Abstract
We used 12 land-based experimental enclosures (6 m × 5 m) in a saline–alkaline pond of shrimp (Penaeus vannamei) to determine the impact of net-isolated polyculture of tilapia (Oreochromis niloticus) on plankton communities for 40 days. Tilapias were stocked in net cages suspended in enclosures, in polyculture ponds including tilapia and shrimp. Four tilapia biomass were tested: 0, 39, 115 and 227 g m−2. Shrimp stocking biomass were 0.7 g m−2 in all treatments. There were three replicates in each treatment. Our results showed that the presence of tilapia significantly reduced phytoplankton biomass directly through predation and indirectly through top-down effect. The stocking of tilapia reduced zooplankton biomass, particularly rotifer biomass. However, copepod biomass was not been significantly affected. So, net-isolated polyculture of tilapia can thus have a strong impact on phytoplankton allowing the co-existence of large numbers of copepods with planktivorous fish and improving the water quality of shrimp ponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrimp farming in saline–alkaline pond has been expanding rapidly in China, where closed or semi-closed culture systems were suggested as an effective strategy to reduce fresh water requirements and to mitigate the environmental impacts of aquaculture. To minimize water exchange, one of the key challenges is to control the excessive phytoplankton growth that is common in shrimp ponds. However, shrimp does not graze directly on phytoplankton. Therefore, filter-feeding animals have been introduced not only into freshwater but also into seawater shrimp ponds to control phytoplankton or to enhance shrimp production in polyculture systems (Lu et al. 1999). Shrimp production has been generally found higher in polyculture than in monoculture (Lu et al. 1999; Li and Dong 2002).
Silver carp (Hypophthalmichthys molitrix), known to be planktivorous and used as a tool to manage water quality in fresh water ponds by many researchers (Kajak et al. 1975; Burke et al. 1986; Starling and Rocha 1990; Starling 1993; Fukushima et al. 1999), cannot live in saline–alkaline ponds due to their high salt content. Nile tilapia (Oreochromis niloticus) has the ability to filter feed on phytoplankton (Turker et al. 2003) and appears to be the most appropriate choice for a polyculture with the shrimp (Penaeus vannamei) in saline–alkaline ponds.
Planktivorous fish may regulate phytoplankton and water quality of aquaculture ponds through their consumption of plankton and alteration of nutrient cycles (Lazzaro 1987; Vanni 1987). Previous attempts to control phytoplankton using tilapia have produced inconclusive results (Diana et al. 1991; Ruan et al. 1993; Zhao et al. 2000), and some observations revealed that stocking tilapia may increase, rather than decrease, phytoplankton biomass (Ruan et al. 1993; Zhao et al. 2000). Also, unconfined free-swimming tilapia in shrimp ponds have the potential of negative species interaction including competition over food and uncontrolled reproduction (Danaher et al. 2007).
Recently, there has been an enormous interest in the polyculture of freshwater prawn with finfish, especially with tilapia (New 2005). However, in saline–alkaline ponds, not only the water quality but also the plankton community structure is different from that in fresh water ponds (Shentu et al. 2000; Zhao et al. 2001; Zhao et al. 2002). So, the impacts and feasibility of shrimp and Nile tilapia polyculture need to be evaluated.
In the present field study, we propose a net-isolated polyculture system of tilapia in saline–alkaline shrimp ponds, which herein are tested in earthen-based enclosures (mesocosms). The objectives were, therefore, (a) to evaluate the impact of tilapia on plankton communities and (b) to optimize the stocking densities of tilapia with shrimp in this polyculture system.
Materials and methods
Study area and experimental design
A 40-day experiment was conducted in land-based experimental mesocosm enclosures (6 m × 5 m) in a saline–alkaline earthen pond at the Breeding Center of Chinese mitten crab nearby the Yellow River Estuary, from July 2 through August 10, 2005. The earthen pond used for the experiment has a surface area of 1,000 m2 and an average depth of 1.3 m.
