Abstract
Trophic status in surface waters has been mostly monitored by measuring soluble reactive phosphorus (SRP) and total phosphorus (TP). Additional to these common parameters, a two-dimensional ion chromatography mass spectrometry (2D-IC-MS) method was used to simultaneously measure soluble phosphate (Pi), pyrophosphate (PPi), and eleven phosphate-containing metabolites (P-metabolites) in Lake Ontario and its tributaries. From the additional P species, PPi, adenosine 5′-monophosphate (AMP), glucose 6-phosphate (G-P), D-fructose 6-phosphate (F-P), D-fructose 1,6-biphosphate (F-2P), D-ribulose 5-phosphate (R-P), D-ribulose 1,5-bisphosphate (R-2P), and D-(-)-3-phosphoglyceric acid (PGA) were detected and quantified in the lake and river samples. The additional multivariate statistical analysis identified similarities between samples collected at different locations. The presence of R-P, R-2P, and F-2P in Lake Ontario tributaries seems to be mainly related to the Calvin cycle, while the lack of all these three P-metabolites and higher PGA levels than G-P in Toronto Harbour samples seems to be the result of depleted Calvin cycle, pentose phosphate, and glycolysis metabolic pathways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human development of the watersheds to Lake Ontario, the most downstream of the Laurentian Great Lakes, has contributed to the stresses on water quality which continue to impact environmental conditions in the rivers and the adjacent coastal areas of the lake. Alteration of river channels and shoreline, clearing of land, and population growth have resulted in ongoing levels of non-point nutrient runoff and loading from wastewater treatment plants (Makarewicz et al. 2012) that impact water quality. Consequently, concentrations of phosphorus and nitrogen-based compounds increased at nearshore locations of Lake Ontario and its tributaries. Eutrophication and nuisance algal concerns due to nutrient enrichment pose a threat to freshwater quality in Lake Ontario as they do globally.
In surface waters, the most biologically active form of phosphorus (P) species is the phosphate ion, Pi (4500-P Phosphorus 2017; Cade-Menun et al. 2006; Garrett et al. 2013). Pi is easily transferred through cell membrane and it can be involved in wide-ranging chemical and biological pathways in the aquatic environment (Garrett et al. 2013; Packa et al. 2019a). It can precipitate in the presence of Ca2+ or Fe3+ and can be incorporated into a host of inorganic and organic complexes (Boujelben et al. 2008; Lin et al. 2020; Packa et al. 2019a). Upon biological uptake, it is a critical component of diverse and multi-function metabolic pathways of fish and algae (Feng et al. 2016b; Polakof et al. 2012), producing a wide range of phosphate-containing metabolites (P-metabolites). In addition to the naturally produced P species loaded into lakes or created within the aquatic environment, anthropogenic sourced P species such as pyrophosphate (PPi) may be detected at higher levels in developed areas (Correll 1998). For theoretical and practical reasons, most of the P species analyzed in lakes are Pi and total phosphorus (TP) (Schindler et al. 2016).
Measurement of additional P species may provide information that can be used to further assess biological availability of phosphorus and potentially infer the operation of biological pathways for example helping to interpret the dynamics of algal blooms, particularly harmful algal blooms (HABs) (Baldwin 2013; Baykov et al. 2017; Cooper et al. 2005; Saiardi 2012; Van Moorleghem et al. 2013; Zhao and Liu 2013). Knowledge of the transitions among inorganic and organic P species over limnologically important events such as spring diatom blooms and river to lake discharge events may potentially provide new insight on how physical and biological processes in the aquatic environment influence the availability of phosphorus. Studies characterizing P species in algae and aquatic macrophytes using 31NMR-based techniques have shown that organic-P compounds can account for up to 80% of TP. Organic P species identified in algal biomass extracts included P monoesters and diesters (Feng et al. 2019). In addition to directly bioavailable inorganic phosphate, pyrophosphate and up to 40 % of the organic phosphate from decaying algal biomass can be hydrolyzed to inorganic phosphate and released to water column supporting further growth of aquatic organisms and potentially augmenting algal blooms (Feng et al. 2016a; Wenqiang et al. 2015). Considering the differences in physical and biological conditions between rivers and lakes, and possibly differences in indirect pollutant loadings, it is possible that contrasts in the distribution of P species between rivers, lake area receiving river loading and broader lake may be used to detect and assist in gauging the extent to which the river loading represents P species that are able to support algal or microbial growth.
