vessel puncture, using a heparin-coated (5000 UI/Roche) 22G needle attached to a 5-mL syringe. Blood samples were centrifuged for 6 min at 587g, and the plasma was transferred to heparin-coated cryotubes and immediately placed at − 80 °C until quantification.
Morphometric data (total length and body mass) were registered; gonads and pituitary were collected for subsequent histological and immunohistochemical analysis. Gonads were weighed to calculate the gonadosomatic index (GSI = [gonad weight/total animal weight] × 100).
All experiments were performed in accordance with the Animal Ethics Committee of the Biosciences Institute of the University of São Paulo (Protocol No. 055/2008). This license was based in the guidelines of the Brazilian Federal Law (11.794, of 10/08/2008), which establishes the procedures for the scientific use of animals, and the São Paulo State Law (11.977, of 08/25/2005), which establishes the Animal Protection Code of the State of São Paulo and other rules applicable to the use of animals for teaching and scientific purposes.
Tissue processing
Gonads were fixed for 24 h in Karnovsky’s solution and then transferred to ethanol (70%). Subsequently, the dehydrated material was embedded in Paraplast® (Sigma-Aldrich, USA) or resin (Leica HistoResin®, USA) to be sectioned at 5 or 2 μm respectively. Sections were stained with Hematoxylin-Eosin or PAS + Metanil Yellow. Histological sections were then analyzed and documented through a computerized system for image analysis (Camera: Leica DFC 295; Program: Leica Application suite V3). Morphological classification for oocytes stages was performed according to Grier et al. (2009), and spermatogenesis stages, according to Grier and Uribe (2009). The presence of male germ cells after 90 days was considered to establish the percentage of sex change.
Histochemistry and immunohistochemistry of gonadotropin- and somatolactin-producing cells
The pituitary was fixed for 24 h in Bouin’s solution and then transferred to ethanol (70%). Subsequently, they were dehydrated and embedded in paraffin Paraplast®. Serial sections of 5 μm were stained with Mallory’s Trichrome.
For immunohistochemical analysis, sections were dewaxed, hydrated in PBS buffer (pH 7.6), and then treated with 3% hydrogen peroxide (H2O2) in PBS buffer for 10 min to decrease endogenous peroxidase activity. After buffer rinse, slides were treated with a protein blocker (5% of non-fat powder milk in PBS) for 15 min. They were subsequently incubated with primary antisera for 24 h at 4 °C at 1:1000 dilution. Antisera (βLH, βFSH, and SL) from chum salmon (Oncorhynchus keta) were donated by Professor Dr. H. Kawauchi (School of Fisheries Sciences, Kitasato University, Japan). After incubation, slides were washed in PBS (pH 7.6), incubated with biotinylated secondary antibody (Universal DakoCytomation LSAB + System-HRP, peroxidase) for 30 min, washed again in PBS, and incubated with the SABC complex (Streptavidin-Biotin-peroxidase Complex DakoCytomation LSAB2® System HRP Liquid DAB – Ref. 0673) reagent for another 30 min. After washing in PBS, immunostaining was visualized using 0.05% DAB (3′, 3′-diaminobenzidine, brownish staining) in PBS buffer containing 0.03% H2O2. Sections were lightly counterstained with Hematoxylin and mounted with Erv-Mount (Erviegas Instrumental Cirúrgico Ltda, SP, Brazil). Negative control sections were treated with PBS instead of the primary antibody.
Slides were analyzed under light microscopy, and the images were obtained by a capture system. After the identification of the different adenohypophysis regions (proximal pars distalis, rostral pars distalis, and pars intermedia) and the regions of the gonadotropic ir-cells, images were captured from left to right and from one end to another in the area of the whole pituitary. The number of images varied according to the size of the pituitary, ranging from 7 to 13 images per pituitary.
Stereological analyses of gonadotropic cells
Five quadrants of three histological sections covering the adenohypophysis regions were analyzed from all animals, from the different experimental groups, for each antibody (anti-βFSH and anti-βLH) (amplification × 1000). In each quadrant, among all the adenohypophyseal cells present, those marked by the antibody and those unlabeled were counted. The quadrants were always in the same position, and in all sections, adenohypophysis and neurohypophysis portions were present. This analysis was performed comparing the animals from all experimental groups after 90 days of treatment. All sections were analyzed using an Axioskop 2 light microscope (Zeiss Inc., USA) coupled with a digital MRC5 camera (Zeiss Inc., USA) and equipped with a Zen 2012 digital image capture software (Axioskop 2, Zeiss Inc., USA).