The experiment consisted of four stocking densities of tilapia calculated in relation to the enclosure: 0 (Control), 39 (T1), 115 (T2) and 227 g m−2 (T3). Enclosures were stocked at a fixed stocking density of 90 shrimp juvenile m−2 (Table 1). Each treatment had three replicates that were randomly assigned to the corresponding enclosure.
Pond preparation and stocking
Twelve experimental enclosures made of impervious polyethylene woven cloth were used. The structure of the enclosure in detail was described by Li et al. (1998) and Wang et al. (1998). Each enclosure was aerated with six pieces of air stone suspended at mid-depth of water column for 6–12 h daily to prevent water stratification and to simulate the natural pond conditions. One net cage (1.2 m × 1.2 m × 0.7 m, 0.5-cm mesh net) was suspended in each enclosure for net-isolated polyculture of tilapia. Enclosures were initially fertilized 15 days prior to shrimp and fish stocking with semi-decomposed chicken manure (3 kg per enclosure) to allow growth of natural food organisms. The water in the enclosures was not been exchanged during the experiment.
All male tilapia were transported from the National Improvement Variety of Fishery Hatchery in Jiaozhou, Shandong. For 20 days prior to the experiment, fishes were gradually acclimatized to enclosure conditions (from fresh water to brackish water) in another earthen pond.
On July 2 (day 0), shrimp juveniles with mean individual weight of 7.9 ± 6.2 mg were released into each enclosure. Tilapia (139.7 ± 4.5 g in size) were stocked into the net cages at the same time. A commercial pellet manufactured by Haiyue Fishery Feed, Qingdao, China, was supplied to shrimp two times a day at a rate of 8% body weight at the beginning, and adjustment was made to actual consumption based on observations in feeding trays outside of the net cages 2 h after each feeding. Tilapia were fed twice a day at a rate of 0.5% body weight throughout 40-day period on a commercial pellet manufactured by Shengli Fishery Feed, Dongying, China.
At the end of the experiment, the pond was completely drained with a pump placed on the bottom and siphon pipes with net on one end were used to remove the water from the enclosures simultaneously. Shrimp and tilapia in each enclosure were collected from a harvesting pit.
Sampling and analysis
On day 0, enclosures were sampled for phytoplankton, zooplankton and chemical parameters to check that initial conditions were similar in all enclosures. After fish and shrimp introduction, enclosures were sampled at 10-day intervals at about 09:00 a.m.
For phytoplankton samples, 1 l of water was preserved with 1% acidified Lugol’s iodine solution and concentrated to 30 ml after 48 h of sedimentation. After complete mixing, identification and counting were accomplished using a counting chamber and an inverted microscope (Olympus IMT-2, Japan). Zooplankton was sampled with vertical tows of 64-μm mesh plankton net and preserved in 4% formalin and counted under a binocular microscope (Olympus BH-2, Japan) in a Dolfuss chamber. Triplicate water samples for phytoplankton and zooplankton were taken with the column sampler from the centre and two diagonal corners of each enclosure. Physicochemical variables (temperature, salt content, alkalinity, pH, total nitrogen and total phosphorus) were monitored on the dates when samples of plankton were taken. A full description of the methods used is given in Huang et al. (1999).
Statistical analysis
The data were first checked for assumptions for the analysis of variance. One-way ANOVA was tested on day 0 to check that the initial values were similar in all enclosures and was used to analyze the effect of tilapia stocking biomass on shrimp growth. Main effects of tilapia stocking biomass on plankton were analyzed with a repeated-measures two-way ANOVA with time, and because fish and shrimp were stocked on day 0, data from day 0 were excluded from the analysis. If significant differences were found in the ANOVA test, Duncan’s multiple range tests were used to rank the groups. Significance was assigned at the 5% level. All statistical analyses were carried out using SPSS 13.0.
Results
On day 0 (pretreatment), no significant difference in any of the measured variables was detected between Control and treatment enclosures. During the experiment, temperature ranged from 22.8 to 31.2°C and the salt content fluctuated between 6.8 and 7.9 g l−1. Other water quality parameters were alkalinity 2.1–3.2 mmol kg−1, pH value 8.2–10.4, average total nitrogen 326.1 μmol l−1 and average total phosphorus 7.8 μmol l−1.
