Abstract
The introduction of omnivorous tilapia into a variety of aquatic systems worldwide has led to a number of serious ecological problems. One of the main issues is an increase in water turbidity, which affects not only light penetration but also primary production and the distribution of phytoplankton and benthic algae in shallow lakes. These changes cause deterioration of water quality in these lakes. A 12-week mesocosm experiment was set up to test the hypotheses that omnivorous Nile tilapia (Oreochromis niloticus) introduced to a shallow water system will increase turbidity and nutrient levels in the water column and thereby boost growth of phytoplankton and depress benthic algae. Relative to the control treatments, the presence of tilapia led to higher concentrations of total nitrogen and total phosphorus in the water column, greater biomass of phytoplankton as measured by chlorophyll a (Chl a), greater concentrations of total suspended solids and inorganic suspended solids, lower light intensity, and lower biomass of benthic algae at the sediment surface. A tube-microcosm experiment using 32P radio tracer indicated that the presence of tilapia accelerated the release of sediment phosphorus (P) into the water column. We conclude that these invasive omnivorous fish not only stimulate growth of phytoplankton in shallow lakes by increasing nutrients in water column, but also depress benthic algal growth by promoting sediment resuspension, leading to increased turbidity of the water. Thus the removal of tilapia could be a useful practice for managers of shallow aquatic ecosystems, promoting benthic primary production and improving water clarity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Nile tilapia (Oreochromis niloticus) is a fast-growing and invasive fish native to Africa, and one of several related species that have been introduced into wild aquatic systems in more than 100 tropical and subtropical areas, including Australia and parts of Asia, Europe and the Americas (Lèveque 2002). The species has a highly omnivorous diet, incorporating phytoplankton, zooplankton, aquatic insects, submersed plants, benthic fauna, detritus and bacterial biofilms, and impacts heavily on the biodiversity, habitats and water turbidity of invaded waters, leading to serious ecological problems (Parker et al. 1999).
Our objective is to better understand the impacts of Nile tilapia on the water quality and nutrient dynamics in shallow lakes. Water turbidity affects both the structure and functions of lake ecosystems, not least by limiting the availability of light at the sediment surface, and thereby strongly influencing benthic production. In clear water systems, light penetrates into deeper layers and benthic algae may account for more than 90% of primary productivity and contribute substantially to whole-lake production (Loeb et al. 1983). In eutrophic systems, overabundant phytoplankton increase turbidity of the water column. Phytoplankton blooms reduce light penetration to deeper water and the sediment surface (Hansson 1992; Havens et al. 2001), limiting the growth of benthic algae. For example, in the shallow Danish lakes studied by Liboriussen and Jeppesen (2003), primary production was almost completely dominated by phytoplankton. Based on known bioturbation effects of tilapia activity, we hypothesize that pelagic primary production will increase, while benthic primary production will decrease in the presence of the species. Although seemingly counteractive, both trends contribute to water quality deterioration in shallow lake systems. The problem of eutrophication in such systems is often attributable to external nutrient sources such as from municipal water treatment facilities or agricultural runoff in watersheds (Gulati and Van Donk 2002), but the potential for introduced fish species to cause to similar impairments is an important consideration for lake managers.
The presence of different functional groups of fish can have a significant influence on water quality (Schindler and Scheuerell 2002; Zhang et al. 2016). Zooplanktivorous fish are considered to promote phytoplankton in shallow water through their predation on herbivorous zooplankton (especially Daphnia spp.). A reduction in Daphnia numbers releases phytoplankton from top-down control, leading to increased turbidity (Carpenter et al. 1985; Scheffer et al. 1993). In addition, such fish contribute considerably to the resuspension of bottom sediments through their foraging activity (Persson 1997). The presence of piscivorous fish is thought to be important in regulating numbers of zooplanktivorous fish, releasing zooplankton from predation and allowing them in turn to control pelagic seston, including phytoplankton (Carpenter et al. 1985; Scheffer et al. 1993) and thereby promote clear water conditions. Benthivorous fish such as common carp are particularly proficient at resuspending particulate material as they forage in bottom sediments and defecate undigested food items (Lammens 1991; Zhang et al. 2016). Some benthivorous fish penetrate up to 12 cm into the bottom substrate while foraging (Panek 1987), disturbing not only surface sediment layers but also the deeper underlying material. The abundance of fine particles resuspended by such fish can severely increase the muddy brown turbidity of lake water (Havens 1991). Thus, the presence of different functional groups of fish in both benthic and pelagic habitats can have a significant influence on water quality (Schindler and Scheuerell 2002; Zhang et al. 2016; Yi et al. 2016).