Plasma levels of gonadal steroids
The plasma level of gonadal steroids was quantified by enzyme-linked immunosorbent assay (ELISA) with the following commercial kits: E2 and T (Interteck, Virginia, USA) and 11-ketotestosterone (11-KT) (Cayman Company, USA). Analyses were performed on microplates (Spectra MAX 250) at 450 nm for E2 and T, and at 405 nm for 11-KT. Kits were previously validated for E. marginatus by Garcia et al. (2013).
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Gonadosomatic index (GSI) and the percentage of ir-cells (anti-βFSH and anti-βLH) were compared between the different treatments after 90 experimental days, using one-way ANOVA followed by the Student-Newman-Keuls test. Steroid plasma levels were compared using two-way ANOVA (variables: time and treatment) followed by the Student-Newman-Keuls test. Values were considered significantly different when P < 0.05.
Results
Gonadosomatic index
The use of MT alone or combined with AI decreased GSI (Fig. 1) (P = 0.022) after 90 days of treatment, while AI alone did not change GSI compared with the CT group (P = 0.314).
Gonadal remodeling
Animals from the initial and control groups
Animals from the initial group were classified as juveniles according to the pattern of gonadal development for females. Ovaries of E. marginatus were paired, saculiform, and short, located dorsally within the coelomic cavity longitudinally (Fig. 2a). The ovary belongs to a cyst-ovarian type, presenting a continuous luminal compartment. Both oviducts join in the caudal portion and flow into the urogenital papilla. Within the germinal compartment, only female germ cells were found: oogonia, oocytes with chromatin-nucleolus in different stages of prophase I, and oocytes in primary growth up to the perinucleolar stage (Fig. 2b–d). Somatic cells with characteristics of steroid-producing cells (SpC) were found in cell clusters immersed within the tunica albuginea near large blood vessels; these cells were oval-shaped, showing a small spherical nucleus with compact chromatin and a homogeneous cytoplasm with numerous acidophilic spots (Fig. 2 e and f). The histological analysis of E. marginatus gonads from the control group, at any time of the experiment, showed primary growth oocytes at the perinucleolar stage (Fig. 2a–f), and none of the animals changed the sex.
Histological sections of the ovary from Epinephelus marginatus before the experimental procedure (Initial group). a General view. b–d Oogonia, oocyte nests, and oocytes in leptotenic chromatin and primary growth stages. e Clusters of potential steroid-producing cells found in the tunica albuginea. Notice the clusters near the blood vessels. f Potential steroid-producing cells. Note the acidophilic granules in the cytoplasm. a–f Ovarian cavity (OC); germinal compartment (CGe); tunica albuginea (TA); stroma (Es); gonia (G); basement membrane (Mb); primary growth (CP); primary growth with multiple nucleoli (CPmn); dipolar nucleolar chromatin (CNd); perinucleolar primary growth (CPpn); cytoplasm (C), nucleus (N); nucleoli (n); oogonia (Og); steroid-producing cells (SpC); blood vessel (VS); red blood cells (H). a–f Staining PAS + Metanil Yellow
After 30 days of treatment
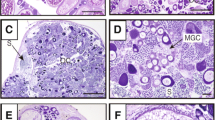
Gonads of the animals treated with aromatase inhibitors (AI) showed slight disorganization of the gonadal architecture and proliferation of the interstitial tissue occurring from the periphery of the tunica albuginea towards the ovarian lamellae, the onset of sex change, and few degenerative oocytes (Fig. 3 a and b). Gonads from the MT and AI + MT groups showed a very similar pattern of sex change and even more advanced stages of sex change than the AI group, evidencing greater gonadal disorganization, an increase of the interstitial tissue proliferation, and the presence of degenerative primary growth oocytes (Fig. 3 c and d). During the gonadal transition, the female germinal epithelium and stroma were progressively replaced by male germ cells and typical interstitial tissue of the testes (Fig. 3e). The gonia found within the germinal epithelium, from the now-presumed testes, proliferate and differentiate into spermatogonia, which are surrounded by the Sertoli cell processes forming the spermatocytes (Fig. 3 e and f) and subsequently initiate spermatogenesis.
Histological sections of Epinephelus marginatus during the beginning of sex transition. Animals treated with an aromatase inhibitor (AI), methyltestosterone (MT), and AI + MT. a, b Disorganization of gonadal architecture, and degeneration of female germ cells and proliferation of interstitial tissue in the AI group. c Gonadal disorganization in the most advanced inversion process in the MT and MT + AI groups. d The proliferation of male germ cells and degenerating oocytes (MT group). e, f Male germ cells in development in the androgen-treated groups. a–f Ovarian cavity (OC); tunica albuginea (TA); gonia (G); disorganization of the gonadal architecture (DAG); degenerative oocytes (OD); proliferation of interstitial tissue (PTI); spermatogonia (Sg); spermatocyst (STC); spermatocyte (Sc). White arrow: Sertoli cells. a–f Staining PAS + Metanil Yellow
After 60 days of treatment
After 60 days, animals implanted only with AI showed greater disorganization of the gonadal architecture, a thicker interstitial compartment, the presence of male germ cells, and several atretic primary growth oocytes. When MT was administered, gonads were in a more advanced stage of sex change, and degenerative oocytes were no longer observed. Male germ cell cysts were visualized, spermatogenesis was active, and the spermatic duct formation was beginning together with the release of sperm (Fig. 4 a and d).