In this study we examine the concentrations of eleven P-metabolites as well as PPi, Pi, and TP in water samples collected in urban rivers and the receiving waters of Lake Ontario. The P-metabolites considered are as follows: adenosine 5′-monophosphate (AMP), D-fructose 6-phosphate (F-P), D-fructose 1,6-biphosphate (F-2P), D-glucose 6-phosphate (G-P), α-D-glucose 1,6-biphosphate (G-2P), α-D-galactose 1-phosphate (Gal-P), D-ribulose 5-phosphate (R-P), D-ribulose 1,5 – bisphosphate (R-2P), D-erythrose 4-phosphate (E-P), D-(-)-3-phosphoglyceric acid (PGA), and uridine 5′- diphosphoglucose (UDPG). These P-metabolites were chosen for specific roles in the metabolic activity of the organisms, abundance in lake water, and biological relevance in lake ecology found in previous NMR studies (Cade-Menun et al. 2006; Feng et al. 2019; Feng et al. 2016a; Feng et al. 2016b; Worsfold et al. 2008; Zhang et al. 2014) and can be grouped in several categories based on their occurrences in cell metabolism. AMP, ADP, and ATP are ubiquitous compounds contributing to many anabolic and catabolic processes, functioning as energy carriers (ATP), signal molecules, and building blocks for a variety of complex organic compounds. UDPG is a nucleotide sugar found in all groups of organisms; F-P, F-2P, G-P, G-2P, and PGA are intermediate products of the glycolysis metabolic pathway. R-P, R-2P, and E-P are intermediate steps in the Calvin cycle of photosynthetic organisms and in the anabolic pentose phosphate pathway. All these compounds are abundant in living cells and can leak into the environment from cell lysis. Stability of these P-metabolites in freshwater environments will vary depending on their structure, but previous studies showed that at least phosphoric anhydrides, such as the phosphate bonds in ATP molecules, can persist in aquatic environments, as they are protected by their negative charges (Westheimer 1987).
The distributions of P-species in Toronto Harbour and Toronto-area rivers discharging to Lake Ontario are compared, to assess the degree to which the P-species distribution can be used to identify phosphorus loading sources and contrasts in aquatic biological activity among sites and over time. Multi-variate descriptive analysis was used to identify location patterns in P-metabolites and potential correlations with other water quality parameters. The premise of this study is that examination of patterns in a broader suite of P-metabolites than routinely measured in eutrophication studies may improve diagnosis of sources of phosphorus enrichment and phosphorus fate in aquatic environments.
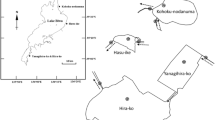
The sampling locations in this study were within Toronto and Region Area of Concern (AOC), a region identified by the International Joint Commission (IJC) in 1985 (Within Reach: 2015 Toronto and Region Remedial Action Plan 2015) as one of the 43 Great Lakes areas impacted by human activity. The Toronto and Region AOC has six major watersheds with a drainage area of 2000 km2, covering a highly urbanized area with over 4 million inhabitants that stretches over 42 km of Lake Ontario shores (Within Reach: 2015 Toronto and Region Remedial Action Plan 2015).
Experimental section
Standards and chemicals
Certified Pi standards were purchased from Inorganic Ventures (Christiansburg, VA, USA) and NSI Lab Solutions (Raleigh, USA), and the isotope-labeled phosphate, H3P18O4, HPLC grade acetonitrile, alkaline phosphatase, sodium pyrophosphate tetrabasic, and sodium triphosphate pentabasic were purchased from Millipore Sigma (Oakville, ON, Canada). Also eleven P-metabolites were purchased from Millipore Sigma as salt species: AMP disodium salt, F-P disodium salt hydrate, F-2P trisodium salt hydrate, G-P sodium salt, G-2P potassium salt hydrate, Gal-P dipotassium salt pentahydrate, R-P disodium salt, R-2P sodium salt hydrate, E-P sodium salt, PGA disodium salt, and UDPG disodium salt hydrate. Separate sets of calibration standards were used for Pi (up to 50 μg L−1 as P), P-metabolites (up to 20 nM each), and additional inorganic P species (pyrophosphate and triphosphate, each up to 50 μg L−1 as P).
Field sampling and storage containers
Surface water samples were collected from 14 sampling sites located in Toronto Harbour and Lake Ontario tributaries, namely Rouge River, Highland Creek, Don River, and Humber River. Two sets of samples were collected from the same locations (Fig. 1), with first set in July 2018 and the second set in October 2018. Samples were obtained as grab samples (0.5 m depth), except for Lake Ontario samples collected in October 2018, which were collected using a flow-through pumping system located on a sampling vessel. For the analysis of Pi and P-metabolites, samples were filtered in the field (Fisherbrand 0.45 μm Durapore filters, Fisher Scientific, Ottawa, ON, Canada) and prepared in 15 mL HDPE amber screw-cap bottles (Thermo Scientific, Mississauga, ON, Canada) by pipetting 10 mL of sample and immediately adding 100 μL of internal standard (IS), H3P18O4 containing 2 mg L−1 as P. Additionally, 500 mL of unfiltered samples was collected in PET bottles for additional analytical parameters described in the instrumental analysis section, with all samples being stored at 5±3 °C until analysis.