Phytoplankton
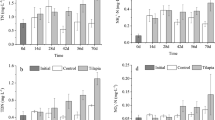
The enclosures in saline–alkaline shrimp pond were dominated by Cyanophyceae and Chlorophyceae, and Cyanophyceae was mainly represented by Spirulina spp. and colonial Microcystis spp. Total phytoplankton biomass data over different sampling dates are shown in Fig. 1. The effect of tilapia on phytoplankton in enclosures was significant (P < 0.01). Duncan’s multiple range test indicated that total phytoplankton biomass in the Control without tilapia was significantly higher than in the treatments with tilapia. But there were no difference between T1, T2 and T3. In the Control, the total phytoplankton biomass showed an increasing trend with time that did not occur in the treatments after the introduction of shrimp to the enclosures.
Total phytoplankton biomass in different treatments throughout the experimental period. Values are means (±SD) of three enclosures (N = 3) per sampling date in each treatment. Control treatment with 90 ind. m−2 shrimp and no tilapia, T1 treatment with 90 ind. m−2 shrimp and 39 g m−2 tilapia, T2 treatment with 90 ind. m−2 shrimp and 115 g m−2 tilapia, T3 treatment with 90 ind. m−2 shrimp and 227 g m−2 tilapia
Cyanophyceae biomass in the enclosures were significantly different among treatments (P < 0.05). No significant difference was observed between the Control and T1, and between T2 and T3. Cyanophyceae biomass in latter treatments were significantly lower than in Control and T1. Cyanophyceae increased in the Control and T1 more than in the other treatments (Fig. 2).
Cyanophyta biomass in different treatment throughout the experimental period. Values are means (±SD) of three enclosures (N = 3) per sampling date in each treatment. For treatment abbreviations, refer to Fig. 1
Zooplankton
Zooplankton was mainly represented by Schmackeria inopinus, Schmackeria forbesi, Sinocalanus spp., copepod nauplii, Brachionus urceus, Rotaria rotatoria, Euchlanis spp. and a few protoza.
Copepod biomass in all treatments showed a decreasing trend from day 0 to the end of the experiment (Fig. 3). The repeated-measures ANOVA revealed no significant differences among the four treatments (P > 0.05).
Copepod biomass in different treatment throughout the experimental period. Values are means (±SD) of three enclosures (N = 3) per sampling date in each treatment. For treatment abbreviations, refer to Fig. 1
During the experiment, rotifer biomass markedly increased with time (Fig. 4). Fish stocking biomass significantly affected rotifer biomass (P < 0.01). With increasing fish stocking biomass, rotifer biomass decreased and was significantly higher in the Control than in any of the treatments with fish. Duncan’s multiple tests did not show difference statistically among treatment T1, T2 and T3.
Rotifer biomass in different treatment throughout the experimental period. Values are means (±SD) of three enclosures (N = 3) per sampling date in each treatment. For treatment abbreviations, refer to Fig. 1
Fish/shrimp yield parameters
Yield parameters of shrimp and fish are shown in Table 1. Survival of tilapia was 100% during the experiment. Individual weight gains of tilapia were similar in T1 and T2 and significantly higher (about double) than in T3.
Shrimp in treatment T2 showed significantly higher survival and net yield than in the other treatments. The effect of stocking density of tilapia on individual harvesting weight of shrimp was significant (P < 0.05) with about 25% increased shrimp weight in T2 and T3 than in the control and T1.