Histological sections of the Epinephelus marginatus gonads showing the development of male germ cells in the group treated with AI and androgens. a Gonadal tissue of the group treated with androgens without the presence of degenerative oocytes after 60 days of treatment. b Detail of the melanomacrophagic centers within the gonadal tissue of the groups treated with AI and MT. c Male germ cells at different stages of development after 90 days of the AI group. d Sperm cyst rupture from the androgen-treated group of males after 60 days. e, f Different cell types during spermatogenesis and sperm release to the spermatic duct in the androgen-treated groups after 90 days. a–f Leydig cells (CL); Melanomacrophagic centers (CMF); tunica albuginea (TA); interstitial tissue (TI); somatic cells (CS); spermatogonia (Sg); spermatocyte (SC); gonia (G); initial spermatids (STi); final spermatids (STf); spermatozoa (SZ). White arrows: Sertoli cells. a–g Staining PAS + Metanil Yellow
In all groups, melanomacrophagic centers were detected within the interstitial tissue: a structure involved in the removal and reabsorption of tissue debris (Fig. 4b). Clusters of SpC, now named Leydig cells (Fig. 4 a and f), were found immersed within the tunica albuginea in greater quantity compared with the former group.
After 90 days of treatment
After 90 days, animals of the AI group showed a more advanced stage of sex change compared with the animals of 60 days of treatment. Also, cysts of male germ cells were found containing different stages of spermatogenesis (spermatogonia, spermatocytes, spermatids) (Fig. 4c). Some degenerating oocytes were found, but 100% of the animals changed sex.
Animals from the experimental groups, where synthetic androgen was administered, showed a similar gonadal pattern to the male, full of cysts at various stages of spermatogenesis, and it was also possible to observe a larger number of germ cell cysts with spermatogonia, spermatocytes, and spermatids and sperm release (Fig. 4 e and f). The percentage of animals that changed sex was 100%.
Histochemical and immunohistochemistry characterization of the pituitary gland
The E. marginatus pituitary gland is divided into two distinct regions: neurohypophysis and adenohypophysis. The neurohypophysis—pars nervosa—penetrates and branches into the adenohypophysis. The adenohypophysis is subdivided into rostral pars distalis (RPD), proximal pars distalis (PPD), and pars intermedia (PI), distinguished according to the staining characteristics of its hormone-producing cells (Fig. 5a). The RPD occupies the rostral or anterior portion of the gland, involving the pituitary stalk as soon as it enters the adenohypophysis. PPD is located between RPD and PI, and is characterized by being more basophilic than the other regions of the pituitary gland. PI is located in the most caudal or posterior portion of the gland and receives a great number of branches of the neurohypophysis (Fig. 5 a and d).
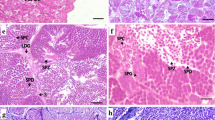
Epinephelus marginatus—Localization of the pituitary gland and histochemical reactions of the histological sections presenting the structures of the control group, and the immunohistochemical reactions of the histological sections presenting the immunostaining of the gonadotropic cells and somatolactin (SL)-producing cells in different parts of the adenohypophysis. a Sagittal section showing the diencephalon, pituitary peduncle, and pituitary gland. Note that the adenohypophysis is divided into three parts according to the staining characteristics of the cells: rostral pars distalis, proximal pars distalis, pars intermedia. b Sagittal section localizing the pars intermedia of the adenohypophysis by immunostaining the SL-producing cells. c A sagittal section demonstrating immunostaining of anti-β-FSH cells. d A sagittal section demonstrating anti-β-LH cell immunostaining. e Anti-β-FSH cells (white arrows). f Anti-β-LH cells (red arrows). Note that the anti-β-FSH and anti-β-LH cells are globose cells, with a very eccentric nucleus and abundant cytoplasm. a–f Diencephalon (De); pituitary peduncle (Pp); neurohypophysis (NH); adenohypophysis (AH); Rostral pars distalis (RPD); Proximal pars distalis (PPD); Pars intermedia (PI); A: Mallory trichrome staining. b–i Immunohistochemical reactions using anti-chum salmon antisera (Dr. H. Kawauchi)
Using immunohistochemistry techniques, immunoreactive somatolactin-producing cells (ir-SL) evidenced the PI region (Fig. 5b), as they are typical cells of this region in teleosts. SL cells were located at the branches of the neurohypophysis peripherally and are easily differentiated from the gonadotropic cells (ir-LH and ir-FSH) because of their smaller size (Fig. 5 b insert; e and f).