Ion chromatography mass spectrometry analysis
A two-dimensional ICS 5000+ ion chromatography (IC) coupled to a QExactive mass spectrometer (MS) was used for P-metabolite analysis (Thermo Scientific, Mississauga, ON), following a recently published method (Packa et al. 2019b). Species analyzed were Pi (based on field spiked H3P18O4 as IS), P-metabolites (AMP, F-P, F-2P, G-P, G-2-P, Gal-P, R-P, R-2-P, E-P, PGA, UDPG), PPi, and triphosphate. The method detection limits (MDLs) and MS-related parameters are included in the SI file. XCalibur software version 3.0 was used for data acquisition and TraceFinder version 3.2 (Thermo Scientific, Mississauga, ON) for quantitation.
Measurement of additional parameters
Ammonia, soluble reactive phosphorus (SRP), nitrite, nitrate, and TP were measured using continuous flow analyzers (Skalar, Breda, Netherlands). Chloride, sulfate, bromide, sodium, potassium, magnesium, and calcium were measured by capillary ion chromatography (Thermo Scientific, Mississauga, ON). Total organic carbon (TOC) was measured by combustion (Skalar, Breda, Netherlands), while pH, alkalinity, and conductivity were measured on an automated Mandel instrument (Guelph, ON, Canada). Additional information is included in the Supplementary Information file.
Multivariate statistical analysis
Correlation coefficients between the variables were generated for the months of July and October. In addition, PCA and hierarchical cluster analysis (HCA) in this study was applied to 23 analytes in total. The number of meaningful principal components (PC) was determined by eigenvalues ≥ 1. Calculations and graphical representations of PCA and HCA were performed using Origin Pro 2018. From the 616 values analyzed by PCA, only 65 (10.5%) were below MDL values. These values below MDL were replaced with randomly generated values between 0 and corresponding MDL. Additional analysis was done using only values corresponding to P-containing species, with 252 values (same 65 values below MDL replaced by randomly generated values between 0 and corresponding MDLs). Concentration units were normalized from data distribution using standardization (z-score) based on mean and standard deviation prior to PCA.
Results and discussions
Phosphorus species in river and lake water
A highly urbanized watershed, mostly situated within the City of Toronto, Don River had the highest levels of inorganic forms and P-metabolites over the study area. Samples collected near the mouth of the Don River in the Keating Channel contained 74 μg L−1 Pi, 87 μg L−1 SRP, 166 μg L−1 TP, and 64 μg L−1 PPi (all reported as P content). In comparison, the other three tributaries contained an average of 9.5 μg L−1 Pi, 13 μg L−1 SRP, 41 μg L−1 TP, and 3.0 μg L−1 PPi (Table 1). Lake samples collected from six Toronto Harbour sites contained on average 6.0 μg L−1 Pi, 5.9 μg L−1 SRP, 13 μg L−1 TP, and 1.0 μg L−1 PPi (all reported as P).
Within Toronto Harbour, levels of P compounds increased towards the mouth of the Don River, with an average concentration increase of 67% for inorganic P-compounds and 69% in organic P-compounds (Fig. 2). Previous studies have found the highest P concentrations in the northeast corner of Toronto Harbour, adjacent to the Keating Channel through which the Don River flows into the harbor (Poulton and Griffiths 1986). TP concentrations in Toronto Harbour were higher than in the adjacent open water of Lake Ontario (Howell et al. 2012), where they ranged between 8 and 11 μg L−1. The Don River watershed, covering the area of 36,000 hectares and 1.4 million residents, is one of the most urbanized in Canada. Paved surfaces and combined sewers, carrying both stormwater and sanitary sewage, may contribute to nutrient pollution due to sewer overflow during large storm events. The Don River, connected to Lake Ontario via Keating Channel, has the greatest effect on Inner Harbour (IH) water quality (Poulton and Griffiths 1986).
The shortest polyphosphate, PPi, can serve as a bioavailable P reservoir for aquatic microorganisms, and can occur in higher quantities than Pi (Sundareshwar et al. 2001). Speciation dynamic studies of sewage sludge by 31P liquid NMR showed that main P-species present were Pi, PPi, other polyphosphates, monoesters, and diesters (Huang and Tang 2015). Average amounts of PPi detected in Lake Ontario tributaries were between 1.7 for Rouge River and 64 μg L−1for Don River. Anthropogenic and biological processes can be the source of the PPi observed in surface water samples (Read et al. 2014; Xie et al. 2019; Zhang et al. 2019). Highland Creek and Don River are the most urbanized watersheds and contained 5 and 64 μg L−1 of PPi, respectively, while the partially urbanized watersheds of the Rouge River and Humber River contained in average only 1.7 and 2.3 μg L−1 of PPi, respectively. This suggests that an anthropogenic source is responsible for increased PPi in Highland Creek and Don River watersheds. At 0.99 μg L−1, the PPi was lower in the lake water than in the tributaries, likely due to diluting effect of the open lake and possibly from biological uptake. As previously noted (Sundareshwar et al. 2001), PPi represents a labile form of phosphorus associated with human activity, which is not evident from the SRP measurements frequently used to infer biologically available phosphorus in algal bloom studies. PPi concentration was similar to Pi (and SRP) on average in Don River samples composing 39% of TP. Biological availability of PPi presumes hydrolysis to phosphate by extra-cellular phosphatases of bacteria or algae. Nonetheless, the supply of readily available P to Toronto Harbour from the Don River to support algal growth is likely underestimated using only Pi and SRP measurements.
