Abstract
Fluoxetine (FLX) is an antidepressant drug that belongs to the class of selective serotonin reuptake inhibitors. FLX is known to induce apoptosis in multiple types of cancer cells. In this study, the molecular mechanisms underlying the anti-cancer effects of FLX were investigated in SK-N-BE(2)-M17 human neuroblastoma cells. FLX induced apoptotic cell death, activation of caspase-4, -9, and -3, and expression of endoplasmic reticulum (ER) stress-associated proteins, including C/EBP homologous protein (CHOP). Inhibition of ER stress by treatment with the ER stress inhibitors, salubrinal and 4-phenylbutyric acid or CHOP siRNA transfection reduced FLX-induced cell death. FLX induced phosphorylation of mitogen-activated protein kinases (MAPKs) family, p38, JNK, and ERK, and an upstream kinase apoptosis signal kinase 1 (ASK1). Inhibition of MAPKs and ASK1 reduced FLX-induced cell death and CHOP expression. We then showed that FLX reduced mitochondrial membrane potential (MMP) and ER stress inhibitors as well as MAPK inhibitors ameliorated FLX-induced loss of MMP. Interestingly, FLX induced hyperacetylation of histone H3 and H4, upregulation of p300 histone acetyltransferase (HAT), and downregulation of histone deacetylases (HDACs). Treatment with a HAT inhibitor anacardic acid or p300 HAT siRNA transfection blocked FLX-induced apoptosis in SK-N-BE(2)-M17 cells. However, FLX did not induce histone acetylation and anacardic acid had no protective effect on FLX-induced cell death and CHOP expression in MYCN non-amplified SH-SY5Y human neuroblastoma and MYCN knockdowned SK-N-BE(2)-M17 cells. These findings suggest that FLX induces apoptosis in neuroblastoma through ER stress and mitochondrial dysfunction via the ASK1 and MAPK pathways and through histone hyperacetylation in a MYCN-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma is a type of cancer that is derived from progenitor cells of the sympathetic nervous system. It is frequently developed in adrenal glands, neck, chest, abdomen, and spine. It is known that neuroblastoma is the most common extracranial solid tumor in children [1]. Although new and better drugs and treatments have been developed for neuroblastoma, the effectiveness of anti-cancer drugs and treatments is hampered by a variety of adverse side effects [2]. Many chemotherapy drugs exert cytotoxic effects in both tumor and normal tissues, leading to indiscriminate tissue necrosis. Thus, development of more effective and specific chemotherapeutic agents is required for the treatment of neuroblastoma.
Fluoxetine (FLX, Prozac®) is one of newer (second generation) and the most popular antidepressant drugs, termed as selective serotonin reuptake inhibitors (SSRIs). SSRIs also include citalopram, fluvoxamine, paroxetine, and sertraline. Compared to the older (first generation) tricyclic antidepressants and monoamine oxidase inhibitors, SSRIs generally have fewer side effects and thus they are well tolerated over extended periods. Therefore, SSRIs are the treatment of choice for a broad range of mental problems, including clinical depression and several anxiety disorders [3]. SSRIs exert an antidepressant function by inhibiting reuptake of serotonin into the presynaptic cells and increasing the serotonin concentration in the synaptic cleft available to bind to the postsynaptic receptor. In addition to their ability to modulate neurotransmission, SSRIs have been shown to exert a range of effects, including immunomodulatory, neuroprotective, and anti-inflammatory activities [3]. It has also been reported that a range of SSRIs including FLX suppress cell cycle progression and induce extensive apoptosis in several cancer cell lines, including Burkitt lymphoma [4, 5], neuroblastoma [6], glioblastoma cell lines [7], and MDA-MB231 breast cancer cells and SiHa cervical cancer cells [8]. Although the elevation of cytosolic Ca2+ concentration, mitochondrial dysfunction, reactive oxygen species (ROS) accumulation, and endoplasmic reticulum (ER) stress have been implicated in FLX-induced cell death [5, 7, 9, 10], the molecular pathways underlying the anti-cancer effect of FLX are dependent on the cellular contexts and thus remains to be further elucidated.
ER stress is one of the molecular mechanisms implicated in apoptotic cell death [11, 12]. Accumulation of unfolded proteins and disturbance of Ca2+ homeostasis can cause abnormalities in the ER function, resulting in ER stress. ER stress responses include activations of a serine/threonine kinase PKR-like ER kinase (PERK) and a type I transmembrane protein inositol-requiring enzyme 1 (IRE1), and translocation of activating transcription factor 6 (ATF6) to the Golgi apparatus. The activated PERK phosphorylates and inactivates eukaryotic initiation factor 2α (eIF2α), thereby inhibiting translation upon ER stress [13]. The activated IRE1 cleaves X-box binding protein and the spliced XBP-1 promotes the expression of ER chaperone genes, including glucose-regulated protein 78 (GRP78) [14]. ATF6 is cleaved by sites 1 and 2 proteases in the Golgi apparatus. The cleaved ATF6 acts as a transcription factor to regulate the expression of ER stress-associated genes, such as C/EBP homologous protein (CHOP). Although these events are implicated in ER stress, they play roles in cellular protection against severe damage. When ER functions are severely impaired, apoptosis occurs to protect the whole organism by eliminating damaged cells [15]. Overexpression of CHOP participates in ER stress-induced apoptosis and cells lacking CHOP are protected from apoptosis [16]. Mitochondrial dysfunction, ROS accumulation, and cytosolic Ca2+ increase crosstalk each other and these factors might play some roles in regulating ER stress-associated apoptotic cell death [17].
Histone proteins are acetylated and deacetylated on the lysine residues within the N-terminal tails protruding from the histone core of the nucleosome [18]. These reactions are catalyzed by histone acetyltransferase (HAT) and histone deacetylase (HDAC), respectively [19]. A balance between these two classes of enzymes controls the net status of histone acetylation, which plays an important role in gene expression by chromatin remodeling and by acting as either transcriptional coactivators or corepressors [20]. When the balance between HATs and HDACs is disrupted, the transcriptional alteration of various genes has been suggested to cause diseases, such as cancer. Histone hyperacetylation by upregulation of p300 HAT is associated with a cancer treatment effect via apoptotic cell death [21, 22]. Histone hyperacetylation by HDAC inhibition also contributes to the regulation of cell differentiation, growth arrest, and apoptosis [23]. Due to their effects on apoptotic cell death, HAT and HDAC family members have been considered as targets for cancer treatment [24]. Because HDAC inhibitors increasing histone acetylation are shown to exert robust antidepressant-like effects similar to the effects of the standard antidepressant fluoxetine [25,26,27], any relationship between the antidepressant effect and histone hyperacetylation might be present.
Therefore, in this study, we investigated how histone hyperacetylation and ER stress are implicated in the molecular mechanisms underlying FLX-induced anti-cancer effect in SK-N-BE(2)-M17 neuroblastoma cells. We found that FLX causes apoptotic cell death by ER stress and mitochondrial dysfunction via apoptosis signal-regulating kinase 1 (ASK1)/MAPKs signaling pathways, and by histone hyperacetylation via p300 HAT upregulation and HDACs downregulation in a MYCN-dependent manner.
Materials and methods
Materials
Dulbecco’s modified Eagles medium (DMEM), fetal bovine serum (FBS), and other cell culture products were purchased from Life Technologies (Grand island, NY, USA). FLX and thapsigargin (TG) were purchased from Biomol Research Labs (Plymouth Meeting, PA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), propidium iodide (PI), Ac-LEVD-CHO, Ac-LEHD-CMK, Z-DEVD-FMK, salubrinal, 4-phenylbutyric acid (4-PBA), 3,3′-dihexyloxacarbocyanine iodide (DiOC6), N-acetylcysteine (NAC), 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (BAPTA-AM), EGTA, anacardic acid, and TRI reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). SB203580, SP600125, PD98059, and 2,7-dihydro-2,7-dioxo-3H-naphtho[1,2,3-de]quinoline-1-carboxylic acid ethyl ester (NQDI-1) were purchased from Tocris (Bristol, UK) and Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was from BD Bioscience (Oakville, ON, Canada). Small interfering RNAs (siRNAs) against scrambled control, caspase-4, and CHOP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and siRNAs for p38α, ERK1/2, JNK1, ASK1, p300 HAT, and MYCN were purchased from M-biotech (Seoul, Korea). Antibodies CHOP, GRP78, sXBP-1, ATF6α (p90), eIF2α, IRE1α, p38, JNK1, ERK1/2, acetylated histone H3 (ac-H3), histone H3, acetylated histone-H4 (Ac-H4), histone H4, p300, histone deacetylases 1-6 (HDACs1-6), and N-Myc were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-IRE1α (P-IRE1α) were purchased from Abcam (Cambridge, MA, USA). Antibodies against caspase-4, -9, -3, poly (ADP-ribose) polymerase (PARP), phospho-eIF2α (P-eIF2α), phospho-JNK(P-JNK), phospho-p38 (P-p38), phospho-ERK (P-ERK), and phospho-ASK1 (P-ASK1) were purchased from Cell Signaling Technology (Beverly, MA, USA). Enhanced chemiluminescence (ECL) system was acquired from Amersham (GE Health, Piscataway, USA) and GeneSilencer siRNA transfection reagent was obtained from Genlantis (San Diego, CA, USA).