Within extensive literature documenting the effects of fish on ecosystem processes (Havens 1991; Milstein et al. 2006), few studies have simultaneously investigated the effect of omnivorous fish on water turbidity and primary production dynamics in shallow lakes. In the present mesocosm experiment, we evaluated the effect of Nile tilapia on water turbidity and the dynamics of phytoplankton and benthic algal production in a shallow lake. A further tube microcosm experiment using 32P-PO4 as a tracer sought to evaluate the effect of the tilapia on sediment P release. The results obtained help resolve the role of tilapia in influencing water turbidity and the primary production dynamics of phytoplankton and benthic algae.
Materials and methods
Effects of tilapia on water turbidity, phytoplankton and benthic algae in mesocosms
Eight mesocosms were established according to Zhang et al. (2014). Each comprised a circular, somewhat conical, plastic tank (upper diameter = 54 cm, bottom diameter = 40 cm, height = 60 cm). Each mesocosm contained a bed of natural sediment and water above. Sediment obtained from Ming Lake, a eutrophic shallow water body in Guangzhou City, China, was air dried, powdered and sieved through a stainless 0.5 mm mesh to remove coarse grains, debris and clumps. The homogenized sediment [total nitrogen (TN) = 1.13 mg g−1; total phosphorus (TP) = 0.56 mg g−1] added to each mesocosm formed a layer ~10 cm thick, after which the tank was filled with filtered (mesh size 0.064 mm) lake water (100 L, TN = 1.67 mg L−1, TP = 0.04 mg L−1), then exposed to natural sunlight and ambient temperatures for 2 weeks.
After the acclimatization period, TN in the mesocosms had declined slightly to 1.58 mg L−1 and TP had increased to 0.06 mg L−1, mostly because of release from the sediment. A petri dish (diameter 5 cm) filled entirely with homogenized sediment was inserted into the bed of each mesocosm such that the sediment surfaces inside and outside the petri dish were level, to allow benthic algal colonization and determination of algal biomass. Tilapias (Oreochromis niloticus) were collected from Ming Lake. The fish were maintained in 100-L tanks for two weeks prior to their introduction to the mesocosms in Jinan University, Guangzhou. One tilapia (11.8 ± 0.2 cm, weighing 28.6 ± 0.8 g per individual fish) was added to each of the four mesocosms for tilapia treatments. One individual that died in the course of the experiment was replaced with a new specimen. The four control mesocosms contained no fish. Nitrogen as KNO3 and phosphorus as NaH2PO4 were added weekly at rates of 1.5 mgN L−1 wk−1 and 0.1 mgP L−1 wk−1 to each mesocosm. During the experiment, any drop in water levels due to evaporation and sampling was compensated by the addition of rain water (TN = 1.95 mg L−1, TP = 0.02 mg L−1). The experiment was run from April 20 to July 26 2014, during which time the mesocosms continued to be exposed to natural sunlight.
Water was sampled (1 L) every two weeks from each mesocosm for analysis of total suspended solids (TSS), inorganic suspended solids (ISS), phytoplankton biomass (Chl a), TN and TP. Concentrations of both nutrients were determined according to APHA (1998). 200 ml water was filtered by GF/C grade filter and the Chl a on the filter was determined spectrophotometrically after ethanol extraction at room temperature according to the method of Jespersen and Christoffersen (1987). TSS and ISS were determined as residual matter from 500 ml retained on GF/C grade filters. The filters were dried at 105 °C for 24 h to calculate TSS then incinerated at 550 °C for 2 h for ISS.
Light intensity at the sediment surface was measured every two weeks between 9 and 12 am, after sampling of phytoplankton, using an underwater irradiance meter (ZDS-10W). The sediment-filled petri dishes were removed from the mesocosms and replaced by fresh dishes filled with homogenized sediment. Benthic algae were collected from the removed samples by scraping the surface of the sediment with a razor blade (Barbour et al. 1999) and their biomass (Chl a) was measured by spectrophotometry, as described for phytoplankton. After the collection of water and benthic algae samples, nutrients were added to each mesocosm.