Past studies have examined the occurrence patterns of organic and inorganic P compounds such as phosphonates, P monoesters, P diesters, Pi, PPi, and polyphosphates in soil, animal manure, and algal biomass by 31P NMR (Feng et al. 2019; He et al. 2007; Turner et al. 2012; Zhu et al. 2013). In freshwater lakes from China (Zhang et al. 2019), PPi was detected by 31P NMR in 7 of the 12 sites at 0.12–13 μg L−1, similar to averages observed in Toronto Harbour (0.35 μg L−1) and Lake Ontario tributaries (3.75 μg L−1), while P monoesters observed at 15–50 μg L−1 were higher than the TP measured in Toronto Harbour at the times of sampling. However, analysis in surface water remains challenging since P species in rivers and lakes are usually found at much lower concentrations. The IC-MS method used in this study allowed quantification of P-metabolites present at ng L−1, reported as corresponding P levels. Previous 31P NMR measurements of surface water samples were based on quantification of functional groups (Read et al. 2014), while IC-MS measures separate P species present at lower concentrations. P-metabolites, including AMP, G-P, G-2P, R-P PGA, and PPi, were identified and quantified for the first time in Lake Ontario and its tributaries. While found in both July and October samples from Lake Ontario tributaries, in terms of maximum values AMP, R-P and PPi increased, while G-P and PGA decreased in October samples. Both F-P and Gal-P were detected only in October. The most abundant metabolite was R-P at a maximum concentration of 360 and 640 ng L−1 P in July and October, respectively. Average concentrations of P-metabolites and other inorganic parameters in individual tributaries are summarized in Tables 1 and 2. A summary of the average concentrations of P-metabolites across tributaries and harbor samples, and corresponding ratios are presented in Table 3. The concentrations of the targeted P-metabolites were low comparing with Pi concentrations (< 1 μg/L P), and excluding R-P, all were lower than 0.1 μg/L, representing only a small fraction of TP or potentially bioavailable P as referenced to Pi concentration. The measured concentrations of P-metabolites represent net levels after in situ biological processing of P-metabolites released into the environment (Garrett et al. 2013). The levels are likely reduced by breakdown of the labile P-metabolites by external phosphatases to meet phosphate needs of biota (Jansson et al. 1988).
The most abundant of the assayed P-metabolites was R-P, a building block in the Calvin cycle and pentose sugar pathways. This P-metabolite is expected as a constituent in tissues of both autotrophic and heterotrophic organisms (Garrett et al. 2013). R-P was found only in the river samples and at higher levels in October than in July samples. The relative abundance of R-P may simply result from contrasting biomass levels between the more nutrient-enriched river than lake sites. The temporal difference observed is due to higher levels of microbial decomposition expected in the fall or simply resulting from difference in loading to the rivers associated with stochastic variability in weather, which is known to strongly effect microbial pollution in the Don River and may affect levels of biological activity (Edge et al. 2020).
Relations between P-metabolites, inorganic P species, and water quality features
Concentration ranges of P-containing inorganic and organic compounds quantified in Toronto Harbour and tributaries are shown in Figs. 3 and 4. The concentrations of P-containing inorganic compounds and the sugar phosphates G-P and G-2P were higher in the tributaries than in Toronto Harbour, ranging from 2.0 to 10.7 times lower in the harbor (Fig. 5), with the widest difference in PPi. Higher concentration of the organic P-containing compounds PGA and AMP in Toronto Harbour compared to the rivers is suggestive of differences in the biological activity of these two environment types. Differences between the river and lake ecosystems in prevalence and intensity autotrophic metabolism are to be anticipated and potentially influence the distribution of P-containing organic species (Lin and Guo 2016).