The gonadotropic cells of E. marginatus were immunoreactive to both antibodies used, salmon anti-gonadotropin: β-FSH (Fig. 5c) and β-LH (Fig. 5d). These cells are globular, with an eccentric nucleus and abundant cytoplasm (Fig. 5 e and f). Immunohistochemistry using serial sections and specific antibodies showed two gonadotropic cell types producing FSH and LH separately. The use of β-FSH (Fig. 5 c and e) and β-LH (Fig. 5d and f) subunit antibodies showed that ir-FSH and ir-LH are also synthesized in different gonadotropic cells; these are located in different regions of the adenohypophysis, independent of the experimental group. ir-FSH cells of E. marginatus were located in PI, an unusual place for these cells that are typically distributed in PPD in teleosts, while ir-LH cells were seen in PI and PPD.
Comparing ir-FSH cells and their hormone accumulation (staining intensity) in the different treatments, under qualitative evaluation, the animals from the AI group, after 90 days of treatment, seem to display a weaker marking (Fig. 6a–d). It was not possible to establish the same relationship for the LH-producing cells.
Epinephelus marginatus—Immunohistochemical reactions of the histological sections showing anti-β-FSH cell immunostaining in the different experimental groups after 90 days. a Sagittal section of pituitary showing anti-β-FSH cell immunostaining in the control group (CT). b Sagittal section of pituitary showing the immunostaining of anti-β-FSH cells in the aromatase inhibitor (AI) group. Note weaker immunostaining than the other experimental groups. c Sagittal section of pituitary showing anti-β-FSH cell immunostaining in the methyltestosterone and aromatase inhibitor (MT + AI) group. d Sagittal section of pituitary showing the immunostaining of anti-β-FSH cells in the methyltestosterone group (MT). a–d Neurohypophysis (NH); Rostral pars distalis (RPD); Proximal pars distalis (PPD); Pars intermedia (PI). Immunohistochemical reactions using anti-chum salmon antiserum (Dr. H. Kawauchi)
The counting of ir-FSH cells showed that animals from the CT group presented a higher percentage of stained cells than AI, MT, and AI + MT groups (P < 0.001) (Fig. 7A). Additionally, the percentage of ir-FSH cells was also higher in animals from MT treatment, compared with AI and MT + AI treatment (Fig. 7A). No differences were observed in ir-LH cells after 90 days (Fig. 7B) (P = 0.131).
A) FSH immunoreactive cells (ir-FSH) and B) LH immunoreactive cells (ir-LH) in Epinephelus marginatus after 90 experimental days. Control (CT), aromatase inhibitor (AI), methyltestosterone (MT), and methyltestosterone + aromatase inhibitor (MT + AI). Different letters indicate a statistically significant difference between treatments
Plasma levels of gonadal steroids
The analysis within each treatment showed that E2 plasma levels decreased in animals from MT and MT + AI groups after 60 and 90 days and remained unchanged in control and AI groups. Within each treatment period, after 30 days, E2 levels were higher in animals treated with MT + AI than animals from control and AI groups, and after 90 days, animals from the AI group presented higher E2 levels than animals from the MT group. No differences were found after 60 days of treatment (Fig. 8).
Plasma levels of estradiol in Epinephelus marginatus throughout the experimental period. Control (CT), aromatase inhibitor (AI), methyltestosterone (MT), and methyltestosterone + aromatase inhibitor (MT + AI). . Different letters indicate a statistically significant difference between treatments within the same period. Asterisk and number sign indicate a statistically significant difference between periods within the same treatment
Within each treatment, T levels decreased in animals treated with AI after 90 days, increased after 60 days in animals treated with MT, and decreased during the experimental period in animals from the MT + AI group. Within each experimental period, after 30 days, animals from the MT + AI group presented higher T levels compared with all experimental groups, and animals from MT and MT + AI groups presented higher T levels when compared with animals from control and AI groups. After 60 days, animals from the MT group presented higher T levels compared with animals from all experimental groups. No statistical differences were found in T levels within 90 days (Fig. 9). 11-KT levels did not change comparing the different treatments but increased in the animals treated with AI after 90 days of treatment (Fig. 10).