Cell culture
SK-N-BE(2)-M17 and SH-SY5Y human neuroblastoma, Neuro 2a mouse neuroblastoma, and U251MG human glioblastoma cells were purchased from American Type Culture Collection (Manassas, VA, USA). SK-N-BE(2)-M17, SH-SY5Y, Neuro 2a, and U251MG cells were grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Exponentially growing cultures were maintained in a humidified atmosphere of 5% CO2 at 37 °C. For treatment, the cells were serum-starved for 3 h and incubated with FLX or other drugs for the indicated times.
MTT assay
Based on conversion of MTT to MTT-formazan by mitochondrial enzymes, cell viability was determined. Briefly, cells were seeded into a 12-well plate at a density of 4 × 105 cells/well in growth medium and cultured to about 60–70% confluency. After serum starvation for 3 h, cells were treated with FLX or TG. After 24 h of incubation at 37 °C, cells were washed three times with PBS and 30 μl MTT solution (5 mg/ml stock) was added to the cells, and these were then incubated for 1 h at 37 °C. The medium was removed carefully and 300 μl dimethyl sulfoxide (DMSO) was then added to resolve the blue formazan in living cells. Finally, the absorbance at 540 nm was read with an ELISA reader (Multiskan EX, Thermo Lab system, Beverly, MA, USA).
Flow cytometry for detection of DNA cleavage and apoptotic cell death
For detection of DNA cleavage, SK-N-BE(2)-M17 cells were seeded at 2 × 105 cells/ml density in 100-mm dishes and treated with 15 μM FLX for 12 or 24 h. After incubation for 24 h, cells were harvested, washed twice with PBS, and then fixed with ice-cold 75% ethanol at 4 °C for 24 h. Cells were then pelleted by centrifugation at 1000×g for 5 min and the ethanol layer was discarded. After washing with PBS, the cells were treated with 0.5 μg/ml RNase A in PI buffer for 30 min. At the end of treatment, the cells were stained with 20 μg/ml PI in the dark for 30 min. The DNA contents were then analyzed using Kaluza flow cytometry software (Beckman Coulter, Orange County, CA, USA). The apoptotic cell death was also detected by flow cytometry using the Annexin V-FITC/PI double-labeling method. Cells were seeded at 2 × 105 cells/ml density in 100-mm dishes and treated with FLX for 12 or 24 h. After incubation, cells were trypsinized and collected by centrifugation at 1000×g for 5 min. After resuspension in Annexin V-FITC binding buffer, cells were incubated with 1 μg/ml annexin V-FITC and 10 μg/ml PI at room temperature in the dark for 15 min. Samples were analyzed with Kaluza flow cytometry.
RNA interference (siRNA)
siRNA transfection was conducted using GeneSilencer siRNA transfection reagent. SK-N-BE(2)-M17 or U251MG cells were plated in six-well plates overnight and the media was replaced with 1 ml of serum-free DMEM before transfection. Scrambled control, caspase-4, CHOP, p38α, JNK1, ERK1/2, ASK1, p300, and MYCN siRNA duplexes (10 nM) were incubated with 5 μl of siRNA transfection reagent for 5 min at room temperature; mixtures were then added to these cells. After 12 h of incubation with siRNAs in the absence of serum, 1 ml DMEM containing 20% bovine serum was added to each well and incubated for additional 24 h. Cells were then treated with FLX or TG for 24 h.
Assessment of MMP
To assess MMP loss, cells were treated with FLX or TG for the indicated times. Cells were washed twice with PBS, resuspended in PBS containing 20 nM DiOC6 and 20 μg/ml PI, and then incubated at 37 °C for 15 min. Fluorescence intensity was examined in cells at channel FL1 for DiOC6 or channel FL3 for PI. Non-apoptotic cells were stained green with DiOC6 and apoptotic cells showed decreased intensity of DiOC6 staining, while necrotic cells were stained red with PI. Fluorescence intensity was then measured by flow cytometry using excitation and emission wavelengths of 482 and 504 nm, respectively. At least 20,000 events were analyzed per sample and each sample was performed in duplicate.
Measurement of ROS production
Cells incubated with 15 μM FLX or 5 μM TG for the indicated times. ROS production was measured in cells treated with 10 μM DCFH-DA at 37 °C for 30 min. Cells were washed twice with ice-cold PBS followed by suspension in the same buffer. Fluorescence intensity was measured by flow cytometry (Beckman Coulter) using excitation and emission wavelengths of 488 and 525 nm, respectively. 10,000 events were analyzed per sample.
Measurement of cytosolic Ca2+
For the spectrofluorimetric measurements, the cells were loaded with 5 μM Fura-2AM for 30 min and treated with 15 μM FLX for the indicated times. Fluorescence was monitored throughout each experiment at 37 °C with a fluorescence plate reader (VICTOR luminometer, Perkin-Elmer). After a 5 min temperature equilibration period, samples were excited at 370 nm and emission was collected at 476 nm as described [28]. The concentrations of intracellular free Ca2+ were calculated by using the following equation:
A value of 224 nM for Kd was added into the calculations. Rmax value was obtained by addition of 5 μM ionomycin and Rmin value was obtained by addition of 2 mM MnCl2, 0.1% Triton X-100, and 2 mM EGTA.
Western blot analysis
Cells were treated with vehicle or FLX for the indicated times and washed twice with ice-cold PBS. Cells were lysed in a lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethanesulfonylfluoride, and 0.5% protease inhibitor cocktail. The whole cell lysates were cleared by centrifugation at 12,000×g for 10 min at 4 °C to remove cellular debris. After protein concentration was determined by a Bio-Rad DC protein assay kit, cell lysates containing equal amounts of protein (50 μg) were resolved by 8–10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and then analyzed by Western blot analysis. Each blot was blocked with 5% skim milk in Tris-buffered saline with 0.05% Tween 20 (TBST) for 1 h at room temperature, and treated with primary antibodies (1:1000) in TBST overnight at 4 °C, washed for 1 h with TBST, and probed with secondary HRP-conjugated anti-rabbit, anti-mouse, or anti-goat IgGs (1:2000) in TBST for 1 h at room temperature. The immune complexes were visualized using an ECL detection system according to the manufacture’s protocols.
Statistical analysis
All data are presented as the mean ± standard deviation of at least three independent experiments. Statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparison (Graphpad Prism, version 5.0, Graphpad Software Inc, San Diego, CA, USA). P values of <0.05 were considered statistically significant.
Results
FLX induces apoptotic cell death in human SK-N-BE(2)-M17 neuroblastoma cells
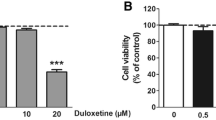
The effect of FLX on cell viability was examined in SK-N-BE(2)-M17 and SH-SY5Y human neuroblastoma, Neuro 2a mouse neuroblastoma, and HT22 normal murine hippocampal neuronal cells. Cells were treated with FLX (5–50 μM) for 24 h, and cell viability was examined by MTT assay. Data from MTT showed that FLX reduced cell viability in a dose-dependent manner and the half maximal inhibitory concentration (IC50) for FLX-induced cell death was 16.1 μM in SK-N-BE(2)-M17, 20.7 μM in SH-SY5Y, and 34.8 μM in Neuro 2a cells (Fig. 1a). The cytotoxic effect of FLX was much greater in neuroblastoma cells compared to that in normal HT22 murine hippocampal neuronal cells.