The Pi, SRP, TP, and PPi forms were strongly correlated (correlation coefficients higher than 0.9) in both sampling events in the beginning of summer as well as in the fall. This confirms their common origin in the system. Conversely, organic P-species were not well correlated between each other or with other parameters. This possibly indicates varying prevalence of production or external loss processes for these compounds among the rivers or over time. PCA analysis of all P-containing compounds found patterns in the distributions of species among rivers, locations within the harbor and between samples collected in July and October. PCA axes with eigenvalues > 1 from the separate analysis of July and October samples accounted for 85% and 92% of the total variance, respectively. The first axis of the analysis for the July samples most strongly related to TP, Pi, SRP, PPi, and G-P (0.40 to 0.46) suggests an increasing influence of external loading of phosphorus among samples. The second axis was positively related to AMP and PGA (both 0.60) and negatively to R-P (− 0.53). Analysis of the October samples also suggested an external loading effect with TP, Pi, SRP, PPi, and F-P (0.37 to 0.41) associated with the axis. The second axis was positively associated with G-P (0.5) and R-P (0.53) and negatively with Pi, AMP, and PPi (− 0.30 to − 0.34). The river and harbor samples fell in separate groups on plots of sample scores on axes 1 and 2 (Fig. 6). In July, lake and river samples are grouped vertically along the second axis. The harbor samples are associated with higher relative levels of AMP and PGA, and lower levels of R-P than the river samples. For October dataset, lake samples and river samples are separated along PC2 with lake samples associated with higher concentration of AMP and river samples with higher G-P, R-P, Gal-P, and PGA levels.
When the October phosphorus dataset was examined using hierarchical cluster analysis, three statistically significant clusters were identified separating samples in similar manner to the second axis of the October PCA analysis. The harbor samples were separated from the river samples and the river samples further split into two groups, one of which consisted of the samples from site R3, the Don River, the more polluted of the rivers examined.
The difference between the harbor and tributary samples was confirmed by repeating PCA analysis with additional water quality variables (Figs. 6 and 7), specific to the time and sample collection locations. PCA axes with eigenvalues > 1 from the separate analysis of July and October samples accounted for 85% and 92% of the total variance, respectively, as observed for the phosphorus-only analysis. The first axis of the analysis for the July samples was positively related to TP, TOC, Pi, SRP, G-P, and PPi (0.28 to 0.31), negatively related to AMP (− 0.14) and pH (− 0.19), and consistent again with an external loading gradient, suggesting that AMP is not an expected component of nutrient-enriched external loading to these rivers. Figure S3 shows corresponding loading graphs, scree diagrams, and additional details of the PCA. The second axis was positively related to SO42−, Mg2+, Ca2+, conductivity, Na+, and Cl− (0.29 to 0.31) and negatively to PGA, AMP, and nitrate (− 0.20 to − 0.24). The second axis again separates the river and harbor locations which is consistent with the higher levels of major ions in the rivers compared with the harbor and the paradoxical contrast of high nitrate levels in Lake Ontario compared with the rivers. Differences were observed between the July and October samples. The first axis was positively related to K+, F-P, Na+, Cl− (all 0.25), and AMP (− 0.24). The second axis is positively associated with nitrite, ammonia, PPi, and Pi (0.3 to 0.34) and negatively associated with G-P, SO4, R-P, and pH (− 0.20 to − 0.25).
Metabolic pathways and P-metabolites in surface water
The metabolites detected in surface waters are either the result of an unchanged release from biological activities or the combined result of a release followed by separate selective biological uptake of specific metabolites. Intermediates present may provide information about the distribution of specific metabolic pathways of biota in tributaries and harbor waters. R-P is a known intermediate in the Calvin cycle, known also as Calvin-Benson cycle, of photosynthetic organisms, but also an intermediate in the anabolic pentose phosphate pathway in algae and other micro-organisms (Garrett et al. 2013). R-P was detected at relatively high levels in Lake Ontario tributaries, but not in Toronto Harbour. The detection of two other specific Calvin cycle intermediates, R-2P and F-2P, indicates the presence of photosynthetic-related P-metabolites. The pentose phosphate pathway uses G-P as starting metabolite, with R-P as an intermediate and F-P as a product. Since all these three metabolites were detected, it suggests that the pentose phosphate pathway may also be a P species source in Lake Ontario tributaries. Pentose phosphate pathway can be a parallel metabolic pathway to glycolysis, with both starting from G-P, but F-P resulting from pentose phosphate pathway can also be used in the glycolysis pathway, producing PGA as an intermediate (Garrett et al. 2013). PGA was observed in samples from all locations. Toronto Harbour samples did not have detectable levels of R-P and seven times higher concentration of the glycolysis intermediate PGA than G-P, the initial metabolite in both pentose phosphate and glycolysis pathways (Fig. 2). This seems to indicate a depleted pentose phosphate pathway with no R-P intermediate and much higher level of PGA, a late stage glycolysis intermediate (Fig. 8). The lack of R-P, R-2P, and F-2P in Toronto Harbour samples suggests that these metabolites may have been preferentially removed by biota. Analyzed by PCA, the July tributary samples showed clustering along Principal Component 2 axis, with R-P separated from the other variables (Fig. 7). Note that R-P was present in higher concentration in tributary samples than R-2P and F-2P. Clustering of tributaries is observed also for the October set, but in this case, R-T, G-P, Gal-P, and PGA have similar influence on clustering.