Discussion
Effects on plankton biomass
The effects of planktivorous fish on phytoplankton are complex. Perschbacher and Lorio (1993) reported that tilapia stocked at a density of 0.5 ind. m−2 promoted a very effective biological control to decrease phytoplankton. Ruan et al. (1993) showed increases in phytoplankton density and primary productivity after stocking tilapia (0.53–2.25 g m−2) in a microcosm study. Datta and Jana (1998) obtained similar results in an enclosure study (stocking tilapia biomass: 48.3–58.3 g m−2) within an artificial lake in India. Our enclosure experiment demonstrated that net-isolated polyculture of tilapia could reduce total phytoplankton biomass significantly. However, the ability to control phytoplankton did not increase as tilapia biomass increased from 39 to 226 g m−2 (Fig. 1).
Focusing on Cyanobacteria of shrimp pond, they are considered a nuisance because they are a relatively poor base for aquatic food chains, may impart unpleasant flavors to water and fish, produce compounds which are toxic to aquatic animals and deteriorate water quality (Paerl and Tucker 1995). Attempts have been made to use filter-feeding fish, such as silver carp and bighead carp, to reduce Cyanobacteria blooms in China and other countries, which has been sometimes proven effective (Starling 1993; Xie and Liu 2001). Tilapia is among the very few fish species which are not only capable of digesting Cyanobacteria but also could be cultured in saline–alkaline pond. In our enclosure experiment, T2 and T3 could control Cyanobacteria effectively but T1 had no significant difference with Control (Fig. 2). This result indicates that Cyanobacteria biomass vary greatly depending on fish stocking densities. Therefore, determination of an optimal stocking density in this type of polyculture is a key in the success of tilapia to reduce Cyanobacteria.
Planktivorous fish are known to be size-selective predators that prey selectively on large zooplankton (Brooks and Dodson 1965). In this field experiment, polyculture treatments reduced rotifer biomass significantly, but copepod biomass had not been affected. It is presumed that compared with rotifer, copepod have stronger escape ability (Drenner et al. 1978) from fish feeding and net-isolated polyculture restricted tilapia activity in net and reserved a space as refuge for zooplankton in the enclosures (Sun et al. 2010). Also, another possibility is that tilapia decreased phytoplankton, which is the main food of rotifers (while advanced Cyclopoid copepods are generally predators), so that the rotifer density was indirectly affected too.
Effects on growth and yield parameters of shrimp and tilapia
Tidwell et al. (2000) reported that polyculture of prawn and tilapia in cages increased total pond productivity by 81%. Similarly, Tian et al. (2001) demonstrated that survival and net yield of shrimp in a polyculture system were higher by 3–16% and 5–17% than that in the monoculture, respectively, probably due to the better water quality in the polyculture system. Wang et al. (1998) also found that the optimum stocking density of Chinese shrimp (0.6 g in size) and Taiwanese red tilapia (126.3 g in size) was 6 and 0.32 ind. m−2, respectively.
Compared to the above results, the presence of tilapia could improve growth of shrimp at early stage of culture in saline–alkaline pond, too. The survival and growth (individual weight gain and net yield) of shrimp was higher in T2. The net yield of T2 was higher by 58.5% than that in Control (monoculture) (Table 1). Therefore, a stocking biomass of 115 g m−2 of tilapia appeared to be the best in terms of shrimp as well as fish production for net-isolated polyculture system in saline-alkaline pond.
Possible mechanisms of effective phytoplankton controlling
Although the effectiveness of using tilapia as a tool for water quality management continues to be a subject of debate (Diana et al. 1991; Ruan et al. 1993; Danaher et al. 2007), our results in the enclosure experiment substantiate that net-isolated polyculture of tilapia in saline–alkaline shrimp pond is effective to control excessive phytoplankton.
Free-swimming fish can influence phytoplankton directly through nutrient regeneration and indirectly through impacts on zooplankton populations (Carpenter et al. 1992). However, few authors have paid much attention to potential bottom-up forces and have related their results to trophic cascade effects that sometimes may not be the main driving forces promoting phytoplankton growth. An important advantage of this type of polyculture is that it can avoid the bioturbation of pond sediments by tilapia.