Plasma levels of testosterone in Epinephelus marginatus throughout the experimental period. Control (CT), aromatase inhibitor (AI), methyltestosterone (MT), and methyltestosterone + aromatase inhibitor (MT + AI). Different letters indicate a statistically significant difference between treatments within the same period. Asterisk and number sign indicate a statistically significant difference between periods within the same treatment
Plasma levels of 11-ketotestosterone in Epinephelus marginatus throughout the experimental period. Control (CT), aromatase inhibitor (AI), methyltestosterone (MT), and methyltestosterone + aromatase inhibitor (MT + AI). Asterisk and number sign indicate a statistically significant difference between periods within the same treatment
Discussion
Sex reversal in E. marginatus juveniles was induced with AI and MT, but the gonadal analyses showed that the final stages of spermatogenesis were obtained using MT, alone or combined, while AI triggered spermatogenesis, but male gametes reached only the spermatid stage. The plasma level of gonadal steroids did not follow the pattern described in adult animals (Sarter et al. 2006; Garcia et al. 2013). The differential distribution pattern and differential counting of ir-FSH cells suggest the involvement of this gonadotropin in sex change.
During the sex change, several simultaneous events were found in the gonads: disorganization of the standard gonadal architecture, massive degeneration of female germ cells, the presence of melanomacrophagic centers, interstitial tissue and the entire process of development and maturation of male germ cells. Several authors report other structures and events during this process, such as the appearance and intense proliferation of Leydig cells, myoid cells, and the presence of structures that are probably related to the removal and reabsorption of tissue remains (granulocytes, melanomacrophagous, and macrophages) (Sadovy and Shapiro 1987; Besseau and Bruslé-Sicard 1991; Grier and Taylor 1998; Mazzoni et al. 2018). Alam et al. (2006), investigating steroid-producing cells (SPCs) in the ovaries of E. coioides, assumed as Leydig cells, showed assemblages of cells strongly immunopositive to the antibodies P450scc and P450 11β (enzymes involved respectively in the initial steps of steroidogenesis and in the final step of 11KT synthesis) during the annual reproductive cycle, near the blood vessels in the tunica albuginea. These cells identified by Alam et al. (2006) were also identified in E. marginatus in the present study, with an expressive increase of these cell agglomerates in the tunica albuginea of the animals that changed the sex.
The difference in the size of the germ cells explains the decrease in GSI in the experimental groups treated with MT and MT + AI, which was documented by histological analyses of E. marginatus gonads, culminating with the degeneration of the gonadal tissue of the first sex (ovary) and the growth and maturation of the gonadal tissue of the opposite sex (testes). The morphological analyses of the animals treated with MT corroborate with the results obtained in E. coiodes that showed that the sex change process could be divided into two phases, an early phase when female and male germ cells coexist and a late phase when only male germ cells are present (Wang et al. 2017), a phase that was not achieved with AI. These authors also found that sex change can be permanent if MT withdrawal occurred just after 96 days in that species.
The result obtained in the plasma profile of the gonadal steroids over the 90 experimental days demonstrated that E2 decreased in the animals that reached the most advanced stages of sex change (i.e., animals from MT and MT + AI groups) and did not change throughout the experimental period in the control and AI groups. However, even considering this profile, E2 levels in juvenile animals were higher when compared with adult females of the same species during sex change with AI (Garcia et al. 2013). E2 has an important role in spermatogonial stem cell renewal, what can explain the maintenance of high levels of this estrogen even after AI administration, a profile that was also observed in adult dusky grouper females treated with E2 (Garcia et al. 2013). Therefore, even in males, this steroid, typically female related, is present (Miura and Miura 2003; Schulz et al. 2010). T levels increased at the beginning and middle of the experimental period in the animals treated with MT + AI and MT, but when the more advanced stage of sex reversal was observed, T levels decreased. This profile was also similar in the induction of sex change in adult E. marginatus using AI during summer, which presented a transient increase in T levels in the middle of the experimental period, but at the end of the experiment, T levels were the same of the animals from the control group (Garcia et al. 2013).
The study of the functional role of androgens in females indicates that 11-KT produced in the ovaries is involved in controlling the growth of pre-vitellogenic oocytes (Lokman et al. 2015), while in males, 11-KT plays a key role in sexual maturation, development of secondary sexual characteristics, reproductive behavior (Schulz et al. 2010), and sex change in protogynic hermaphrodites (Alam et al. 2006). The data from this study show that in AI-treated animals, the final drop in T concentration is associated with an increase in 11-KT levels, evidencing the important role of this androgen in the proliferation of spermatogonia and spermiogenesis. In the groups treated with synthetic androgens (MT and MT + AI), the decrease in T levels after 90 days of the experiment also occurred; however, elevations of 11-KT were not observed, suggesting that in these animals, the production of male gametes occurred mainly due to the action of the synthetic androgen.