Summary of P-metabolites related to the Calvin cycle, pentose phosphate, and glycolysis pathways (cycle/pathway-specific P metabolites in red font), as measured in Lake Ontario tributaries and Toronto Harbour. The P metabolites identified in higher concentrations are circled, while non-detected P metabolites are covered with blue boxes
Differences in P-metabolites between the rivers and lake conditions of Toronto Harbour were anticipated based on differences in the relative proportions of autotrophic to heterotrophic organisms. While not quantified, the fluvial river environment of urban areas is expected to be more heterotrophic, dominated by external loading of organic material and microbial activity than the downstream lake where the slower flushing rates of the water mass allow algal assemblages to grow and accumulate to a greater extent. The high autotrophic productivity of the Toronto Harbour as inferred from concentrations of chlorophyll a (Howell and Benoit 2020) and the relative degree of microbial pollution of the Don River compared with Toronto Harbour (Edge et al. 2020) support this view. While there were differences in the P-metabolites detected between the river and the Toronto Harbour sites, the patterns were not easily interpreted. The two P-metabolites specific to the Calvin cycle (Garrett et al. 2013), R-2P and F-2P, likely to be exclusively associated with algal growth, were not detected in Toronto Harbour, but were found at relatively low concentrations in river samples. PGA, AMP, and G-P were found at all lake sites and G-2P at two sites, including the site most strongly influenced by the discharge of the Don River into the harbor. Only AMP was disproportionally more abundant in lake than in river samples, while PGA was found on average at roughly the same concentrations in both the river and lake samples. The explanation for why AMP, a molecule associated with energy regulation in plants and animals (Garrett et al. 2013), should be most abundant in the lake samples is not obvious. Possibilities include both differential production and breakdown of the molecule depending on the environment type. The higher levels of autotrophic productivity in the lake may result in more gross production of AMP, in contrasts with cycling of AMP in a more heterotrophic system. Alternatively, higher levels of heterotrophic activity in the rivers than in the lake may hydrolyze AMP more rapidly. In the case of PGA, the slightly higher levels in the river samples than AMP may reflect a higher contribution from autotrophic production related to the Calvin cycle in the lake, augmenting what might be a lower production from the non-photosynthesis related pathways in the lake relative to those anticipated in the rivers.
The key P-metabolites detected in current study G-P, R-P, R-2P, F-P, F-2P, and PGA, and other not monitored metabolites can be used in future field studies as endpoint parameters to examine physiological responses to environmental stimulus or gradients.
Conclusion
PPi was identified as a likely source of biologically available P loaded from river to the lake that is not typically considered in nutrient studies. The concentrations of the measured targeted P-metabolites were relatively low compared with the Pi concentrations, which implies that either the production of these compounds viewed from the perspective of leakage and release into the environment is low or the hydrolysis of P and molecular modification in the environment is extensive resulting in their observed low concentrations. The lower ratios for AMP and PGA concentrations (tributaries/harbor) suggest contrasting levels of autotrophic vs heterotrophic processes.
A range of P-metabolites in photosynthetic and energy pathways were detected in Toronto Harbour and area rivers. The contrasting pattern and concentrations of metabolites among locations suggest that the occurrence feature of the P-metabolites may be used to detect and diagnose the outcomes of phosphorus enrichment in aquatic environments. However, the complexity of the metabolic pathways that produce these compounds precluded clear associations between the levels of metabolites and biological activity. Further study over temporal cycles of biological activity and incorporating biological measurements will likely be required to interpret the ecological relevance of the occurrence features of these and other P-metabolites.
Data availability
The data used in this paper is available on request from the corresponding author.