Effects of FLX on cell viability and apoptosis in neuroblastoma cells. a SK-N-BE(2)-M17, SH-SY5Y, and Neuro 2a neuroblastoma cells and normal hippocampal HT22 neuronal cells were treated with DMSO (Vehicle) or various concentrations (5–50 μM) of FLX for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments. *P < 0.01 compared with vehicle-treated control cells. b SK-N-BE(2)-M17 cells were treated with vehicle (Control) or 15 μM FLX for 12 or 24 h. FLX-treated cells were stained with PI and evaluated by flow cytometry. c Vehicle- or FLX-treated cells were stained with annexin V-FITC and PI, and evaluated by flow cytometry. n = 3 for each experimental group. d SK-N-BE(2)-M17 cells were treated with 15 μM FLX for the indicated times. Cells were lysed and total cell extracts were resolved by SDS–PAGE. Protein levels were detected by Western blot analysis using antibodies against pro- and cleaved form (C) of caspase-4, -9, and -3, the full length PARP(F), cleaved PARP(C), and β-actin. e After pretreatment with caspase inhibitors (10 μM Ac-LEVD-CHO for caspase-4, 1 μM Ac-LEHD-CMK for caspase-9, and 1 μM Z-DEVD-FMK for caspase-3) for 1 h, SK-N-BE(2)-M17 cells were incubated with FLX for 24 h. Cell viability was determined as described in a. f SK-N-BE(2)-M17 cells were transfected with scrambled (Scr) siRNA as a control and caspase-4 siRNA for 24 h and then treated with FLX for 24 h. Cell viability was determined as described in a. Cell lysates were analyzed by Western blot with antibodies against caspase-4 and β-actin. Blots are representative of those obtained in more than three independent experiments. *P < 0.01 compared with vehicle- or Scr siRNA-treated control cells. #P < 0.01 compared with FLX-treated control cells with vehicle (e) or Scr siRNA transfection (f)
To ascertain whether the effect of FLX on cell viability was caused by apoptotic cell death, SK-N-BE(2)-M17 cells were selected and treated with 15 μM FLX for 12 or 24 h and their DNA contents were examined by flow cytometry. This analysis demonstrated that FLX induced an increase in the first peak (sub-G1) with a lower DNA content from 2.1 to 25.3 and 3.5 to 51.9% at 12 and 24 h, respectively (Fig. 1b). Apoptotic cell death was also examined by an annexin V-FITC/PI double labeling assay. The percentages of early apoptotic (annexin V-positive/PI negative) were significantly increased from 2.7 to 22.8% at 12 h and 3.7 to 34.1% at 24 h. Under this condition, the fractions of late apoptotic cells (annexin V-positive/PI-positive) were increased from 1.4 to 7.4 and 1.8 to 9.3% at 12 and 24 h, respectively (Fig. 1c). The percentages of necrotic cells (annexin V-negative /PI-positive) were not altered by FLX treatment for 24 h. Collectively, these results suggest that FLX induces apoptotic cell death in SK-N-BE(2)-M17 human neuroblastoma cells.
Because caspases are critical molecules of apoptotic cell death, we investigated the effect of FLX on caspase activation. SK-N-BE(2)-M17 cells were treated with FLX for the indicated times (2–24 h), and activation (cleavage) of caspase-4, -9, and -3 and PARP cleavage were detected by Western blot analysis. An increase in the cleaved fragments of caspase-4, -9, -3 and PARP was observed upon FLX treatment for 6–24 h (Fig. 1d). To investigate whether caspase activation affected FLX-induced apoptotic cell death, cells were preincubated with caspase inhibitors (Ac-LEVD-CHO for caspase 4, Ac-LEHD-CMK for caspase 9, and Z-DEVD-FMK for caspase 3) for 1 h, followed by treatment with FLX for 24 h. All of caspase inhibitors significantly inhibited FLX-induced cell death in SK-N-BE(2)-M17 cells (Fig. 1e). To confirm whether caspase-4 is a critical factor in FLX-induced apoptosis in SK-N-BE(2)-M17 cells, cells were transfected with caspase-4 siRNA for 24 h, followed by treatment with FLX for 24 h. Compared with those of scrambled siRNA-transfected cells, siRNA knockdown of caspase-4 significantly reduced FLX-induced cell death (Fig. 1f). This result implicates that caspase-4 plays an important role in FLX-induced cell death in SK-N-BE(2)-M17 cells.
FLX induces ER stress-associated proteins in SK-N-BE(2)-M17 cells
Because caspase-4 is shown to be closely related to ER stress-induced cell death [29], we postulated that FLX causes ER stress. To determine whether FLX can increase the expression and activation of ER stress-associated proteins, SK-N-BE(2)-M17 cells were treated with FLX or TG as a positive control for ER stress induction. Western blot analysis was performed to examine the effects of FLX on the expression of ER stress-associated proteins, such as CHOP, GRP78, and sXBP-1, and the cleavage of ATF6α. Results showed the increased expression of CHOP as well as GRP78 and sXBP-1, and cleavage of ATF6α at 12–24 h in cells treated with FLX (Fig. 2a). In addition, the effects of FLX on the ER stress-derived initial unfolded protein response were examined, including the phosphorylation of eIF2α and IRE1α. FLX induced an increase in the phosphorylation of eIF2α and IRE1α at 0.5–2 h (Fig. 2b).
Effect of FLX on ER stress-associated proteins in SK-N-BE(2)-M17 and SH-SY5Y neuroblastoma cells. a, b SK-N-BE(2)-M17 cells were treated with vehicle, 15 μM FLX, or 5 μM TG for the indicated times. Cell lysates were resolved by SDS–PAGE and analyzed by Western blot with antibodies against CHOP, GRP78, sXBP-1, ATF6α (p90), and β-actin in a and P-eIF2α, eIF2α, P-IRE1α, IRE1α, and β-actin in b. c–h SK-N-BE(2)-M17 (c–f) or SH-SY5Y (g, h) cells were preincubated with vehicle, 10 μM salubrinal, or 1 mM 4-PBA for 1 h (c, d, g, h) or transfected with scrambled (Scr) control and CHOP siRNA for 24 h (e, f) and then treated with FLX or TG for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments (c, e, g). Cell lysates were separated by SDS–PAGE and the protein levels of CHOP, cleaved form (C) of caspase-4, or β-actin were detected by Western blot analysis (d, f, h). *P < 0.01 compared with vehicle- or Scr siRNA-treated control cells. #P < 0.01 compared with FLX- or TG-treated cells with vehicle (c, g) or Scr siRNA transfection (e). Results shown are representative of those obtained in more than three independent experiments
To investigate the relationship between FLX-induced ER stress and apoptotic cell death, we used chemical inhibitors of ER stress: salubrinal, a selective inhibitor of eIF2α dephosphorylation, or 4-PBA, a chemical chaperone. SK-N-BE(2)-M17 cells were pretreated with 10 μM salubrinal or 5 mM 4-PBA for 1 h and incubated with FLX or TG for 24 h. Salubrinal or 4-PBA significantly reversed FLX- or TG-induced cell death in SK-N-BE(2)-M17 cells (Fig. 2c), presumably by inhibiting CHOP induction and caspase-4 activation (Fig. 2d). To confirm the role of ER stress and CHOP expression in FLX-induced apoptotic cell death, CHOP expression was blocked by transfection with CHOP siRNA for 24 h, the effects of FLX on cell viability were then examined at 24 h. CHOP knockdown resulted in a significant reduction of FLX- or TG-induced cell death (Fig. 2e, f). These data suggest that FLX-induced CHOP expression might be responsible for apoptotic cell death. We also examined the role of ER stress in FLX-induced cell death in another type of human neuroblastoma SH-SY5Y cells using ER stress inhibitors. The results showed that pretreatment with salubrinal or 4-PBA significantly reversed FLX-induced cell death and CHOP expression as well as caspase-4 cleavage in SH-SY5Y cells (Fig. 2g, h), similarly as shown in SK-N-BE(2)-M17 cells (Fig. 2c, d). Collectively, these results suggest that ER stress plays a key role in FLX-induced cell death in human neuroblastoma cells, including SK-N-BE(2)-M17 and SH-SY5Y cells.
The ASK1/MAPK signaling pathway plays a role in FLX-induced CHOP induction and cell death in SK-N-BE(2)-M17 cells
Previous studies have demonstrated an involvement of MAPKs, such as p38, JNK, and ERK, in ER stress-induced apoptosis [30,31,32]. Therefore, we attempted to determine whether FLX regulates MAPKs by Western blot analysis in SK-N-BE(2)-M17 cells. As shown in Fig. 3a, FLX induced an increase in phosphorylation (activation) of p38, JNK, and ERK within 30 min after FLX treatment. When cells were pretreated with specific inhibitors of p38 (10 μM SB203580), JNK (10 μM SP600125), and MAPK/ERK kinase (MEK) (20 μM PD98059) for 1 h, FLX-induced cell death was partially recovered (Fig. 3b). Consistently, treatment with MAPK inhibitors reduced FLX-induced CHOP expression and caspase-4 cleavage (Fig. 3c). To confirm the roles of MAPK inhibition in FLX-induced apoptosis, cells were transfected with MAPK siRNAs (p38α, JNK1, and ERK1/2) for 24 h and followed by treatment with FLX. The results showed that knockdown of MAPKs partially recovered FLX-induced cell death as well as CHOP expression (Fig. 3d, e). These data suggest that MAPK activation plays a role in FLX-induced ER stress and cell death in SK-N-BE(2)-M17 cells. To confirm the role of MAPKs in FLX-induced ER stress and cell death, we also examined the effect of MAPK inhibitors on FLX-induced cell death, CHOP expression, and caspase-4 cleavage in SH-SY5Y cells. The results showed that the MAPK inhibitors significantly reversed FLX-induced cell death and CHOP expression as well as caspase-4 cleavage in SH-SY5Y cells (Fig. 3f, g). These data suggest that MAPK activation might be responsible for FLX-induced ER stress and subsequent apoptotic cell death in human neuroblastoma.