Effects of fish on sediment P release
Microcosms were established as described in Zhang et al. (2014). Eight sediment cores incorporating 10 cm sediment and overlying water were collected from Ming Lake using perspex tubes (40 cm in length, 12.6 cm internal diameter). The core tubes were sealed at the top and bottom with silicone rubber stoppers to preserve sediment structure during transportation to the laboratory at Jinan University in Guangzhou. A 5-L sample of surface lake water was also collected from Ming Lake. All samples arrived in the lab within an hour of collection and the top rubber stoppers were removed immediately to allow gas exchange with air. Prior to the start of experiment, water overlying the sediment in each tube (microcosm) was siphoned off and the depth of the core samples was adjusted to a standard upper 10 cm by removing sediment below from the bottom of the tubes. A plastic tube (1.0 cm internal diameter) was inserted 1.0 cm deep into the centre of the sediment core. Then it was retracted, with the top of the plastic tube sealed with a thumb, resulting in the removal of a sediment plug and the creation of a round hole. 5 μCi NaH 322 PO4 (Perkin Elmer, Inc. USA) was added to each hole before the sediment in the plastic tube was replaced. 2500 mL lake water was added to each of the eight microcosm tubes. One tilapia (5.1 ± 0.2 cm) was placed in each of four tubes, while the other four served as fish-free controls. Thereafter, 1.0 ml water was sampled from the center of the water column after 24, 36, 48, 60, 96 and144 h to determine 32P activity (Zhang et al. 2013). The experiment was run at room temperature and ambient room light intensity (15.8 ± 9.4 μmol photons m−2 s−1 in the daytime and dark at night), with a dark:light cycle of 12:12 h.
To measure 32P activity in the water column, at each sampling 1.0 ml water was collected from middle of each tube and transferred to a 10 ml scintillation vial containing 10 ml of scintillation cocktail (5.0 g 2,5-diphenyloxazole + 0.5 g 1,4-bis (5-phenyloxazol-2-yl) benzene + 1000 mL dimethylbenzene + 400 g tritonX-100; (Zhang et al. 2013, 2014). 32P activity was recorded for 1 min and expressed as dpm mL−1 (Hansson 1988) using a liquid scintillation counter (Beckman Model LS6500, Beckman Coulter, Inc., Fullerton, CA). The measured activity was corrected for loss due to the standard 32P decay provided by Perkin Elmer, Inc. USA.
Statistical analyses
The effects of tilapia presence on nutrient concentrations, phytoplankton and benthic algal biomass, TSS, ISS, light intensity and the 32P activity of water were determined using repeated measures analyses of variance (RM-ANOVAs), with time as the repeated factor. If a significant difference was found, a Least Significant Difference (LSD) test was used to detect which treatments differed. One-way ANOVA was performed to detect differences among treatments on each sampling occasion. If a significant difference emerged, LSD test was used to detect the differing treatments. All statistical analyses were conducted using SPSS 16.0 software. Data are presented as mean ± SD.
Results
Nutrients of TN and TP, and phytoplankton in mesocosms
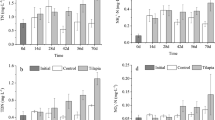
Values for both nutrients of TN and TP and Chl a of phytoplankton (Fig. 1) were higher in mesocosms with tilapia than in the controls (RM-ANOVAs, treatment effect, p < 0.05). In addition, TP and Chl a of phytoplankton varied significantly over time (RM-ANOVAs, time effect, p < 0.05), while TN did not (p > 0.05), though significant differences were detected between 14 and 28 d and between 14 and 42 d for TN (p < 0.05). Values for both nutrients of TN and TP and Chl a of phytoplankton were higher in the tilapia treatments than in the controls on every sampling occasion, except for a lower TN on day 14 (one-way ANOVA, treatment effect, p < 0.05).
Total nitrogen (TN, mean ± SD), total phosphorus (TP, mean ± SD) and Chl a of phytoplankton (mean ± SD) in different treatments over time. Asterisk indicates significant (p < 0.05) differences between treatments and controls. Note on day 14, TN was lower in the tilapia treatment than in the controls (upper panel)
TSS, ISS and light intensity in mesocosms
TSS and ISS (Fig. 2) were higher in the tilapia treatments than in the controls (RM-ANOVAs, treatment effect, p < 0.05). Overall, the values changed significantly with time (RM-ANOVAs, time effect, p < 0.05). These values were also higher in the tilapia treatments than in the controls on every sampling occasion except for TSS at 56 d (one-way ANOVA, treatment effect, p < 0.05).