References
4500-P Phosphorus (2017) In: Standard methods for the examination of water and wastewater. https://doi.org/10.2105/smww.2882.093
Baldwin DS (2013) Organic phosphorus in the aquatic environment. Environ Chem 10:439–454. https://doi.org/10.1071/EN13151
Baykov AA, Anashkin VA, Salminen A, Lahti R (2017) Inorganic pyrophosphatases of Family II—two decades after their discovery. FEBS Lett 591:3225–3234. https://doi.org/10.1002/1873-3468.12877
Boujelben N, Bouzid J, Elouear Z, Feki M, Jamoussi F, Montiel A (2008) Phosphorus removal from aqueous solution using iron coated natural and engineered sorbents. J Hazard Mater 151:103–110. https://doi.org/10.1016/j.jhazmat.2007.05.057
Cade-Menun BJ, Navaratnam JA, Walbridge MR (2006) Characterizing dissolved and particulate phosphorus in water with 31P nuclear magnetic resonance spectroscopy. Environ Sci Technol 40:7874. https://doi.org/10.1021/es061843e
Cooper WT, Llewelyn JM, Bennett GL, Salters VJM (2005) Mass spectrometry of natural organic phosphorus. Talanta 66:348–358. https://doi.org/10.1016/j.talanta.2004.12.028
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27:261–266. https://doi.org/10.2134/jeq1998.00472425002700020004x
Edge TA, Boyd RJ, Shum P, Thomas JL (2020) Microbial source tracking to identify fecal sources contaminating the Toronto Harbour and Don River watershed in wet and dry weather. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2020.09.002
Feng W, Zhu Y, Wu F, He Z, Zhang C, Giesy JP (2016a) Forms and lability of phosphorus in algae and aquatic macrophytes characterized by solution 31P NMR coupled with enzymatic hydrolysis. Sci Rep 6:37164. https://doi.org/10.1038/srep37164
Feng W, Zhu Y, Wu F, Meng W, Giesy JP, He Z, Song L, Fan M (2016b) Characterization of phosphorus forms in lake macrophytes and algae by solution31P nuclear magnetic resonance spectroscopy. Environ Sci Pollut Res 23:7288–7297. https://doi.org/10.1007/s11356-015-5913-5
Feng W, Li C, Zhang C, Liu S, Song F, Guo W, He Z, Li T, Chen H (2019) Characterization of phosphorus in algae from a eutrophic lake by solution 31P nuclear magnetic resonance spectroscopy. Limnology 20:163–171. https://doi.org/10.1007/s10201-018-0562-2
Garrett RH, Grisham CM, Andreopoulos S, Willmore W, Gallouzi I-E (2013) Biochemistry, 1st Canadian Edition. Nelson Education, Toronto
He Z, Cade-Menun BJ, Toor GS, Fortuna A-M, Honeycutt CW, Sims JT (2007) Comparison of phosphorus forms in wet and dried animal manures by solution phosphorus-31 nuclear magnetic resonance spectroscopy and enzymatic hydrolysis. J Environ Qual 36:1086. https://doi.org/10.2134/jeq2006.0549
Howell ET, Benoit N (2020) Nutrient footprints on the Toronto-Mississauga waterfront of Lake Ontario. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2020.11.009
Howell ET, Chomicki KM, Kaltenecker G (2012) Patterns in water quality on Canadian shores of Lake Ontario: correspondence with proximity to land and level of urbanization. J Great Lakes Res 38:32–46. https://doi.org/10.1016/j.jglr.2011.12.005
Huang R, Tang Y (2015) Speciation dynamics of phosphorus during (hydro)thermal treatments of sewage sludge. Environ Sci Technol 49:14466–14474. https://doi.org/10.1021/acs.est.5b04140
Jansson M, Olsson H, Pettersson K (1988) Phosphatases; origin, characteristics and function in lakes. In: Phosphorus in Freshwater Ecosystems. Springer, Dordrecht, pp 157–175. https://doi.org/10.1007/BF00024903
Lin P, Guo L (2016) Dynamic changes in the abundance and chemical speciation of dissolved and particulate phosphorus across the river-lake interface in southwest Lake Michigan. Limnol Oceanogr 61:771–789. https://doi.org/10.1002/lno.10254
Lin J, Zhao Y, Zhan Y, Wang Y (2020) Influence of coexisting calcium and magnesium ions on phosphate adsorption onto hydrous iron oxide. Environ Sci Pollut Res 27:11303–11319. https://doi.org/10.1007/s11356-020-07676-w
Makarewicz JC, Booty WG, Bowen GS (2012) Tributary phosphorus loading to Lake Ontario. J Great Lakes Res 38:14–20. https://doi.org/10.1016/j.jglr.2012.08.001
Packa V, Bostan V, Furdui VI, (2019a) Analysis of phosphorus species in water. In: Encyclopedia of Water. pp 1–14. https://doi.org/10.1002/9781119300762.wsts0049
Packa V, Maedler S, Howell T, Bostan V, Diep N, Tooley R, Furdui VI (2019b) Unbiased measurement of phosphate and phosphorus speciation in surface waters. Environ Sci Technol 53:820–828. https://doi.org/10.1021/acs.est.8b05089
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182:1015–1045. https://doi.org/10.1007/s00360-012-0658-7