Effect of FLX on the MAPK family in SK-N-BE(2)-M17 and SH-SY5Y cells. a SK-N-BE(2)-M17 cells were treated with vehicle, 15 μM FLX, or 5 μM TG for the indicated times. Cell lysates were resolved by SDS–PAGE and analyzed by Western blot with antibodies against P-p38, p38, P-JNK, JNK1, P-ERK, ERK, and β-actin. b, f SK-N-BE(2)-M17 (b) or SH-SY5Y (f) cells were preincubated with the MAPK inhibitors (10 μM SB203580 for p38, 10 μM SP600125 for JNK, and 20 μM PD98059 for ERK) for 1 h and then treated with FLX for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments. c, g SK-N-BE(2)-M17 (c) or SH-SY5Y (g) cells were preincubated with vehicle or the MAPK inhibitors for 1 h and then treated with FLX for 12 or 24 h. Cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), or β-actin. d, e SK-N-BE(2)-M17 cells were transfected with scrambled (Scr) control, p38α, JNK1, or ERK siRNAs for 24 h and then treated with FLX for 12 or 24 h. Cell viability was determined by MTT assay (d). Cell lysates were analyzed by Western blot with antibodies against CHOP, p38, JNK1, ERK, and β-actin (e). *P < 0.01 compared with vehicle- or Scr siRNA-treated control cells (b, d). #P < 0.01 compared with FLX-treated cells with vehicle (b, f) or Scr siRNA transfection (d)
ASK1 has been shown to be involved in cancer, diabetes, cardiovascular and neurodegenerative disease [33]. ASK1 is an upstream kinase of MAPK, also called as mitogen-activated protein kinase kinase kinase 5 (MAP3K5) that has previously demonstrated to induce apoptosis by activating p38, JNK, and ERK under various stimuli conditions [33, 34]. To determine the effect of FLX on ASK1 activity, SK-N-BE(2)-M17 cells were treated with FLX for the indicated times (1–24 h), and phosphorylation of ASK1 was detected by Western blot analysis. We found that FLX induced ASK1 phosphorylation at Thr845 (Fig. 4a). Next, to determine the functional role of ASK1 in FLX-induced cell death, cells were pretreated with ASK1 inhibitor 5 μM NQDI-1 followed by treatment with FLX for 24 h. Inhibition of ASK1 significantly blocked FLX-induced cell death, CHOP expression, and caspase-4 cleavage (Fig. 4b, c). To confirm the role of ASK1 in FLX-induced apoptotic cell death, cells were transfected with ASK1 siRNA for 24 h and followed by treatment with FLX for 24 h. The results showed that knockdown of ASK1 blocked FLX-induced cell death and CHOP expression (Fig. 4d, e). The results suggest that ASK1 is involved in FLX-induced apoptosis and ER stress. We then examined whether ASK1 acts upstream of MAPKs in FLX-treated cells. The results showed that inhibition of ASK1 by NQDI-1 and ASK1 siRNA transfection reduced phosphorylation of p38, JNK, and ERK (Fig. 4f, g). These suggest that ASK1 is an upstream of MAPKs in FLX-treated SK-N-BE(2)-M17 cells.
Effect of FLX on ASK1 phosphorylation in SK-N-BE(2)-M17 cells. a Cells were treated with vehicle (−) or 15 μM FLX for the indicated times. Cell lysates were resolved by SDS–PAGE and analyzed by Western blot with antibodies against P-ASK1 (Thr845), ASK1, and β-actin. b–g Cells were preincubated with 10 μM NQDI-1 (ASK1 inhibitor) for 1 h (b, c, f) or transfected with scrambled (Scr) control or ASK1 siRNA for 24 h (d, e, g) and then treated with FLX for the indicated times. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments (b, d). Cell lysates were analyzed by Western blot with antibodies against CHOP, caspase-4(C), ASK1, and β-actin (c, e). Cell lysates were also analyzed by Western blot with antibodies against P-ASK1, ASK-1, P-p38, P-JNK, P-ERK, and β-actin (f, g). *P < 0.01 compared with vehicle- or Scr siRNA-treated control cells. #P < 0.01 compared with FLX-treated cells with vehicle (b) or Scr siRNA transfection (d)
FLX induces mitochondrial dysfunction via ER stress responses in SK-N-BE(2)-M17 cells
Previously, it has been shown that mitochondrial dysfunctions are responsible for ER stress-associated apoptotic cell death [17]. In addition, FLX was shown to cause a loss of mitochondrial membrane potential in Burkitt lymphoma cells [4]. Therefore, to understand whether mitochondrial dysfunction is involved in FLX-induced apoptosis and how they interact each other, we examined the effect of FLX on mitochondrial membrane potential (MMP) loss in SK-N-BE(2)-M17 cells. For the measurement of MMP loss, cells were treated with FLX for 2–12 h. After incubation, cells were treated with DiOC6 for 30 min and MMP was measured using flow cytometry. The data showed that the levels of MMP gradually decreased and reached to 45% at 12 h treatment with FLX (Fig. 5a).
Effect of FLX on MMP loss in SK-N-BE(2)-M17 cells. a Cells were incubated with vehicle or 15 μM FLX for the indicated times. b Cells were preincubated with 10 μM salubrinal or 1 mM 4-PBA for 1 h and followed by treatment with 15 μM FLX or 5 μM TG for 12 h. c Cells were transfected with either the Scr control or CHOP siRNA for 24 h. Cells were then treated with FLX or TG for 12 h. d Cells were preincubated with vehicle or MAPK inhibitors (10 μM SB203580, 10 μM SP600125, 20 μM PD98059) for 1 h and followed by FLX or TG for 12 h. After each treatment, cells were incubated with 20 nM DiOC6 for 30 min and MMP was measured using flow cytometry. The percent MMP was calculated and plotted as the mean ± standard deviation of at least three experiments. *P < 0.01 compared with vehicle- or Scr siRNA-treated cells. #P < 0.01 compared with FLX-or TG-treated cells with vehicle (b, d) or Scr siRNA transfection (c)
To determine whether ER stress affects FLX-induced mitochondrial dysfunction, cells were preincubated with the ER stress inhibitors, salubrinal or 4-PBA, followed by treatment with FLX or TG for 12 h. The results showed that salubrinal or 4-PBA recovered the MMP levels when compared with FLX and TG-treated groups (Fig. 5b). These results suggest that ER stress may contribute to FLX-induced mitochondrial dysfunction and subsequent cell death. To confirm that ER stress is responsible for FLX- or TG-induced MMP loss, SK-N-BE(2)-M17 cells were transfected with CHOP siRNA for 24 h and followed by FLX or TG treatment for 12 h. Compared with scrambled control siRNA-transfected cells, CHOP siRNA transfection significantly recovered MMP loss induced by FLX or TG (Fig. 5c). These results suggest that ER stress has a critical role in FLX-induced mitochondrial damage and subsequent apoptosis in SK-N-BE(2)-M17 cells. Next, to examine whether MAPKs play roles in FLX-induced MMP loss, cells were treated with inhibitors of MAPKs (SB203580, SP600125, and PD98059) and followed by FLX or TG for 12 h. The data showed that treatment with MAPK inhibitors partially blocked FLX-induced MMP loss in SK-N-BE(2)-M17 cells (Fig. 5d). Collectively, our data suggest that all three types of MAPKs, p38, JNK, and ERK, and ER stress might play a role in FLX-induced mitochondrial dysfunction.