In such polyculture system, tilapia may control phytoplankton through two paths. First, tilapia feed phytoplankton directly. Tilapias are selective plankton grazers and feed primarily on zooplankton and large size phytoplankton (Drenner et al. 1984; Vinyard et al. 1988). In the present experiment, the saline–alkaline shrimp pond was dominated by Cyanophyceae and Chlorophyceae, and Cyanophyceae was mainly represented by Spirulina spp. and colonial Microcystis spp. Hence, the presence of tilapia could regulate the large size and colonial phytoplankton biomass. Secondly, a top-down effect resulted from suppression of zooplankton grazing. Net-isolated polyculture of tilapia can thus have a strong impact on phytoplankton permitting the coexistence of large numbers of copepods with planktivorous fish by excluding the fish from most of the water column. Copepod, which feed on smaller phytoplankton, and tilapia, which feed on large and colonial phytoplankton, together can consume almost all sizes of phytoplankton, thus regulating phytoplankton biomass. The success of phytoplankton control by tilapia is probably largely dependent on fish stocking densities, plankton community structure and structure of the polyculture system.
Therefore, net-isolated polyculture of shrimp with tilapia may provide an alternative approach for shrimp farming in saline–alkaline pond, which could ultimately lead to a more sustainable shrimp industry. However, further research is needed on the merits of converting from monoculture to polyculture of shrimp for commercial scale.
References
Brooks J, Dodson S (1965) Predation, body size, and composition of plankton. Science 150:28–35
Burke JS, Bayne DR, Rea H (1986) Impact of silver and bighead carps on plankton communities of channel catfish pond. Aquaculture 55:59–68
Carpenter SR, Cottingham KL, Schindler DE (1992) Biotic feedbacks in lake phosphorus cycles. Trends Ecol Evol 7:332–336
Danaher JJ, Tidwell JH, Coyle SD et al (2007) Effects of two densities of caged monosex Nile tilapia, Oreochromis niloticus, on water quality, phytoplankton populations, and production when polycultured with macrobrachium rosenbergii in temperate ponds. J World Aquacult Soc 38:367–382
Datta S, Jana B (1998) Control of bloom in a tropical lake: grazing efficiency of some herbivorous fishes. J Fish Biol 53:12–24
Diana JS, Dettweiler DJ, Lin CK (1991) Effect of Nile tilapia (Oreochromis niloticus) on the ecosystem of aquaculture ponds, and its significance to the trophic cascade hypothesis. Can J Fish Aquat Sci 48:183–190
Drenner RW, Strickler JR, O’Brien WJ (1978) Capture probability: the role of zooplankter escape in the selective feeding of planktivorous fish. J Fish Res Board Can 35:1370–1373
Drenner R, Taylor SB, Lazzaro X et al (1984) Particle-grazing and plankton community impact of an omnivorous cichlid. T Am Fish Soc 113:397–402
Fukushima M, Takamura N, Sun L et al (1999) Changes in the plankton community following introduction of filter-feeding planktivorous fish. Freshwater Biol 42:719–735
Huang XF, Chen WM, Cai QM (1999) Survey, observation and analysis of lake ecology. Standards Press of China, Beijing (in Chinese)
Kajak Z, Rybak JI, Spodniewska I et al (1975) Influence of the planktonivorous fish Hypophthalmichthys molitrix (Val.) on the plankton and benthos of the eutrophic lake. Pol Arch Hydrobiol 22:301–303
Lazzaro X (1987) A review of planktivorous fishes: their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146:97–167
Li DS, Dong SL (2002) Summary of studies on closed-polyculture of penaeid shrimp with tilapia and molluscans. Oceanol Limnol Sin 33:90–96 (in Chinese, with English abstract)
Li DS, Yang HS, Wang JQ et al (1998) A device of land-based experimental enclosure used in ponds. Ocean Univ Qingdao J 28:199–204 (in Chinese, with English abstract)
Lu J, Li D, Dong S (1999) The impact of the polycultured filter-feeding animals with penaeid shrimp in plankton community. Ocean Univ Qingdao J 29:243–248 (in Chinese, with English abstract)