Poulton DJ, Griffiths M (1986) Toronto waterfront general water quality, 1976–1983. Great Lakes Section. Ontario Ministry of the Environment, Ontario
Read EK, Ivancic M, Hanson P, Cade-Menun BJ, McMahon KD (2014) Phosphorus speciation in a eutrophic lake by 31P NMR spectroscopy. Water Res 62:229–240. https://doi.org/10.1016/j.watres.2014.06.005
Saiardi A (2012) How inositol pyrophosphates control cellular phosphate homeostasis? vol 52. https://doi.org/10.1016/j.jbior.2012.03.002
Schindler DW, Carpenter SR, Chapra SC, Hecky RE, Orihel DM (2016) Reducing phosphorus to curb lake eutrophication is a success. Environ Sci Technol 50:8923–8929. https://doi.org/10.1021/acs.est.6b02204
Sundareshwar PV, Morris JT, Pellechia PJ, Cohen HJ, Porter DE, Jones BC (2001) Occurrence and ecological implications of pyrophosphate in estuaries. Limnol Oceanogr 46:1570–1577. https://doi.org/10.4319/lo.2001.46.6.1570
Turner BL, Cheesman AW, Godage HY, Riley AM, Potter BVL (2012) Determination of neo- and D-chiro-inositol hexakisphosphate in soils by solution 31P NMR spectroscopy. Environ Sci Technol 46:4994–5002. https://doi.org/10.1021/es204446z
Van Moorleghem C, De Schutter N, Smolders E, Merckx R (2013) The bioavailability of colloidal and dissolved organic phosphorus to the alga Pseudokirchneriella subcapitata in relation to analytical phosphorus measurements. Hydrobiologia 709:41–53. https://doi.org/10.1007/s10750-013-1442-8
Wenqiang Z, Baoqing S, Hong Z, Wenzhong T (2015) Characteristics of phosphorus compounds and their effects in sediments of an Eutrophic Chaohu Lake, China. CLEAN Soil Air Water 43:544–550. https://doi.org/10.1002/clen.201300254
Westheimer FH (1987) Why nature chose phosphates. Science 235:1173–1178. https://doi.org/10.1126/science.2434996
Within Reach: 2015 Toronto and Region Remedial Action Plan (2015). Toronto, Ontario, https://torontorap.ca/app/uploads/2019/12/2015-RAP-Progress-Report.pdf
Worsfold PJ, Monbet P, Tappin AD, Fitzsimons MF, Stiles DA, McKelvie ID (2008) Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: a review. Anal Chim Acta 624:37–58. https://doi.org/10.1016/j.aca.2008.06.016
Xie F, Li L, Song K, Li G, Wu F, Giesy JP (2019) Characterization of phosphorus forms in a Eutrophic Lake, China. Sci Total Environ 659:1437–1447. https://doi.org/10.1016/j.scitotenv.2018.12.466
Zhang YM, Chen J, Gao YX, Wang LM, Yang F (2014) Characteristics of organic phosphorus in a shallow lake Gehu investigated by sequential fractionation and 31P-NMR. Tech Adv Mater Res 955–959:3529–3539. https://doi.org/10.4028/www.scientific.net/AMR.955-959.3529
Zhang C, Feng W, Chen H, Zhu Y, Wu F, Giesy JP, He Z, Wang H, Sun F (2019) Characterization and sources of dissolved and particulate phosphorus in 10 freshwater lakes with different trophic statuses in China by solution 31P nuclear magnetic resonance spectroscopy. Ecol Res 34:106–118. https://doi.org/10.1111/1440-1703.1006
Zhao J, Liu X (2013) Organic and inorganic phosphorus uptake by bacteria in a plug-flow microcosm. Front Environ Sci Eng 7:173–184. https://doi.org/10.1007/s11783-013-0494-3
Zhu Y, Wu F, He Z, Guo J, Qu X, Xie F, Giesy JP, Liao H, Guo F (2013) Characterization of organic phosphorus in lake sediments by sequential fractionation and enzymatic hydrolysis. Environ Sci Technol 47:7679–7687. https://doi.org/10.1021/es305277g
Acknowledgements
Special thanks to Andrew Laursen (Ryerson University) for helpful recommendations in manuscript preparation. Stefanie Maedler (MECP) is thanked for manuscript suggestions. Hany Eskander, Shelly Chan, Andrew Senyi, Carlos Jurado, Sajid Feeroze, and Mark Duric (MECP) are thanked for the analysis of additional parameters by accredited methods.
Author information
Authors and Affiliations
Contributions
Vlastimil Packa: resources, investigation, formal analysis, visualization, writing–original draft. Todd Howell: conceptualization, resources, writing–review and editing. Vadim Bostan: conceptualization, supervision, resources, writing–review and editing. Vasile Furdui: conceptualization, supervision, visualization, writing–review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Additional information about analytical methods of Pi, TP, and sulfate, MDLs for species, schematic of the 2D-IC configuration and Pi loss (%) in samples spiked 48 h after collection are available at https://doi.... (DOCX 382 kb)
Rights and permissions
About this article
Cite this article
Packa, V., Howell, T., Bostan, V. et al. Phosphorus-based metabolic pathway tracers in surface waters. Environ Sci Pollut Res 28, 29498–29508 (2021). https://doi.org/10.1007/s11356-021-12697-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12697-0