Involvement of ROS accumulation and cytosolic Ca2+ signals in the regulation of ER stress-induced apoptosis has also been demonstrated [35]. In addition, FLX was shown to increase ROS and intracellular Ca2+ concentration in Burkitt lymphoma cells [4, 5]. Therefore, we determined whether FLX could induce accumulation of ROS and cytosolic Ca2+ in SK-N-BE(2)-M17 cells. Cells were treated with FLX. Cellular ROS levels were measured using flow cytometry after treatment with DCFH-DA for 30 min. As shown in Fig. 6a, FLX induced ROS generation and pretreatment with a chemical antioxidant N-acetyl cysteine (NAC) at a concentration of 5 mM abrogated FLX-induced ROS production. In addition, we also measured the level of cytosolic Ca2+ with a fluorescent plate reader after cells were stained with Ca2+-sensitive fluorescent dye Fura-2AM for 30 min. We found that FLX markedly induced cytosolic Ca2+ elevation and showed substantial reduction of fluorescent signals in the absence of Ca2+ in extracellular medium (Fig. 6b), suggesting that FLX induces extracellular Ca2+ influx.
Effects FLX on ROS generation and intracellular Ca2+ concentration in SK-N-BE(2)-M17 cells. a Cells were incubated with vehicle or 15 μM FLX for 6 h in the presence or absence of 5 mM NAC. Cells were then treated with 10 μM DCFH-DA for 30 min. Generation of ROS was measured using flow cytometry. Percent ROS generation was calculated from DCF fluorescence and plotted as the mean ± standard deviation of at least three experiments. b Cells were incubated with FLX for the indicated times with or without extracellular Ca2+ and then loaded with Fura-2AM for 30 min. Fluorescence was monitored at 37 °C with a fluorescent plate reader. Intracellular Ca2+ changes (Δ increase in [Ca2+]i) were calculated and plotted. c Cells were preincubated with 5 mM NAC, 20 μM BAPTA-A, or 1 mM EGTA for 1 h and followed by treatment with FLX for 24 h. The cell viability was assessed by MTT assay. The percent viabilities are plotted as the mean ± standard deviation of at least three experiments. *P < 0.01 compared with vehicle-treated cells. #P < 0.01 compared with FLX-treated cells without NAC, BAPTA-AM, or EGTA. d Cells were preincubated with NAC, BAPTA-A, or EGTA for 1 h and followed by FLX for 12 h. Cells were then assessed for MMP by flow cytometry. *P < 0.01 compared with vehicle-treated cells. #P < 0.01 compared with FLX-treated cells without NAC, BAPTA-AM, or EGTA. e Cells were preincubated with NAC, BAPTA-AM, or EGTA for 1 h and followed by FLX for 24 h and cell lysates were analyzed by Western blot with antibodies against CHOP and β-actin. Results shown are representative of those obtained in more than three independent experiments
To investigate whether ROS and Ca2+ release and/or influx are involved in FLX-induced ER stress, mitochondrial dysfunction, and cell death in SK-N-BE(2)-M17 cells, cells were incubated with 5 mM NAC, 20 μM BAPTA-AM (an intracellular Ca2+ chelator), or 1 mM EGTA (an extracellular Ca2+ chelator) for 1 h followed by treatment with FLX. NAC and BAPTA-AM did not affect FLX-induced cell viability, while EGTA reduced FLX-induced cell death (Fig. 6c). Consistently, EGTA but not NAC and BAPTA-AM reduced FLX-induced MMP loss and CHOP expression (Fig. 6d, e).
FLX induces cell death through histone hyperacetylation in SK-N-BE(2)-M17 cells but not in SH-SY5Y cells
HATs and HDACs play important roles in the histone hyperacetylation [20]. Histone hyperacetylation by upregulation of p300 HAT and by downregulation of HDACs is associated with a cancer treatment effect via apoptotic cell death [21,22,23]. To examine whether histone hyperacetylation is involved in FLX effect on cell death, we first examined the effects of FLX and TG on the levels of acetylated histone H3 and H4. Cells were treated with FLX or TG for 6 and 12 h and histone H3 and H4 acetylation levels were examined by Western blot analysis. Treatment with FLX but not TG markedly increased the levels of acetylated H3 and H4 (Fig. 7a), while both FLX and TG showed the elevation of CHOP expression (Fig. 2a).
Effect of FLX on histone hyperacetylation in SK-N-BE(2)-M17 cells. a Cells were treated with vehicle, 15 μM FLX or 5 μM TG for 6 or 12 h. Cell lysates were subjected to Western blot analysis using antibodies against acetylated histone H3 (Ac-H3), H3, acetylated histone H4 (Ac-H4), H4, and β-actin. b, c Cells were pretreated with 5 μM anacardic acid (AA) for 1 h and followed by FLX or TG for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments (b). Cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), and β-actin (c). d, e Cells were transfected with Scr control or p300 HAT (p300) siRNA for 24 h and then treated with FLX or TG for 24 h. Cell viability was determined by MTT assay (d). e The cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), p300, and β-actin. f, g Cells were pretreated with AA, SB203580 (SB), SP600125 (SP), PD98059 (PD) for 1 h and treated with FLX for 24 h. Cell lysates were analyzed by Western blot with antibodies against p300, Ac-H3, Ac-H4, and β-actin in f, and antibodies against HDACs 1-6 and β-actin in g. *P < 0.01 compared with vehicle-treated or Scr siRNA-treated cells. #P < 0.01 compared with FLX- or TG-treated cells with vehicle (b) or Scr siRNA transfection (d)
To determine whether histone acetylation plays a role in FLX-induced apoptotic cell death, cells were preincubated with 5 μM anacardic acid, a HAT inhibitor, and followed by treatment with FLX or TG for 24 h. The results showed that anacardic acid significantly blocked cell death by FLX but not by TG in SK-N-BE(2)-M17 cells (Fig. 7b). Consistently, anacardic acid reduced FLX-induced CHOP induction and caspase-4 cleavage (Fig. 7c). These data suggest that histone acetylation is involved in FLX-induced cell death and ER stress. To confirm the role of HAT in FLX-induced cell death and ER stress, the expression of p300 HAT was inhibited by siRNA transfection. Knockdown of p300 significantly blocked FLX-induced cell death (Fig. 7d) and ER stress-associated CHOP expression and caspase-4 cleavage (Fig. 7e). The results suggest that FLX induces apoptotic cell death by histone hyperacetylation in SK-N-BE(2)-M17 cells.
Previously, it is reported that MAPKs, including p38 and ERK, regulate the activity and expression of p300 HAT [36,37,38]. Thus, we examined the role of MAPKs in FLX-induced p300 HAT expression by using pharmacological MAPK inhibitors. The results showed that MAPK inhibitors (SB203580, SP600125, and PD98059), but not anacardic acid, reduced FLX-induced p300 expression as well as hyperacetylation of H3 and H4 (Fig. 7f). These data suggest that MAPKs are upstream of FLX-induced p300 HAT expression and histone hyperacetylation in SK-N-BE(2)-M17 cells.
HDACs are an enzyme family repressing gene expression by removing acetyl groups from histones to produce a less accessible chromatin structure [39]. HDAC inhibitors have been shown to decrease the expression of HDACs and induce apoptosis in several cancer cells [24]. Thus, to examine whether FLX affects the expression of HDACs in SK-N-BE(2)-M17 cells, cells were treated with FLX and the expression levels of HDAC1-6 were analyzed by Western blot. FLX significantly reduced HDAC1-6 protein expression, which might be in part responsible for the induced hyperacetylation of H3 and H4 in SK-N-BE(2)-M17 cells (Fig. 7g). In order to clarify the involvement of MAPKs in the regulation of HDAC expression, cells were pretreated with the MAPK inhibitors (SB203580, SP600125, and PD98059) and the expression of HDACs was examined. As shown in their effects on p300 HAT, the MAPK inhibitors reversed the FLX-induced downregulation of HDACs, indicating that the MAPK pathways are upstream of histone hyperacetylation via reduction of HDAC expression in SK-N-BE(2)-M17 cells (Fig. 7g).
To investigate whether the histone hyperacetylation is a common pathway for FLX-induced apoptotic cell death in all human neuroblastoma cells, the effects of FLX on histone acetylation and p300 HAT and HDACs expression were examined in another human neuroblastoma SHSY5Y cells. Interestingly, FLX did not show any effects on H3 and H4 hyperacetylation, p300 HAT expression, and HDACs 1-4 protein expression in SH-SY5Y cells (Fig. 8a). Consistently, anacardic acid had no protective effect on FLX-induced cell death, CHOP expression, and caspase-4 cleavage in SH-SY5Y cells (Fig. 8b, c). These data suggest that histone hyperacetylation may be differentially involved in FLX-induced cell death, depending on cell types in human neuroblastoma.