The pattern of FSH and LH levels during the reproductive cycle has been studied in different teleost species, and the most common pattern was described in salmonids since the 1990s, in which high plasma levels of FSH are observed during the onset of gametogenesis, decreasing during vitellogenesis and maturation, and increasing again during the preovulatory period, whereas LH is undetectable during gametogenesis, only increasing slightly prior to ovulation/spermiation (Swanson et al. 1991; Slater et al. 1994). However, different patterns of gonadotropin synthesis and plasma levels were detected in other gonochoristic species (Levavi-Sivan et al. 2010). In protogynous hermaphrodite fish species, gonadotropin synthesis and/or plasma levels also present different patterns. In E. coiodes, the βfsh and βlh genes present low expression levels when animals present ovaries in the resting stage, but higher gene expression levels of both gonadotropins were detected during ovary development (Li et al. 2005). Gonadotropins are also involved in the control of sex change in groupers, as observed in Epinephelus merra that were implanted with FSH and LH (purified from cattle) and reached complete sex change in 3 weeks (Kobayashi et al. 2010). During testis development, levels of βfsh gene expression in the pituitary increased, while blh gene expression levels did not change, suggesting that FSH may trigger sex change in this species.
The data obtained in our study showed the distribution pattern of FSH, LH, and SL in E. marginatus pituitary, evidencing that salmon antibodies are able to recognize these pituitary hormones in this species. After 90 experimental days, animals from all treatments decreased the number of ir-FSH cells, corroborating the study carried out in adult E. marginatus that showed decreased βfsh gene expression after 9 weeks treated with AI (Garcia et al. 2013). However, these authors also observed a decrease in the gene expression levels of βlh in AI-treated animals, which was not observed in the present study performed with juveniles, which can be explained by the lower LH role in juvenile teleost species (Yaron et al. 2003). The combined analysis of gonadal steroids and gonadotropins shows that the decrease in ir-FSH cells in treated animals can be a result of the negative feedback loop caused by the steroids increase, 11-KT in AI-treated animals, and T in MT and MT + AI groups.
Conclusions
In juveniles, E. marginatus sex reversal was most efficiently induced with MT (combined with AI or alone) after 90 experimental days. In the gonads, the differentiation of germinative cells to enter into the spermatogenesis starts from a stock of steam cells, followed by the following events: apparent disorganization of the gonadal architecture, massive degeneration of female germ cells, presence of melanomacrophage centers, proliferation of male-associated structures (interstitial tissue and male germ cells), and maturation of male germ cells. The use of AI was efficient at starting the sex change process. Nevertheless, the experimental period was probably not sufficient to progress the spermatogenesis until the final steps, while MT-treated animals showed a shift from E2 to T levels, followed by a complete spermatogenesis process. Pituitary ir-FSH cells decreased at the end of the experimental period in all treatments, and this pattern can be related with a negative feedback loop triggered by the increase in natural and/or synthetic androgens, while LH was not altered during the sex change, such as observed in adult animals of the same species.
References
Alam MA, Bhandari RK, Kobayashi Y, Soyano K, Nakamura M (2006) Induction of sex change within two full moons during breeding season and spawning in grouper. Aquaculture 255:532–535
Andrade AB, Machado LF, Hostim-Silva M, Barreiros JP (2003) Reproductive biology of the dusky grouper Epinephelus marginatus (LOWE, 1834). Braz Arch Biol Technol 46:373–381
Araújo BA, Wade NW, Mello PH, Rodrigues-Filho JA, Garcia CEO, Campos MF, Botwright NA, Hashimoto DT, Moreira RG (2018) Characterization of lipid metabolism genes and the influence of fatty acid supplementation in the hepatic lipid metabolism of dusky grouper (Epinephelus marginatus). Comp Biochem Physiol A-Mol Integ Physiol 219-220:1–9
Barreiros JP (1998) Sexual inversion in Epinephelus marginatus (Lowe, 1834) (Pisces: Serranidae, Epinephelinae) nos Açores. Rev Portug de Zootec 5:81–90