In contrast, light intensity at the sediment surface in mesocosms was lower in the tilapia treatments than in the controls (RM-ANOVAs, treatment effect, p < 0.05) and changed significantly with time due to adverse weather (RM-ANOVAs, time effect, p < 0.05). In the tilapia treatments, the light intensity recorded in each sampling event decreased throughout the experiment relative to that in the controls (one-way ANOVA, treatment effect, p < 0.05; Fig. 2).
Benthic algae in mesocosms
The Chl a levels recorded in benthic algae samples were lower in the tilapia treatments than in the controls (RM-ANOVAs, treatment effect, p < 0.05). Chl a values also varied significantly with time (RM-ANOVAs, time effect, p < 0.05), being lower on day 28, day 42 and day 84 in the tilapia treatments than in the controls (one-way ANOVA, treatment effect, p < 0.05; Fig. 3).
Effects of tilapia on sediment P release
32P activities in the water were elevated in the tilapia treatments compared with the controls (RM-ANOVAs, treatment effect, p < 0.05), and increased significantly over time (RM-ANOVAs, time effect, p < 0.05). Analyses of the effects of tilapia at each sampling event revealed that the 32P increased in the water from tilapia treatments relative to the controls at 36 h and 60 h (one-way ANOVA, treatment effect, p < 0.05; Fig. 4).
Discussion
Our data suggest that stocking of water bodies with tilapia can result in a measurable increase in phytoplankton Chl a and a concomitant deterioration in water clarity. Furthermore, our study reveals that biomass of benthic algae decreases in the presence of tilapia, as a consequence of grazing and of light limitation due to increased turbidity of water. Thus, tilapia populations contribute to marked decreases of water clarity and water quality in general.
Fish can directly and indirectly mobilize nutrients through multiple pathways: by depositing feces, excreting dissolved nutrients and stimulating the release of sediment nutrients into the water column (Diana et al. 1991; Carpenter et al. 1992; Vanni 1996). Higher 32P values in the microcosms with tilapia indicate that the swimming and foraging activities of the fish enhance the transport of P from sediment to the water above. These pathways are known to stimulate the production of phytoplankton (Elser and Urabe 1999; Adámek and Maršálek 2013). Our study demonstrates an overall increase in water of TN and TP concentrations in the presence of fish, as well as an increase in the phytoplankton biomass. The result is acceleration in eutrophication, i.e. through an increase in phytoplankton concentration.
In addition to increasing the concentration of phytoplankton, our study also shows that tilapia increase levels of both ISS and TSS. The increases are likely due to the resuspension of particles. Since tilapias forage actively for benthic fauna, detritus and bacterial biofilms, the effect is particularly apparent. Increased resuspension leads to high TSS and ISS in the water column, turning the water muddy brown and increasing light attenuation. If light penetration is severely reduced, it may become a limiting factor for benthic algae, leading to poor growth and decreasing abundance (Zhang et al. 2015). Because benthic algae are an important food source for tilapia, their grazing will also impact directly on biomass (van Dam et al. 2002). Thus, the loss of benthic algae further promotes growth conditions for phytoplankton, simultaneously enhancing nutrient release from the sediment and reducing competition for these nutrients (Zhang et al. 2013). Furthermore, because benthic algae can release extracellular material that acts as an adhesive to stabilize the sediment surface and reduce sediment and nutrient resuspension, the loss of biomass may reduce benthic resilience to fish bioturbation (Lubarsky et al. 2010).
In our study, the light intensity at the sediment surface in mesocosms with fish was lower than in the controls. The presence of tilapia also led to a decline in the biomass of benthic algae. We therefore conclude that tilapia enhance water turbidity both by increasing nutrient concentrations in water column and by resuspending particles from the sediment, resulting in a higher biomass of phytoplankton and higher concentrations of TSS and ISS, while simultaneously reducing growth of benthic algae and negatively affecting water quality. These conditions are reminiscent of the eutrophy that invariably prevails in shallow lakes with similar omnivorous fish.