Effects of FLX on histone hyperacetylation in SH-SY5Y cells. a Cells were treated with vehicle or 15 μM FLX for the indicated times. Cell lysates were subjected to Western blot analysis using antibodies against acetylated histone H3 (Ac-H3), acetylated histone H4 (Ac-H4), p300, HDACs 1-4, and β-actin. b SH-SY5Y cells were pretreated with 5 μM AA for 1 h and followed by FLX for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments. *P < 0.01 compared with vehicle-treated cells. c The lysates prepared from cells in b were treated with FLX for 12 or 24 h in the presence of vehicle or AA and cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), and β-actin
MYCN plays a role in FLX-induced histone hyperacetylation and cell death in SK-N-BE(2)-M17 cells
In the present study, it was observed that FLX induces cell death through histone hyperacetylation in SK-N-BE(2)-M17 cells but not in SH-SY5Y cells. These cells are different in terms of MYCN amplification:MYCN is amplified in SK-N-BE(2)-M17 cells but not in SH-SY5Y cells. MYCN amplification occurs in ~25% of human neuroblastomas and is associated with high-risk disease and poor prognosis [13]. MYCN gene product N-Myc can be acetylated by HATs and plays a role in epigenome regulation and HDACs expression [40,41,42]. Therefore, MYCN might play a role in FLX-induced histone hyperacetylation and cell death in SK-N-BE(2)-M17 cells. To confirm the role of MYCN in these FLX-induced changes in SK-N-BE(2)-M17 cells, cells were transfected with MYCN siRNA for 24 h and cells were pretreated with anacardic acid for 1 h and followed by FLX for 24 h. The results showed that MYCN knockdown did not affect FLX-induced cell death but it abrogated the protective effects of anacardic acid on FLX-induced cell death, CHOP expression, caspase-4 cleavage, and p300 HAT expression in SK-N-BE(2)-M17 cells (Fig. 9a, b). Consistently, MYCN siRNA transfection blocked FLX-induced histone H3 and H4 hyperacetylation and HDACs 1-4 downregulation (Fig. 9c). These data suggest that MYCN plays a role in histone hyperacetylation via p300 HAT upregulation and HDACs downregulation in MYCN amplified SK-N-BE(2)-M17 cells.
Effects of MYCN on FLX-induced histone hyperacetylation in SK-N-BE(2)-M17 cells. a, b Cells were transfected with Scr control or MYCN siRNA for 24 h. Cells were then treated with 5 μM anacardic acid (AA) for 1 h and followed by 15 μM FLX for 24 h. Cell viability was determined by MTT assay. *P < 0.01 compared with vehicle-treated cells with Scr siRNA transfection. #P < 0.01 compared with FLX-treated cells with Scr siRNA transfection (a). The cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), p300, N-Myc, and β-actin (b). c After MYCN siRNA transfection for 24 h, cells were treated with 15 μM FLX for the indicated times. Cell lysates were subjected to Western blot analysis using antibodies against acetylated histone H3 (Ac-H3), acetylated histone H4 (Ac-H4), HDACs 1-4, and β-actin
MYCN is also overexpressed in several types of cancer including glioblastoma [43]. Thus, to examine the role of MYCN in FLX-induced histone hyperacetylation and cell death, the effects of FLX on N-Myc overexpressed U251MG human glioblastoma cells were further studied and the data are presented as supplementary figures. As judged by Western blot analysis, it is shown that FLX increases the expression of ER stress-associated proteins, CHOP and sXBP-1, and cleavage of ATF6α and caspase-4 at 6–24 h in U251MG cells (Supplementary Fig. S1a). In addition, ER stress inhibitors, such as salubrinal or 4-PBA, significantly reversed FLX-induced cell death and CHOP expression in U251MG cells (Supplementary Fig. S1b, c), suggesting that FLX can induce cell death via ER stress, similarly as shown in human neuroblastoma cells (Fig. 2). FLX also induced apoptotic cell death via histone hyperacetylation and treatment with anacardic acid as well as knockdown of p300 HAT blocked FLX-induced cell death, CHOP induction, caspase-4 cleavage, H3/H4 histone acetylation, and HDACs downregulation in U251MG cells (Supplementary Fig. S1d–f). In addition, MYCN knockdown by siRNA transfection did not affect FLX-induced cell death and CHOP expression, and caspase-4 cleavage but significantly abrogated the protective effect of anacardic acid on FLX-induced cell death in U251MG cells, similarly as shown in SK-N-BE(2)-M17 cells (Supplementary Fig. S2a). MYCN knockdown inhibited FLX-induced H3 and H4 hyperacetylation, p300 HAT expression and HDACs 1-4 downregulation (Supplementary Fig. S2b). These data suggest that MYCN plays a role in FLX-induced histone hyperacetylation and cell death in N-Myc overexpressed glioblastoma cells.
Discussion
FLX has been shown to inhibit proliferation and induce apoptosis in various cancer cells, including colon, breast, ovarian, lymphoma cells [5, 9, 44, 45]. In this study, we also showed that FLX induces apoptotic cell death in SK-N-BE(2)-M17 and SH-SY5Y human neuroblastoma cells and its cytotoxic effect was much greater in neuroblastoma cells than in normal hippocampus neuronal HT22 cells (Fig. 1a). As shown in the previous studies on FLX, the executive caspase-3 and intrinsic caspase-9 were also activated in SK-N-BE(2)-M17 cells (Fig. 1d). Involvement of these caspases in FLX-induced apoptotic cell death was clarified by using caspase inhibitors, Ac-LEHD-CMK for caspase-9 and Z-DEVD-FMK for caspase-3 (Fig. 1e). In addition to caspase-3 and -9, FLX also induced cleavage and activation of ER stress-associated caspase-4 (Fig. 1d). Caspase-4 inhibition by specific chemical inhibitor Ac-LEVD-CHO (Fig. 1e) and its knockdown by siRNA transfection blocked FLX-induced cell death (Fig. 1f). Furthermore, FLX induced phosphorylation, expression or cleavage/activation of various ER stress-associated proteins, such as eIF2α, IRE1α, CHOP, GRP78, sXBP-1 and ATF6α (Fig. 2a, b). Inhibition of ER stress by the ER stress inhibitors, salubrinal and 4-PBA, and by CHOP siRNA transfection, reduced FLX-induced apoptotic cell death (Fig. 2c–f). Similar to SK-N-BE(2)-M17 cells, FLX induced ER stress associated proteins (data not shown) and the ER stress inhibitors salubrinal and 4-PBA reduced FLX-induced cell death, CHOP expression, and caspase-4 cleavage in another human neuroblastoma SH-SY5Y cells (Fig. 2g, h). These data suggest that ER stress might be involved in FLX-induced apoptotic cell death in human neuroblastoma cells.
FLX was shown to induce apoptosis through mitochondrial dysfunction in Burkitt lymphoma cells [4], in glioma and neuroblastoma cells [6], and ovarian carcinoma cell lines [9]. Similarly, we found that FLX reduced MMP in SK-N-BE(2)-M17 cells (Fig. 5a), suggesting that mitochondrial dysfunction may be a generalized mechanism in FLX-induced apoptosis. It is known that there is a cross-talk between ER and mitochondria during apoptosis and ER stress-induced cell death requires mitochondrial membrane permeabilization [46]. Because both mitochondrial and ER events are implicated in FLX-induced cell death, we examined how ER stress and mitochondrial dysfunction cross-talk each other in FLX-induced death pathways. We demonstrated that an inhibition of ER stress by pharmacological and molecular means prevented FLX-induced MMP reduction (Fig. 5b, c). This suggests that ER stress plays a role in mitochondrial dysfunction in FLX-treated SK-N-BE(2)-M17 cells.
The MAPKs pathways including p38, JNK, and ERK have been shown to play essential roles in apoptotic cell death [47]. Similarly, we found that FLX induced the activation and phosphorylation of p38, JNK, and ERK (Fig. 3a). Inhibition of MAPKs by MAPK inhibitors SB203580 for p38, SP600125 for JNK, and PD98059 for ERK reduced FLX-induced caspase-4 cleavage/activation, CHOP expression, and subsequent cell death in both SK-N-BE(2)-M17 and SH-SY5Y cells (Fig. 3b, c, f, g). Furthermore, knockdown of MAPKs by MAPKs siRNA transfection reduced FLX-induced CHOP expression and cell death (Fig. 3d, e). Consistent with its effects on cell viability, treatment with MAPK inhibitors partially blocked FLX-induced MMP loss in SK-N-BE(2)-M17 cells (Fig. 5d), suggesting that three MAPKs, p38, JNK, and ERK, are involved in FLX-induced mitochondrial dysfunction. In this study, we also found that FLX induced phosphorylation on Thr845 of ASK1, an upstream kinase of MAPKs (Fig. 4a). Inhibition of ASK1 by pharmacological inhibitor NQDI-1 or ASK1 knockdown by siRNA transfection reduced FLX-induced activation of ERK as well as p38 and JNK (Fig. 4f, g), CHOP expression (Fig. 4c, e), and cell death (Fig. 4b, d). These results suggest that activation of ASK1 and subsequent MAPKs activation might be responsible for FLX-induced ER stress, MMP loss, and cell death in SK-N-BE(2)-M17 cells.
