Abstract
As a glycol-protein located in extracellular matrix (ECM), tenascin-C (TNC) is absent in most normal adult tissues but is highly expressed in the majority of malignant solid tumors. Pancreatic cancer is characterized by an abundant fibrous tissue rich in TNC. Although it was reported that TNC’s expression increased in the progression from low-grade precursor lesions to invasive cancer and was associated with tumor differentiation in human pancreatic cancer, studies on the relations between TNC and tumor progression in pancreatic cancer were rare. In this study, we performed an analysis to determine the effects of TNC on modulating cell apoptosis and chemo-resistance and explored its mechanisms involving activation in pancreatic cancer cell. The expressions of TNC, ERK1/2/p-ERK1/2, Bcl-xL and Bcl-2 were detected by immunohistochemistry and western blotting. Then the effects of exogenous and endogenous TNC on the regulation of tumor proliferation, apoptosis and gemcitabine cytotoxicity were investigated. The associations among the TNC knockdown, TNC stimulation and expressions of ERK1/2/NF-κB/p65 and apoptotic regulatory proteins were also analyzed in cell lines. The mechanism of TNC on modulating cancer cell apoptosis and drug resistant through activation of ERK1/2/NF-κB/p65 signals was evaluated. The effect of TNC on regulating cell cycle distribution was also tested. TNC, ERK1/2/p-ERK1/2, and apoptotic regulatory proteins Bcl-xL and Bcl-2 were highly expressed in human pancreatic cancer tissues. In vitro, exogenous TNC promoted pancreatic cancer cell growth also mediates basal as well as starved and drug-induced apoptosis in pancreatic cancer cells. The effects of TNC on anti-apoptosis were induced by the activation state of ERK1/2/NF-κB/p65 signals in pancreatic cell. TNC phosphorylate ERK1/2 to induce NF-κB/p65 nucleus translocation. The latter contributes to promote Bcl-xL, Bcl-2 protein expressions and reduce caspase activity, which inhibit cell apoptotic processes. TNC mediated gemcitabine chemo-resistance via modulating cell apoptosis in pancreatic cancer. TNC resulted in the enrichment of pancreatic cancer cells in S-phase with a concomitant decrease in number of cells in G1 phase. The present study indicated TNC in cellular matrix induces an activation of ERK1/2/NF-κB/p65 signaling cascade and thereby mediates resistance to apoptosis in pancreatic cancer. TNC could serve as a diagnostic marker and predictor of gemcitabine response and potentially as a target for chemotherapy of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the fourth most common cause of cancer death across the globe [1]. It is characterized by a rapid disease progression, high metastatic property, and difficult early diagnosis and treatment [2, 3]. Therefore, a better understanding of the mechanisms involved in tumor progression is needed to develop a new effective target for the treatment of this fatal disease. The characteristic feature of pancreatic cancers is the desmoplastic/stromal reaction [4, 5]. The pathogenesis of the desmoplastic reaction consists of abundant fibrous tissue composed of ECM proteins, new blood vessels, and stromal cells [6]. The development of the desmoplastic reaction is induced by the pancreatic stellate cells (PSCs). PSCs transform from a quiescent into an active myofibroblast like phenotype and secrete ECM proteins during tumor development, which establishes a microenvironment to promote cancer progression and produce the barrier to inhibit tumor chemo-sensitivity [7, 8].
As a ECM glycol-protein, tenascin-C (TNC) is absent in most normal adult tissues but is highly expressed in tumor tissue in the majority of malignant solid tumors, including brain, breast, uterus, ovaries, prostate, pancreas and colon [9]. Several studies have reported that TNC is the important determinant of the tumor stroma and plays a significant role in the regulation of the tumor development. TNC’s high expression is correlated with low survival rates in several tumors, such as prostate carcinoma, lung cancer, osteosarcoma, glioma, colorectal carcinoma and breast cancer [10].