In contrast to the situation as described above, when light intensity reaching the sediment surface is adequate, growth of benthic algae can substantially decrease the availability and transfer of nutrients from sediments to phytoplankton in the water column above (Dodds 2003). Such conditions limit the growth of phytoplankton (Genkai-Kato et al. 2012) and improve water clarity. Due to directly sequestering P from sediment interstitial water, benthic algae can reduce the availability of sediment nutrients to phytoplankton (Hansson 1988, 1989). In addition, by oxidizing the topmost sediment layer (the algal colonization zone) via photosynthetic activity, benthic algae promote conversion of Fe2+ ions to Fe3+ ions and the subsequent formation of insoluble compounds with phosphate ions in sediments. This leads in turn to a reduction in the release of P to the water column (Dodds 2003). Also, benthic algae can stabilize sediment surfaces by releasing extracellular material that acts as glue, further inhibiting resuspension and the release of sediment P (Spears et al. 2008; Tolhurst et al. 2008). Thus, growth of benthic algae can help maintaining a clear-water state and improve water clarity (Genkai-Kato et al. 2012; Zhang et al. 2014).
There is some experimental evidence to suggest that tilapia can help improve water quality by feeding on phytoplankton (Datta and Jana 1998; Turker et al. 2003; Lu et al. 2006; Torres et al. 2016), but whether this would hold under natural lake conditions is controversial (Menezes et al. 2010). Although tilapia can affect phytoplankton abundance by grazing, there are reports that they are unable to filter or graze efficiently on small phytoplankton (Figueredo and Giani 2005). In contrast, we know that tilapia can and do predate heavily on large Daphnia, reducing the grazing pressure of daphnids on algae to such an extent that algal numbers are likely to increase even when they continue to be consumed by tilapia (Vanni 2002).
Tilapia densities in some aquatic ecosystems can be very high, reaching 390–810 g m−2 (Suresh and Lin 1992). In Huizhou West lake, the density of tilapia is 160 g m−2 (Liu et al. 2014). We used a more realistic density for shallow lakes of 124.9 ± 3.5 g m2 as a guide for our study. The tilapias used in the tube-microcosm experiment were smaller (5.1 ± 0.2 cm) than those in the mesocosm (11.8 ± 0.2 cm) because a smaller size is more appropriate for the tracer study. However, since Nile tilapia have similar feeding behavior in the size range 5–12 cm, this difference was not considered a major factor.
Tilapias of this and other species have been widely introduced around the world and are present in all continents, except Antarctica, due to their ability to tolerate a broad range of environmental conditions, their fast growth, successful reproductive strategies, and ability to feed omnivorously at different trophic levels (Zambrano et al. 2006). As the presence or absence of a single species can dramatically alter the ecological processes of an ecosystem (Covich et al. 1999), it seems reasonable to assume that colonization by or removal of tilapia will significantly impact on the ecological processes and functioning of shallow lake systems (Casal 2006). Our study shows that omnivorous fish such as tilapia not only stimulate growth of phytoplankton, but also promote turbidity of water by resuspending sediment and reducing benthic algal biomass. The result was water quality deterioration. From a management point of view, removal of tilapia from invaded shallow lakes, especially in tropical and subtropical areas where the species can reproduce prolifically, may promote benthic primary production and reduce water turbidity. These changes in turn will accelerate the establishment of a clear-water state, thereby improving water clarity and quality.
The removal approaches can be mechanical, biological or chemical. Mechanical removal techniques such as netting, electro fishing and controlled angling are time-consuming and not considered as a cost-effective measure. In contrast, biological approaches such as stocking with piscivorous fish is the most frequently used ecological method and has proved successful in small shallow lakes (Jeppesen et al. 2012). Alternatively, a piscicide such as rotenone can be used to control Nile tilapia populations. However, such action raises ethical considerations as rotenone affects all fish species as well as invertebrates, and legal permission may be difficult to obtain (Jeppesen et al. 2012). Although controlling Nile tilapia is thought to be difficult (Leung et al. 2002), there are a few cases in which removal of tilapia from invaded ecosystems has been successful (GISD 2012).
Conclusions
Invasive omnivorous fish such as tilapia not only stimulate growth of phytoplankton in shallow lakes by increasing nutrients in water column, but also depress benthic algal growth by promoting sediment resuspension leading to increased turbidity of the water, deteriorating water quality. Removal of tilapia could be a useful practice for managers of shallow aquatic ecosystems in tropical and subtropical areas, promoting benthic primary production and improving water clarity.