Cytosolic ROS and Ca2+ are cross-talking messengers and their downstream signals play important roles in various cellular processes including apoptotic cell death and survival [48]. Certain apoptotic stimuli can cause mitochondrial dysfunction by inducing Ca2+ release from the ER [17] or importing Ca2+ from the extracellular medium via activation of ion channels or receptors [7]. Previously, it was reported that FLX-induced cell death was independent on ROS accumulation and intracellular Ca2+ release in the chemoresistant Burkitt lymphoma DG-75 cells and was dependent on extracellular Ca2+ influx in chemosensitive MUTU-1 cells [5]. In agreement with the Cloonan’s reports, although FLX increased ROS and cytosolic Ca2+ concentration (Fig. 6a, b), EGTA but not NAC and BAPTA-AM could block the FLX-induced CHOP expression, MMP loss, and cell death in SK-N-BE(2)-M17 cells (Fig. 6c–e). Similar to our results, FLX-induced cell death was not altered by chelating cytosolic Ca²+ with BAPTA-AM, indicating that cytosolic Ca2+ is not involved in FLX-induced cell death in OC2 human oral cancer cells [49]. However, FLX caused apoptotic cell death in human glioblastoma cell lines by directly binding to α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) receptor, inducing transmembrane Ca2+ influx and mitochondrial damage [7]. Collectively, these results suggest that extracellular Ca2+ influx rather than intracellular Ca2+ release is responsible for ER stress and apoptotic cell death in FLX-treated SK-N-BE(2)-M17 cells.
It has been reported that histone hyperacetylation via either upregulation of p300 HAT or inhibition of HDACs is associated with proapoptotic effects in various cancer cells [21, 22, 24] and neuronal cells [50]. The HDAC inhibitors displayed antidepressant-like functions and improved behavioral disturbance in mouse chronic mental disorder models [25,26,27, 51,52,53]. In addition, the HDAC inhibitor SAHA partially rescues the depressive-like behavior in the Crtc1−/− mice [54]. In this study, we found that FLX, as a type of antidepressants, induced hyperacetylation of histones H3 and H4 in SK-N-BE(2)-M17 (Fig. 7a) and U251MG cells (Supplementary Fig. S1e, f), presumably via overexpression of p300 HAT and downregulation of HDACs. Involvement of p300 HAT was confirmed by the inhibitory effects of anacardic acid, a HAT inhibitor, on FLX-induced apoptosis, CHOP expression, and caspase-4 cleavage/activation in SK-N-BE(2)-M17 (Fig. 7b, c) and U251MG cells (Supplementary Fig. S1d, e). Their recovery after p300 HAT knockdown also demonstrated that p300 HAT is responsible for FLX-induced hyperacetylation (Fig. 7, S1). Because FLX also reduced the expression of HDAC 1-6 proteins in SK-N-BE(2)-M17 and U251MG cells (Fig. 7, S1), FLX might exert its effect on hyperacetylation via both p300 HAT and HDACs in SK-N-BE(2)-M17 and U251MG cells. The roles of individual HAT and HDAC isoforms in FLX-induced histone hyperacetylation and cell death remained to be clarified.
Interestingly, FLX did not induce histone H3 and H4 hyperacetylation, p300 HAT induction, and HDACs 1-4 protein downregulation in SH-SY5Y cells (Fig. 8a). Consistently, HAT inhibition by anacardic acid had no effect on FLX-induced cell death, CHOP expression, and caspase-4 cleavage in SH-SY5Y cells (Fig. 8b, c). It is shown that SK-N-BE(2)-M17 and U251MG cells are MYCN amplified and overexpressed but SH-SY5Y cells are not. MYCN is a member of the MYC family of proto-oncogenes, stimulates tumorigenesis, and regulates epigenome and HDACs expression [40,41,42,43]. In the present study, it was observed that MYCN siRNA transfection did not affect FLX -induced cell death, CHOP induction, and caspase-4 cleavage (Fig. 9b), but it reduced the effect of FLX on H3/H4 acetylation and HDACs downregulation in MYCN amplified SK-N-BE(2)-M17 and overexpressed U251MG cells (Fig. 9c, S2b, respectively). Moreover, MYCN knockdown abrogated the protective effect of anacardic acid on FLX-induced cell death in SK-N-BE(2)-M17 and U251MG cells. The inhibitory effects of anacardic acid on FLX-induced CHOP expression and caspase-4 cleavage were blocked in the MYCN knockdown cells (Fig. 9b, S2b). These data suggest that MYCN plays a role in FLX-induced histone hyperacetylation and cell death in MYCN amplified and overexpressed tumor cells. In agreement with our study, N-Myc downstream target gene NDRG2 has been suggested to regulate histone acetylation in glioblastoma cells [55]. In the present study, Because FLX treatment did not affect the N-Myc protein expression levels in SK-N-BE(2)-M17 and U251MG cells (Fig. 9b, S2b), the regulatory mechanisms of MYCN for FLX-induced changes in p300 HAT and HDAC isoforms remain to be clarified in MYCN amplified cells.
Previously, it was reported that p300 HAT can be phosphorylated and potentiated by p38 signaling in organochlorine-stimulated cells [36]. ERK1/2 was also implicated in the phosphorylation and expressional regulation of p300 HAT [36,37,38]. In this study, we found that the inhibition of MAPKs reduced FLX-induced p300 HAT expression and histone hyperacetylation of H3 and H4 (Fig. 7f). This suggests that MAPKs mediate FLX-induced p300 HAT expression and hyperacetylation of H3 and H4. Interestingly, we also found that the MAPK inhibitors reversed the inhibitory effect of FLX on HDACs expression (Fig. 7g). These data suggest that MAPKs activation by FLX can cause HDACs downregulation as well as p300 HAT induction, resulting in hyperacetylation of H3 and H4 and cell death in human neuroblastoma cells. The phosphorylation status of HAT and HDAC isoforms and their transcription factors including N-Myc in FLX-treated cells should be clarified by further studies.
In conclusion, FLX induces apoptosis in SK-N-BE(2)-M17 cells via ER stress and mitochondrial dysfunction through ASK1 and MAPKs pathway. Moreover, FLX increases levels of acetylated H3 and H4 and inhibition of HAT activity and p300 HAT expression reduces FLX-induced cell death and ER stress in SK-N-BE(2)-M17 cells in a MYCN-dependent manner. These results suggest that FLX induces apoptosis through disturbing the balance between acetylation and deacetylation under MYCN amplification/overexpression. Further studies are required to determine the anti-cancer effect and action mechanisms of FLX on in vivo human neuroblastoma xenograft model and the possibility of its development as an anti-tumor agent for the malignant brain carcinoma.