TNC is mainly secreted by PSCs and tumor cell in pancreatic cancer and constitute an important factor in the tumor-specific microenvironment. High protein levels of TNC have been detected and were associated with tumor differentiation in pancreatic cancer [11]. TNC’s mRNA and protein levels increase in the progression from low-grade precursor lesions [pancreatic intraepithelial neoplasia (PanIN)] to pancreatic ductal adenocarcinoma (PDAC). TNC’s mRNA also expresses in pancreatic cancer cell lines. In addition, it was found that TNC is mainly expressed in the stroma around the neoplastic glands in PDAC [11, 12]. Indeed, TNC is involved in modulating tumor progression in pancreatic cancer, but the underlying mechanism responsible for its effects still needs to be explored. TNC is a huge extracellular matrix molecule and is composed of six monomers linked at their N-termini with disulfide bonds to form a 1080–1500 kDa hexamer. The multidomain molecule consists of an N-terminal assembly domain, followed by 14 1/2 EGF-like repeats, eight constant and up to nine alternatively spliced fibronectin type-III repeats and a C-terminal fibrinogen-like globular domain, each subdomain potentially has a distinct function. TNC interacts with the ECM molecules and several cell surface receptors to active molecular pathways and affects cell functions, such as proliferation, invasion, apoptosis and migration [13]. TNC was found to be able to affect the proliferation, migration and adhesion of poorly differentiated pancreatic cancer cell lines through activation of the integrin/AKT pathway [14]. TNC also stimulates colon carcinoma cell invasion through blocking RhoA and activating Rac, which is consistent with their postulated roles in cell migration [15]. Additionally, in vascular smooth muscle cells, TNC can protect cells against apoptosis through interacting with the αvβ3 integrin and regulate growth factor [16]. Apoptosis or programmed cell death plays an important role in tissue homeostasis regulation. The resistance to apoptosis is regarded as one of the hallmarks of cancer cells and contributes to anticancer treatment failure. TNC can be induced in a tumor by various pro- and anti-inflammatory cytokines such as TNFa, IFNg and by growth factors such as EGF, TGF-β, and CTGF [17], and NF-κB [18], which are mostly secreted by the stromal cells. It has been found that TNC cytotactin-encoded EGF-like repeat protein affects proliferation through the activation of ERK1/2/MAPK in NR6 WT cell line [19]. Several studies also have reported that activation of ERK/NF-κB single down-modulates tumor cell apoptotic response [20–23]. PSCs create a reactive microenvironment to induce the evasion of cancer cell apoptosis [24]. Consequently, as the specific factor existed in tumor ECM, TNC may be involved in the functions of modulating tumor cell apoptosis.
In this study, we performed an analysis to determine the effects of TNC on modulating cell apoptosis and explored its mechanisms involving activation in pancreatic cancer. Our results show the important role of TNC in the regulation of tumor cell apoptosis and drug resistance and indicate the signaling events downstream of TNC.
Results
Overexpression of TNC, ERK1/2/p-ERK1/2, Bcl-xL and Bcl-2 protein in pancreatic cancer tissues
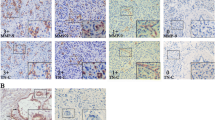
We performed immunohistochemical (IHC) analysis to test the expression levels of TNC, ERK1/2, p-ERK1/2, Bcl-xL and Bcl-2 protein in pancreatic cancer tissues and paracarcinoma tissues. Higher expression levels of TNC, ERK1/2, p-ERK1/2, Bcl-xL and Bcl-2 were found in pancreatic cancer tissues and were also expressed more intensely in pancreatic cancer than in paracarcinoma tissues. In addition, TNC’s expression is intensely localized in tumor stroma. The stains of ERK1/2, p-ERK1/2, Bcl-xL and Bcl-2 were only in the intracellular fraction and found in both the cytoplasm and nucleus of cancer cells (Fig. 1a).
The expression patterns of TNC, ERK1/2, p-ERK1/2, Bcl-2, Bcl-xL, NF-κB/p65 proteins in pancreatic cancer and paracarcinoma tissues. a Representative immunohistochemistry staining of TNC (n = 25), ERK1/2, p-ERK1/2, Bcl-xL and Bcl-2 proteins (n = 15) (all brown) in human pancreatic cancer and paracarcinoma tissues (Original magnification ×40); b The relative rate of TNC positive and negative expressions in pancreatic cancer and paracarcinoma tissues (n = 25); c Scatter plot evaluated the scores of TNC protein expressed in pancreatic adenocarcinoma and paracarcinoma tissues; d Western blotting results of TNC, ERK1/2, p-ERK1/2, Bcl-xL and Bcl-2 proteins. *p < 0.05 (Color figure online)
The score of TNC’s expression was predominantly positive (90 %), while the negative-expression (scores = 0) was mostly found in paracarcinoma tissue (Fig. 1b).The average score of TNC in pancreatic cancer tissue was increased more than 3-fold (Fig. 1c). The protein’s expression was further confirmed using western blot analysis (Fig. 1d). The results indicated that TNC, ERK1/2, p-ERK1/2, Bcl-xL, and Bcl-2 might play important roles in the tumor development of pancreatic cancer.