Besseau L, Bruslé-Sicard S. (1991) Sex inversion in a prontandric hermaphodite Lithgnathus mormayrus (L., 1758) (Teleostei, Sparidae): histocytological peculiarities. In: International Symposium on The Reproductive Physiology of Fish, 4, Sheffield. Proceedings. Sheffield: Fish, 95
Bhandari RK, Komuro H, Nakamura S, Higa M, Nakamura M (2003) Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra). Zoolog Scie 20:1399–1404
Bhandari RK, Komuro H, Mikihiko H, Massaru N (2004) Sex inversion of sexually immature honeycomb grouper (Epinephelus merra) by aromatase inhibitor. Zoolog Scie 21:305–310
Cabrita E, Engrola S, Conceição LEC, Pousão-Ferreira P, Dinis MT (2009) Successful cryopreservation of sperm from sex-reversed dusky grouper, Epinephelus marginatus. Aquaculture 287:152–157
Cunha ME, Ré P, Quental-Ferreira H, Gavaia PJ, Pousão-Ferreira P (2013) Larval and juvenile development of dusky grouper Epinephelus marginatus reared in mesocosms. J Fish Biol 83:1–18
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Eschmeyer, WN and Fong, JD (2018) Catalog of Fishes. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp#Serranidae
Garcia CEO, Mello PH, Araújo BC, Narcizo AM, Rodrigues-Filho JA, Medrado AT, Zampieri RA, Floeter-Winter LM, Moreira RG (2013) Involvement of pituitary gonadotropins, gonadal steroids and breeding season in sex-change of protogynous dusky grouper, Epinephelus marginatus (Teleostei: Serranidae), induced by a non-steroidal aromatase inhibitor. Gen Comp Endocrinol 192:170–180
Grier HJ, Taylor RG (1998) Testicular maturation and regression in the common snook. J Fish Biol 53:521–542
Grier HJ, Uribe MC (2009) The testis and spermatogenesis in teleosts. In: Jamieson, BJM (Ed): Reproductive biology and phylogeny of fishes (agnathans and bony fishes) phylogeny reproductive system viviparity spermatozoa, 802
Grier HJ, Uribe MC, Patiño R (2009) The ovary, folliculogenesis and oogenesis in teleosts. In Jamieson, B.J.M (Ed): Reproductive biology and phylogeny of fishes (agnathans and bony fishes) phylogeny reproductive system viviparity spermatozoa, 802
Guiguen Y, Fostier A, Piferrer F, Chang CF (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165:352–366
Heemstra PC, Randall JE (1993) FAO species catalogue. Groupers of the world (Family Serranidae, Subfamily Epinephelinae). FAO, Rome 16: 382
IUCN (2019) The IUCN Red List of Threatened Species. Version 2019-2. <www.iucnredlist.org>. Downloaded on 08 September 2019
Kobayashi Y, Alam MA, Horiguchi R, Shimizu A, Nakamura M (2010) Sexually dimorphic expression of gonadotropin subunits in the pituitary of protogynous honeycomb grouper (Epinephelus merra): evidence that follicle-stimulating hormone (FSH) induces gonadal sex change. Biol of Reprod 82(6):1030–1036
Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165:412–437
Li CJ, Zhou L, Wang Y, Hong YH, Gui JF (2005) Molecular and expression characterization of three gonadotropin subunits common α, FSHß and LHß in groupers. Mol Cel Endocrinol 233:33–46
Lokman PM, Wylie MJ, Downes M, Di Biase A, Damsteegt EL (2015) Artificial induction of maturation in female silver eels, Anguilla australis: the benefits of androgen pre-treatment. Aquaculture 437:111–119
Lubzens E, Young G, Cerda J (2010) Oogenesis in teleosts: how eggs are formed. Gen Comp Endocrinol 165:367–389
Marino G, Azzurro E, Finoia MG, Messina MT, Massari A, Mandich A (2000) Recent advances in induced breeding of the dusky grouper Epinephelus marginatus (Lowe, 1834). Ciheam Options Mediterraneennes 47:215–225
Marino G, Di Marco P, Massari A, Bottero S, Mandich A (2002) Effects of temperature and social control on sexual maturity and sex inversion in captive dusky grouper Epinephelus marginatus. In Proceedings of the International Conference of Aquaculture Europe (Basurco, B. & Saroglia, M., eds), pp. 331–332
Marino G, Panini E, Longobardi A, Mandich A, Finoia MG, Zohar Y, Mylonas CC (2003) Induction of ovulation in captive-reared dusky grouper, Epinephelus marginatus (Lowe, 1834) with a sustained-release GnRHa implant. Aquaculture 219:841–858
Mazzoni T, Lo Nostro F, Antoneli F, Quagio-Grassiotto I (2018) Action of the metalloproteinases in gonadal remodeling during sex reversal in the sequential hermaphroditism of the teleostei fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells 7:34
Mello PH, Araújo BC, Campos MF, Rodrigues-Filho JA, Garcia CEO, Moreira RG (2018) Embryonic and larval development and fatty acid profile of the dusky grouper (Epinephelus marginatus) reproduced in captivity: tools applied to captive rearing. J Fish Biol 92:1126–1148