New MB (2005) Freshwater prawn farming: global status, recent research and a glance at the future. Aquac Res 36:210–230
Paerl HW, Tucker CS (1995) Ecology of blue-green algae in aquaculture ponds. J World Aquacult Soc 26:109–131
Perschbacher PW, Lorio WJ (1993) Filtration rates of catfish pond phytoplankton by Nile tilapia Oreochromis niloticus. J World Aquacult Soc 24:434
Ruan JR, Rong KW, Wang SM et al (1993) Effect of Nile tilapia on plankton community and primary productivity of freshwater microcosms. Chin J Appl Ecol 4:65–73 (in Chinese, with English abstract)
Shentu Q, Dong S, Zhao W et al (2000) Effects of salinity and alkalinity on plankton and water chemical factors. Chin J Appl Ecol 11:449–454 (in Chinese, with English abstract)
Starling F (1993) Control of eutrophication by silver carp (Hypophthalmichthys molitrix) in the tropical Paranoá (Brasília, Brazil): a mesocosm experiment. Hydrobiologia 257:143–152
Starling F, Rocha AJA (1990) Experimental study of the impacts of planktivorous fishes on plankton community and eutrophication of a tropical Brazilian reservoir. Hydrobiologia 200(201):581–591
Sun W, Dong S, Zhao X et al (2010) Effects of zooplankton refuge on the growth of tilapia (Orecohromis niloticus) and plankton dynamics in pond. Aquacult Int 18:647–655
Tian X, Li D, Dong S et al (2001) An experimental study on closed-polyculture of penaeid shrimp with tilapia and constricted tagelus. Aquaculture 202:57–71
Tidwell J, Coyle S, VanArnum A et al (2000) Growth, survival, and body composition of cage-cultured Nile tilapia Oreochromis niloticus fed pelleted and unpelleted distillers grains with solubles in polyculture with freshwater prawn Macrobrachium rosenbergii. J World Aquacult Soc 31:627–631
Turker H, Eversole AG, Brune DE (2003) Effect of temperature and phytoplankton concentration on Nile tilapia Oreochromis niloticus (L.) filtration rate. Aquac Res 34:453–460
Vanni MJ (1987) Effects of nutrients and zooplankton size on the structure of a phytoplankton community. Ecology 68:624–635
Vinyard GL, Drenner RW, Gophen M et al (1988) An experimental study of the plankton community impacts of two omnivorous filter-feeding cichlids, Tilapia galilaea and Tilapia aurea. Can J Fish Aquat Sci 45:685–690
Wang JQ, Li D, Dong S et al (1998) Experimental studies on polyculture in closed shrimp ponds: I. Intensive polyculture of Chinese shrimp (Penaeus chinensis) with tilapia hybrids. Aquaculture 163:11–27
Xie P, Liu J (2001) Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: a synthesis of decades of research and application in a subtropical hypereutrophic lake. Sci World J 1:337
Zhao W, Dong S, Zheng W et al (2000) Effects of Nile tilapia on plankton in enclosures with different treatments in saline-alkaline ponds. Zool Res 21:108–114 (in Chinese, with English abstract)
Zhao W, Dong S, Zhang M et al (2001) Species composition and biomass of zooplankton in saline alkaline ponds. J Fish China 25:27–32 (in Chinese, with English abstract)
Zhao W, Dong S, Shentu Q et al (2002) Studies on the species composition, biomass and species diversity of phytoplankton in saline-alkaline ponds with chloride typed. Acta Hydrobiolo Sin 26:31–38 (in Chinese, with English abstract)
Acknowledgments
This study was supported by the National Key Technologies R&D Program (2004BA526B0402) and the Chinese National Agricultural Development Project (Grant no. K2002-15). We would like to thank Prof. Xiulan Yang of Yantai University for her help in specimen identification of phytoplankton.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, W., Dong, S., Jie, Z. et al. The impact of net-isolated polyculture of tilapia (Oreochromis niloticus) on plankton community in saline–alkaline pond of shrimp (Penaeus vannamei). Aquacult Int 19, 779–788 (2011). https://doi.org/10.1007/s10499-010-9394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9394-8