Effects of TNC on pancreatic cancer cell growth and apoptosis
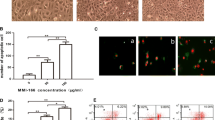
Because TNC exerts its function as an ECM-protein, we tested whether TNC could affect cell viability when cells were grown on a TNC-rich matrix. PANC-1, Capan-2 and SW1990 cells were seeded on TNC-coated plates, grown up to 72 h in serum-free medium and then measured with the CCK8 assay. Cells showed approximately a 50–70 % increase in cell viability, with PANC-1 cells increasing more than Capan-2 and SW1990 cells (Fig. 2a). We further evaluated the effect of exogenous TNC on drug-induced apoptosis in pancreatic cancer cell lines. It was shown that TNC significantly reduced starved-induced apoptosis rates in PANC-1, Capan-2 and SW1990 cells, as determined by assaying the effector caspase-3 activity using a specific assay that measures cleavage of a luminogenic caspase-3 substrate and cleavage of PARP (Fig. 2b). In addition, the result of the IC50 detection showed that there were no significant differences among the three cell lines, and PANC-1 was chosen for further study (Fig. 2c). Gemcitabine, an apoptosis-inducing drug, is commonly applied in pancreatic cancer patients. We used the nucleoside analog gemcitabine to evaluate drug-induced apoptosis of cancer cells. We found that TNC can inhibit the gemcitabine’s effect on cell proliferation by a CCK8 assay (Fig. 3a). Then, using toxicology testing, the IC50 determination indicated that TNC reduced gemcitabine sensitivity of PANC-1 cell (Fig. 3b). For cell apoptosis detection, it was shown that exogenous TNC reduced basal as well as starved-and drug-induced apoptosis (Fig. 3c, d). TNC’s protein can’t be detected in pancreatic cell line [12], so we knocked down the TNC and tested mRNA level for confirming interference efficiency by RT-PCR and DNA gel electrophoresis in PANC-1-siRNA. The interference efficiency was showed a reduction of approximately 60 % (Fig. 4a). Furthermore, we found that knockdown of TNC didn’t significantly affect the cell proliferation (Fig. 4e).
Cellular response to TNC and gemcitabine stimulations of different pancreatic carcinoma cell lines (PANC-1, Capan-2, SW1990). a Effects of exogenous TNC stimulation on pancreatic cancer cells growth. TNC has more effect on PANC-1 cell. Cells were cultured in serum free medium onto TNC coated plates (1.0 mg/cm2) for 72 h. Then, TNC’s effect on tumor cells growth was measured using a CCK8 assay. Data was calculated as mean ± SD of three experiments and were expressed as percentage compared to the uncoated controls; b Effects of TNC on apoptosis of pancreatic cancer cells. Cells were grown on TNC (0.5 mg/cm2) coated plates and uncoated control plates in serum free medium for 72 h. The western blotting results of apoptosis-regulatory proteins including Caspase-3 cleavage and PARP cleavage were detected. The protein levels of Caspase-3 cleavage and PARP cleavage were normalized to those of β-actin. Bar diagrams summarizing the western blotting results of relative expressions of Caspase-3 cleavage and PARP cleavage were presented; c The cytotoxicity effects of Gem (0, 0.2, 1, 5, 25, 125 μM) on PANC-1, Capan-2 and SW1990 and IC50 of Gem in three pancreatic cancer cell lines were shown. Data were calculated as mean ± SD of four experiments. *p < 0.05
The effects of TNC against gemcitabine-induced apoptosis and starved-induced apoptosis on PANC-1. a The relative viability rates of different treatment: control, Gem (20 µM), Gem (20 µM) + TNC (1.0 mg/cm2), starved, starved + TNC (1.0 mg/cm2) (n = 3); b The IC50 and cytotoxicity effects of gemcitabine on PANC-1 of control and TNC coated (1.0 mg/cm2) were tested. Data were calculated as mean ± SD of three experiments; c Western blotting results of Caspase-3 cleavage and PARP cleavage treated with control, starved, starved + TNC (0.5 mg/cm2). Levels were normalized to β-actin separately. Bar diagram illustrating the western blot results (n = 3); d Western blotting results of Caspase-3 cleavage and PARP cleavage with treatment of control, Gem (20 µM), Gem (20 µM) + TNC (0.5 mg/cm2). Levels were normalized to β-actin separately. Bar diagram illustrating the western blot results (n = 3). p < 0.05