Miura T, Miura CI (2003) Molecular control mechanisms of fish spermatogenesis. Fish Physiol and Biochem 28:181–186
Reñones O, Grau A, Mas X, Riera F, Saborido-Rey F (2010) Reproductive pattern of an exploited dusky grouper Epinephelus marginatus (Lowe 1834) (Pisces: Serranidae) population in the western Mediterranean. Sci Mar 74:523–537
Rodrigues-Filho JA, Sanches EG, Garcia CEO, Pannuti CV, Sebastiani EF, Moreira RG (2009) Threatened fishes of the world: Epinephelus marginatus (Lowe, 1834) (Serranidae: Epinephelinae). Environ Biol of Fish 85:301–302
Russo T, Boglione C, De Marzi P, Cataudella S (2009) Feeding preferences of the dusky grouper (Epinephelus marginatus, Lowe 1834) larvae reared in semi-intensive conditions: a contribution addressing the domestication of this species. Aquaculture 289:289–296
Sadovy Y, Shapiro DY (1987) Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1:136–156
Sanches EG, Oliveira IR, Serralheiro PCS (2009) Inversão sexual da garoupa-verdadeira Epinephelus marginatus. R Bras Saúde e Produt 10:198–209
Sarter K, Papadaki M, Zanuy S, Mylonas C (2006) Permanent sex inversion in 1-year –old juveniles of the protogynous dusky grouper (Epinephelus marginatus) using controlled-release 17α-methyltestosterone implants. Aquaculture 256:443–456
Schulz RW, França LR, Lareyre JJ, Legac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Slater CH, Schreck CB, Swanson P (1994) Plasma profiles of the sex steroids and gonadotropins in maturing female spring chinook salmon Oncorhynchus tshawytscha. Comp Biochem Physiol A-Mol Integ Physiol 109:167–175
Spedicato MT, Lembo G, Di Marco P, Marino G (1995) Preliminary results in the breeding of dusky grouper Epinephelus marginatus (Lowe, 1834). Cah Options Méditerr 16:131–148
Swanson P, Suzuki K, Kawauchi H, Dickhoff WW (1991) Isolation and characterization of two coho salmon gonadotropins, GTH I and GTH II. Biology Reproduction 44:29–38
Vaini JO, Mota KG, Ojeda AP, Barreiros JP, Moreira RG, Hilsdorf AWS (2019) Development and characterization of 20 polymorphic microsatellite markers for Epinephelus marginatus (Lowe, 1834) (Perciformes: Epinephelidae) using 454 pyrosequencing. Genet Mol Biol 42(1):74–79. https://doi.org/10.1590/1678-4685-gmb-2018-0067
Wang Q, Liu Y, Peng C, Wang X, Xiao L, Wang D, Chen J, Zhang H, Zhao H, Li SS, Zhang Y, Lin H (2017) Molecular regulation of sex change induced by methyltestosterone-feeding and methyltestosterone-feeding withdrawal in the protogynous orange-spotted grouper. Biol Reprod 97:324–333
Wu GC, Li HW, Tey WG, Lin CJ, Chang CF (2017) Expression profile of amh/Amh during bidirectional sex change in the protogynous orange-spotted grouper Epinephelus coioides. Plos One 12(10):1–18
Yaron Z, Gur G, Melamed P, Rosenfeld H, Elizur A, Levavi-Sivan B (2003) Regulation of fish gonadotropins. Int Rev Cytol 225:131–185
Acknowledgments
The authors would like to thank Instituto de Pesca (Ubatuba/SP) that provided the logistics and facilities for the experiment, Dr. Irani Quagio-Grassiotto (UNESP) for her contribution in gonadal histological analysis, and Dr. H. Kawauchi (School of Fisheries Sciences, Kitasato University, Japan) for donating βLH, βFSH, and SL antisera from chum salmon.
Funding
This study was financially supported by a research grant from FAPESP (2007/59122-7) and CNPq (558992/2009-9). J.A.Rodrigues-Filho also had a FAPESP scholarship (2007/57106-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the Animal Ethics Committee of the Biosciences Institute of the University of São Paulo (Protocol No. 055/2008). This license was based in the guidelines of the Brazilian Federal Law (11.794, of 10/08/2008), which establishes the procedures for the scientific use of animals, and the São Paulo State Law (11.977, of 08/25/2005), which establishes the Animal Protection Code of the State of São Paulo and other rules applicable to the use of animals for teaching and scientific purposes.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodrigues-Filho, J.A., Garcia, C.E.O., Chehade, C.G. et al. Gonadal remodeling and hormonal regulation during sex change of juvenile dusky grouper Epinephelus marginatus (Teleostei, Serranidae), an endangered protogynous hermaphrodite fish. Fish Physiol Biochem 46, 1809–1824 (2020). https://doi.org/10.1007/s10695-020-00830-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00830-8