References
Adámek Z, Maršálek B (2013) Bioturbation of sediments by benthic macroinvertebrates and fish and its implication for pond ecosystems: a review. Aquacult Int 21:1–17
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers. USEPA, Washington
Carpenter SR, Kitchell JF, Hodgson JR (1985) Cascading trophic interactions and lake productivity. Bioscience 35:634–639
Carpenter SR, Cottingham KL, Schindler DE (1992) Biotic feedbacks in lake phosphorus cycles. Trends Ecol Evol 7:332–336
Casal CMV (2006) Global documentation of fish introductions: the growing crisis and recommendations for action. Biol Invasions 8:3–11
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49:119–127
Datta S, Jana BB (1998) Control of bloom in a tropical lake: grazing efficiency of some herbivorous fishes. J Fish Biol 53:12–24
Diana JS, Dettweiler DJ, Lin CK (1991) Effect of Nile tilapia (Oreochromis niloticus) on the ecosystem of aquaculture ponds, and its significance to the trophic cascade hypothesis. Can J Fish Aquat Sci 48:183–190
Dodds WK (2003) The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. J Phycol 39:840–849
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Figueredo CC, Giani A (2005) Ecological interactions between Nile Tilapia (Oreochromis niloticus, L.) and phytoplanktonic community of the Furnas Reservoir (Brazil). Freshw Biol 50:1391–1403
Genkai-Kato M, Vadeboncoeur Y, Liboriussen L, Jeppesen E (2012) Benthic-planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology 93:619–631
GISD (2012) Global invasive species database—Oreochromis niloticus. Available from: http://www.issg.org/database/species/ecology.asp?si=1322&fr=1&sts=sss&lang=EN
Gulati RD, Van Donk E (2002) Lakes in the Netherlands, their origin, eutrophication and restoration: state-of-the-art review. Hydrobiologia 478:73–106
Hansson LA (1988) Effects of competitive interactions on the biomass development of planktonic and periphytic algae in lakes. Limnol Oceanogr 33:121–128
Hansson LA (1989) The influence of a periphytic biolayer on phosphorus exchange between substrate and water. Arch Hydrobiol 115:21–26
Hansson LA (1992) Factors regulating periphytic algal biomass. Limnol Oceanogr 37:322–328
Havens KE (1991) Fish-induced sediment resuspension: effects on phytoplankton biomass and community structure in a shallow hypereutrophic lake. J Plankton Res 13:1163–1176
Havens KE, Hauxwell J, Tyler AC, Thomas S, McGlathery KJ, Cebrian J, Valiela I, Steinman AD, Hwang SJ (2001) Complex interactions between autotrophs in shallow marine and freshwater ecosystems: implications for community responses to nutrient stress. Environ Pollut 113:95–107
Jeppesen E, Søndergaard M, Lauridsen TL, Davidson TA, Liu ZW, Mazzeo N, Trochine C, Özkan K, Jensen HS, Trolle D, Starling F, Lazzaro X, Johansson LS, Bjerring R, Liboriussen L, Larsen SE, Landkildehus F, Egemose S, Meerhoff M (2012) Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Adv Ecol Res 47:411–488
Jespersen AM, Christoffersen K (1987) Measurements of chlorophyll a from phytoplankton using ethanol as extraction solvent. Arch Hydrobiol 109:445–454
Lammens EHRR (1991) Diets and feeding behaviour. In: Winfield IJ, Nelson JS (eds) Cyprinid fishes: systematics, biology, and exploitation. Chapman and Hall, London, pp 127–155
Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G (2002) An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc R Soc Lond B 269:2407–2413
Lèveque C (2002) Out of Africa: the success story of tilapias. Environ Biol Fish 64:461–464
Liboriussen L, Jeppesen E (2003) Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshw Biol 48:418–431
Liu Z, Zhong P, Zhang X, Ning J, Larsen SE, Jeppesen E (2014) Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded (submitted)
Loeb SL, Reuter JE, Goldman CR (1983) Littoral zone production of oligotrophic lakes. Periphyton of freshwater ecosystems. Springer, Netherlands, pp 161–167
Lu K, Jin C, Dong S, Gu B, Bowen SH (2006) Feeding and control of blue-green algal blooms by tilapia (Oreochromis niloticus). Hydrobiologia 56:111–120
Lubarsky HV, Hubas C, Chocholek M, Larson F, Manz W, Paterson DM, Gerbersdorf SU (2010) The stabilization potential of individual and mixed assemblages of natural bacteria and microalgae. PLoS ONE 5:e13794