TNC against gemcitabine-induced apoptosis through the activation of ERK1/2 and NF-κΒ/p65 in PANC-1. a The knockdown efficiency of TNC by transient transfection with hTNC-siRNA was detected by RT-PCR for mRNA level (n = 3) and DNA gel electrophoresis for DNA level; b Western blotting results of ERK1/2, p-ERK1/2 expressions after knockdown of TNC. The expression levels of ERK1/2 and p-ERK1/2 were normalized to β-actin (left panel).The different expressions of NF-κB/p65 protein in cell nuclear and cytoplasmic fractions after knockdown of TNC (right panel); Bar diagram illustrating the western blot results (n = 3); c Western blotting results of ERK1/2, p-ERK1/2 expressions with TNC stimulation (0.5 mg/cm2) (0, 10, 20, 30, 45 min and 2 h). The expression levels of ERK1/2 and p-ERK1/2 were normalized to β-actin. Chart illustrating the variation curve of ERK1/2 and p-ERK1/2 expressions (left panel). The different expressions of NF-κB/p65 proteins in cell nuclear and cytoplasmic fractions of different TNC treatment time (right panel); d The knockdown efficiency of ERK1/2 by transient transfection with h ERK1/2-siRNA was detected by RT-PCR for mRNA level (n = 3) and western blotting for protein level; e The relative viability of cells in five groups: Gem (20 µM), siTNC, Gem (20 µM) + TNC (1.0 mg/cm2), Gem (20 µM) + TNC (1.0 mg/cm2) + PD98059 (100 µM), Gem (20 µM) + TNC (1.0 mg/cm2) + siERK, Gem + TNC (1.0 mg/cm2) + PDTC (100 µM)were detected by using a CCK8 method and were calculated as mean ± SD of five experiments and were expressed as percentages. *p < 0.05
TNC affected apoptosis through regulation of ERK1/2 and NF-κB/p65 molecules in pancreatic cancer cells
To verify the regulation of NF-κB/p65 and ERK1/2 by TNC on protein level, we knocked down the TNC or cultured the cells on the TNC coated-plate and analyzed NF-κB/p65 and ERK1/2 protein expression by western blot. It was found that knockdown of TNC reduced the phosphorylation level of ERK1/2 (Fig. 4b, left panel) and the NF-κB/p65 protein accumulation in the cell nucleus (Fig. 4b, right panel). While adding exogenous TNC increased the phosphorylation level of ERK1/2 (Fig. 4c, left panel) and increased the NF-κB/p65 protein accumulation in the cell nucleus (Fig. 4c, right panel), and those regulated functions were shown to be time-dependent. ERK1/2 was knocked down for further testing. The interference efficiency in PANC-1-siRNA-ERK was confirmed by western blot, and RT-PCR showed a reduction of approximately 70 % (Fig. 4d).We performed a CCK8 assay to exclude the possibility that exogenous TNC promotes cell proliferation properties by activation of NF-κB/p65and ERK1/2. The result showed that TNC can significantly resist gemcitabine-induced cell viability by activation of NF-κB/p65 and phosphorylation of ERK1/2 molecules. To investigate the role of TNC in the development of pancreatic cancer, we induced apoptosis in PANC-1 using gemcitabine in the presence or absence of TNC. The FACS results showed that the viability of TNC-knockdown-PANC-1 cells reduced approximately 2-fold compared to those of control. Moreover, the viability of PANC-1 treated with TNC increased approximately 1-fold compared to those cells without treatment. Additionally, decreased ERK1/2 and NF-κB/p65 expression reduced the relative cell viability in the gemcitabine IC50 of pancreatic cells. The TNC regulation functions were shown both in early apoptosis and late apoptosis of cells (Fig. 5a). These findings indicate that TNC has a protective effect against drug–induced apoptosis, which was related to ERK1/2 and NF-κB/p65 molecules, consequently, we further tested the mechanisms of TNC’s effect on pancreatic cell apoptosis and proliferation.