Change history
30 September 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10495-022-01772-7
References
Dasgupta R, Billmire D, Aldrink JH, Meyers RL (2017) What is new in pediatric surgical oncology? Curr Opin Pediatr 29:3–11
Schor NF (2009) New approaches to pharmacotherapy of tumors of the nervous system during childhood and adolescence. Pharmacol Ther 122:44–55
Bielecka AM, Obuchowicz E (2013) Antidepressant drugs as a complementary therapeutic strategy in cancer. Exp Biol Med 238:849–858
Serafeim A, Holder MJ, Grafton G et al (2003) Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood 101:3212–3219
Cloonan SM, Williams DC (2011) The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int J Cancer 128:1712–1723
Levkovitz Y, Gil-Ad I, Zeldich E, Dayag M, Weizman A (2005) Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J Mol Neurosci 27:29–42
Liu KH, Yang ST, Lin YK et al (2015) Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget 6:5088–5101
Krishnan A, Hariharan R, Nair SA, Pillai MR (2008) Fluoxetine mediates G0/G1 arrest by inducing functional inhibition of cyclin dependent kinase subunit (CKS)1. Biochem Pharmacol 75:1924–1934
Lee CS, Kim YJ, Jang ER, Kim W, Myung SC (2010) Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-κB. Basic Clin Pharmacol Toxicol 106:446–453
Bowie M, Pilie P, Wulfkuhle J et al (2015) Fluoxetine induces cytotoxic endoplasmic reticulum stress and autophagy in triple negative breast cancer. World J Clin Oncol 6:299–311
Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22:8608–8618
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21:871–877
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11:372–380
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Zinszner H, Kuroda M, Wang X et al (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
Choi JH, Lee JY, Choi AY et al (2012) Apicidin induces endoplasmic reticulum stress- and mitochondrial dysfunction-associated apoptosis via phospholipase Cγ1- and Ca(2+)-dependent pathway in mouse Neuro-2a neuroblastoma cells. Apoptosis 17:1340–1358
Grunstein M (1997) Histone acetylation in chromatin structure and transcription. Nature 389:349–352
Wade PA, Pruss D, Wolffe AP (1997) Histone acetylation: chromatin in action. Trends Biochem Sci 22:128–132
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Kaur J, Tikoo K (2013) p300/CBP dependent hyperacetylation of histone potentiates anticancer activity of gefitinib nanoparticles. Biochim Biophys Acta 1833:1028–1040
Wu TC, Lin YC, Chen HL, Huang PR, Liu SY, Yeh SL (2016) The enhancing effect of genistein on apoptosis induced by trichostatin A in lung cancer cells with wild type p53 genes is associated with upregulation of histone acetyltransferase. Toxicol Appl Pharmacol 292:94–102
Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277:8–21
Somech R, Izraeli S, A JS (2004) Histone deacetylase inhibitors—a new tool to treat cancer. Cancer Treat Rev 30:461–472
Covington HE 3rd, Maze I, LaPlant QC et al (2009) Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29:11451–11460
Han A, Sung YB, Chung SY, Kwon MS (2014) Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology 81:292–302
Jochems J, Boulden J, Lee BG et al (2014) Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology 39:389–400
Aires V, Hichami A, Filomenko R et al (2007) Docosahexaenoic acid induces increases in [Ca2+]i via inositol 1,4,5-triphosphate production and activates protein kinase Cγ and -δ via phosphatidylserine binding site: implication in apoptosis in U937 cells. Mol Pharmacol 72:1545–1556
Di Fazio P, Ocker M, Montalbano R (2012) New drugs, old fashioned ways: ER stress induced cell death. Curr Pharm Biotechnol 13:2228–2234
Darling NJ, Cook SJ (2014) The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta 1843:2150–2163
Wang XZ, Ron D (1996) Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272:1347–1349
Urano F, Wang X, Bertolotti A et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666
Hattori K, Naguro I, Runchel C, Ichijo H (2009) The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal 7:9
Lin T, Chen Y, Ding Z, Luo G, Liu J, Shen J (2013) Novel insights into the synergistic interaction of a thioredoxin reductase inhibitor and TRAIL: the activation of the ASK1-ERK-Sp1 pathway. PLoS ONE 8:e63966
Choi AY, Choi JH, Hwang KY et al (2014) Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cgamma1-, Ca(2+)-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis 19:682–697
Bratton MR, Frigo DE, Vigh-Conrad KA et al (2009) Organochlorine-mediated potentiation of the general coactivator p300 through p38 mitogen-activated protein kinase. Carcinogenesis 30:106–113
Meissner JD, Freund R, Krone D et al (2011) Extracellular signal-regulated kinase 1/2-mediated phosphorylation of p300 enhances myosin heavy chain I/β gene expression via acetylation of nuclear factor of activated T cells c1. Nucleic Acids Res 39:5907–5925
Tsai YJ, Tsai T, Peng PC, Li PT, Chen CT (2015) Histone acetyltransferase p300 is induced by p38MAPK after photodynamic therapy: the therapeutic response is increased by the p300HAT inhibitor anacardic acid. Free Radic Biol Med 86:118–132
Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ (2007) Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci 31:47–58
He S, Liu Z, Oh DY, Thiele CJ (2013) MYCN and the epigenome. Front Oncol 3:1
Marshall GM, Gherardi S, Xu N et al (2010) Transcriptional upregulation of histone deacetylase 2 promotes Myc-induced oncogenic effects. Oncogene 29:5957–5968
Sun Y, Liu PY, Scarlett CJ et al (2014) Histone deacetylase 5 blocks neuroblastoma cell differentiation by interacting with N-Myc. Oncogene 33:2987–2994
Pession A, Tonelli R (2005) The MYCN oncogene as a specific and selective drug target for peripheral and central nervous system tumors. Curr Cancer Drug Targets 5:273–283
Kannen V, Hintzsche H, Zanette DL et al (2012) Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS ONE 7:e50043
Zhou T, Duan J, Wang Y et al (2012) Fluoxetine synergys with anticancer drugs to overcome multidrug resistance in breast cancer cells. Tumour Biol 33:1299–1306
Boya P, Cohen I, Zamzami N, Vieira HL, Kroemer G (2002) Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. Cell Death Differ 9:465–467
Choi AY, Choi JH, Yoon H et al (2011) Luteolin induces apoptosis through endoplasmic reticulum stress and mitochondrial dysfunction in Neuro-2a mouse neuroblastoma cells. Eur J Pharmacol 668:115–126
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565
Lin KL, Chou CT, Cheng JS et al (2014) Effect of fluoxetine on [Ca(2)(+)]i and cell viability in OC2 human oral cancer cells. Chin J Physiol 57:256–264
Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG (2010) Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol 77:621–632
Covington HE 3rd, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ (2011) Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett 493:122–126
Covington HE 3rd, Maze I, Vialou V, Nestler EJ (2015) Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience 298:329–335
Schroeder FA, Lin CL, Crusio WE, Akbarian S (2007) Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62:55–64
Meylan EM, Halfon O, Magistretti PJ, Cardinaux JR (2016) The HDAC inhibitor SAHA improves depressive-like behavior of CRTC1-deficient mice: possible relevance for treatment-resistant depression. Neuropharmacology 107:111–121
Li L, Qin X, Shi M et al (2012) Regulation of histone acetylation by NDRG2 in glioma cells. J Neurooncol 106:485–492
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (Nos. 2011-0030721; 2015R1D1A1A01057508) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (No. HI14C2700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Effects of FLX on ER stress and histone acetylation in U251MG human glioblastoma cells. a Cells were treated with vehicle or 25 μM FLX for the indicated times. Cell lysates were analyzed by Western blot with antibodies against CHOP, sXBP-1, ATF6α (p90), cleaved caspase-4(C), and β-actin. b, c cells were preincubated with 10 μM salubrinal or 1 mM 4-PBA for 1 h and then treated with FLX for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments (b). The protein levels of CHOP and β-actin were detected by Western blot analysis (c). d, e Cells were pretreated with 5 μM anacardic acid (AA) for 1 h or transfected with Scr control or p300 siRNA for 24 h and then followed by FLX for 24 h. Cell viability was determined by MTT assay and the percent viabilities are plotted as the mean ± standard deviation of at least three experiments (d). Cell lysates were analyzed by Western blot with antibodies against CHOP, cleaved caspase-4(C), Ac-H3, Ac-H4, p300, and β-actin (e). f Cells were treated with FLX for the indicated times. Cell lysates were analyzed by Western blot with antibodies against HDACs 1-6, Ac-H3, Ac-H4, and β-actin. *P < 0.01 and **P < 0.01 compared with vehicle- or Scr siRNA-treated cells. #P < 0.01 and ##P < 0.01 compared with FLX-treated cells with vehicle (b) or Scr siRNA transfection (d) (JPG 72 kb)
Fig. S2
Effects of MYCN on FLX-induced histone hyperacetylation in U251MG cells. a Cells were transfected with Scr control or MYCN siRNA for 24 h. Cells were then treated with vehicle or 5 μM anacardic acid (AA) for 1 h and followed by 15 μM FLX for 24 h. Cell viability was determined by MTT assay. *P < 0.01 compared with vehicle-treated cells with Scr siRNA transfection. #P < 0.01 compared with FLX-treated cells with Scr siRNA transfection. b After Scr or MYCN siRNA transfection for 24 h, cells were treated with 15 μM FLX for 12 or 24 h. Cell lysates were subjected to Western blot with antibodies against CHOP, cleaved caspase-4(C), Ac-H3, Ac-H4, HDACs 1-4, p300, N-Myc, and β-actin (JPG 42 kb)
About this article
Cite this article
Choi, J.H., Jeong, Y.J., Yu, AR. et al. RETRACTED ARTICLE: Fluoxetine induces apoptosis through endoplasmic reticulum stress via mitogen-activated protein kinase activation and histone hyperacetylation in SK-N-BE(2)-M17 human neuroblastoma cells. Apoptosis 22, 1079–1097 (2017). https://doi.org/10.1007/s10495-017-1390-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1390-2