Menezes RF, Attayde JL, Vasconcelos RF (2010) Effects of omnivorous filter-feeding fish and nutrient enrichment on the plankton community and water transparency of a tropical reservoir. Freshw Biol 55:767–779
Milstein A, Ahmed AF, Masud OA, Kadir A, Wahab MA (2006) Effects of the filter feeder silver carp and the bottom feeders mrigal and common carp on small indigenous fish species (SIS) and pond ecology. Aquaculture 258:439–451
Panek FM (1987) Biology and ecology of carp. In: Cooper EL (ed) Carp in North America. American Fisheries Society, Bethesda, pp 1–15
Parker IM, Simberloff D, Lonsdale WM et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Persson A (1997) Phosphorus release by fish in relation to external and internal load in a eutrophic lake. Limnol Oceanogr 42:577–583
Scheffer M, Hosper SH, Meijer M-L, Moss B, Jeppesen E (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279
Schindler DE, Scheuerell MD (2002) Habitat coupling in lake ecosystems. Oikos 98:177–189
Spears BM, Carvalho L, Perkins R, Paterson DM (2008) Effects of light on sediment nutrient flux and water column nutrient stoichiometry in a shallow lake. Water Res 42:977–986
Suresh AV, Lin CK (1992) Tilapia culture in saline waters: a review. Aquaculture 106:201–226
Tolhurst TJ, Consalvey M, Paterson DM (2008) Changes in cohesive sediment properties associated with the growth of a diatom biofilm. Hydrobiologia 596:225–239
Torres GS, Silva LS, Rangel LM, Attayde JL, Huszar VLM (2016) Cyanobacteria are controlled by omnivorous filter-feeding fish (Nile tilapia) in a tropical eutrophic reservoir. Hydrobiologia 765:115–129
Turker H, Eversole AG, Brune DE (2003) Filtration of green algae and cyanobacteria by Nile tilapia, Oreochromis niloticus, in the Partitioned Aquaculture System. Aquaculture 215:93–101
van Dam AA, Beveridge MC, Azim ME, Verdegem MC (2002) The potential of fish production based on periphyton. Rev Fish Biol Fisheries 12:1–31
Vanni MJ (1996) Nutrient transport and recycling by consumers in lake food webs: implications for algal communities. In: Polis GA, Winemiller KO (eds) Food webs: Integration of patterns and dynamics. Chapman and Hall, New York, pp 81–95
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Yi C, Guo L, Ni L, Luo C (2016) Silver carp exhibited an enhanced ability of biomanipulation tocontrol cyanobacteria bloom compared to bighead carp inhypereutrophic Lake Taihu mesocosms. Ecol Eng 89:7–13
Zambrano L, Martinez-Meyer E, Menezes N, Peterson AT (2006) Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can J Fish Aquat Sci 63:1903–1910
Zhang XF, Liu ZW, Gulati RD, Jeppesen E (2013) The effect of benthic algae on phosphorus exchange between sediment and overlying water in shallow lakes: a microcosm study using 32P as tracer. Hydrobiologia 710:109–116
Zhang XF, Liu ZW, Jeppesen E, Taylor WD (2014) Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic–pelagic coupling: implications for the restoration of eutrophic shallow lakes. Water Res 50:135–146
Zhang XF, Mei XY, Gulati RD, Liu ZW (2015) Effects of N and P enrichment on competition between phytoplankton and benthic algae in shallow lakes: a mesocosm study. Environ Sci Pollut Res 22:4418–4424
Zhang XF, Liu ZW, Jeppesen E, Taylor WD, Rudstam LG (2016) Effects of benthic-feeding common carp and filter-feeding silver carp on benthic-pelagic coupling: implications for shallow lake management. Ecol Eng 88:256–264
Acknowledgements
We thank Professor Lars G. Rudstam in Cornell University for his comments and the comments of editor and anonymous reviewers substantially improved the paper. The authors are also grateful to Dr. Ken Chan for improving the English of this manuscript. This study was sponsored by the National Natural Science Foundation of China (No: 31570456) and Provincial Natural Science Foundation of Anhui (No. 1608085MD85) and Guangdong (No. 2016A030313103). It was completed while the senior author was a visiting scientist at Cornell University, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Mei, X. & Gulati, R.D. Effects of omnivorous tilapia on water turbidity and primary production dynamics in shallow lakes: implications for ecosystem management. Rev Fish Biol Fisheries 27, 245–254 (2017). https://doi.org/10.1007/s11160-016-9458-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-016-9458-6