TNC regulate cell apoptosis and cell cycle through the activation of ERK1/2 and NF-κΒ/p65 molecules in PANC-1. a PANC-1 were treated with gemcitabine (20 µM) or Gem (20 µM) + TNC (0.5 mg/cm2) for 48 h. After PANC-1 were treated with PD98059 (an inhibitor of ERK, 100 µM, 1 h), siTNC, siERK1/2 or PDTC (an inhibitor of NF-κB, 100 µM, 1 h), cells were cultured with gemcitabine (20 µM) or Gem (20 µM) + TNC (0.5 mg/cm2) for 48 h. Both supernatant and attached cells were harvested and stained with PI and AnV followed by subsequent FACS analysis. The averaged percentage of cells in two quadrants was demonstrated. The early apoptotic cells (PI−/AnV+) and late apoptotic cells (PI+/AnV+) were shown as percentages of events. The statistical results of percentage of early apoptotic cells (PI−/AnV+) and late apoptotic cells (PI+/AnV+)of the different treatments were presented; b Flow cytometry cell cycle analyses of PANC-1 being treated by TNC additions with or without treatment of PD98059 or PDTC. Bar diagram illustrating the cell cycle results of G0/G1 phase rate, S phase rate and G2/M phase rate (n = 3). *p < 0.05
TNC functionally activated ERK1/2/NF-κB/p65 signal pathway to inhibit cell apoptosis in pancreatic cancer
TNC’s functions in increasing the accumulation of nuclear NF-κB/p65 (Fig. 6a), indicated that TNC activates NF-κB/p65 by phosphorylation of ERK1/2. Because the ERK1/2/NF-κB/p65 molecule regulates cell apoptosis, we consequently tested the function of ERK1/2/NF-κB/p65 on expression of apoptosis related proteins. It was revealed TNC increased Bcl-2, Bcl-xL expression and inhibited early cleavage of caspase-3 and PARP induced by gemcitabine through activation of ERK1/2/NF-κB/p65 signal (Fig. 6b). To investigate whether the treatment of pancreatic cancer cells with TNC has an impact on NF-κB/p65 activation in pancreatic cancer cells, we further examined the effect of TNC on the transcriptional activity of NF-κB—responsive promoter in a luciferase reporter assay. Our data indicated TNC can increase the transcriptional activity of NF-κB/p65, however, this effect can be blocked by PD98059 and siTNC (Fig. 6c). Because TNC obviously increased the growth rate of PANC-1 cells, we determined whether TNC contributes to the chemo-resistant nature of pancreatic cancers. We found that TNC led to resistance to gemcitabine’s effect on cytotoxicity, which was consistent with TNC’s function on anti-apoptosis by activation of ERK1/2/NF-κB/p65 signal (Fig. 6d). To observe the subcellular distribution of NF-κB/p65, we used confocal images for detection. The translocation of NF-κB/p65 protein from the nucleus to cytoplasm in PANC-1 was observed under the treatment of TNC (Fig. 7).
TNC activates ERK1/2/NF-κB/p65 signal to inhibit apoptosis-regulatory protein’s expression and reduces gemcitabine sensitive in PANC-1. a NF-κB/p65 proteins expressions of different treatments: TNC (0.5 mg/cm2), TNC (0.5 mg/cm2) + PD98059 (100 µM) and TNC (0.5 mg/cm2) + siERK. The chart illustrating the variation curve of NF-κB/p65 protein expressions in cell nuclear and cytoplasmic; b The expression of Bcl-xL, Bcl-2, Caspase-3 cleavage and PARP cleavage proteins with different treatments of: Gem (20 µM), Gem (20 µM) + siTNC, Gem (20 µM) + TNC (1.0 mg/cm2), Gem (20 µM) + TNC (1.0 mg/cm2) + PD98059 (100 µM), Gem (20 µM) + TNC (1.0 mg/cm2) + siERK, Gem + TNC (1.0 mg/cm2) + PDTC (100 µM). The expression was normalized to β-actin separately. Bar diagram illustrating the western blot result separately. Data were representative for three independent experiments; c PANC-1 cells (0.5 × 106 cells/well) with or without knockdown of TNC were seeded in 12-well plate. Next day at 60 % confluence, cells were co-transfected with NF-κB luciferase reporter and Psv-β-galactosidase control vector. 24 h post-transfection, cells were transferred to TNC coated plate or TNC coated-PD98059 plate for next 24 h. Luciferase and β-galactosidase activities were measured using an Orion luminometer. Knockdown of TNC can reduce the transcriptional activity of NF-κB, while exogenous TNC can increase the transcriptional activity of NF-κB, this effect can be blocked by PD98059; Data is presented as normalized fold-change in luciferase activity (mean ± SD; n = 3). d The IC50 and cytotoxicity effect of gemcitabine on PANC-1 cell growth were evaluated including control, TNC coated (1.0 mg/cm2), PD98059 (100 µM) + TNC (1.0 mg/cm2) and PDTC (100 µM) + TNC (1.0 mg/cm2) stimulations. Data were calculated as mean ± SD of three experiments. *p < 0.05
Cellular distribution of NF-κB/p65 proteins in PANC-1. Gave PANC-1 different treatments: siTNC, TNC (1.0 mg/cm2), PD98059 (100 µM, 1 h) + TNC (1.0 mg/cm2), PD98059 (100 µM,1 h) + Gem (20 µM,72 h) TNC(1.0 mg/cm2). The distribution of NF-κΒ/p65 proteins in the cytoplasm and nucleus were observed under by taking the co-focal images (objective ×40)
TNC enhanced G1/S transition
Suppression of cancer cell apoptosis can be caused either by fasting of cell cycle progression or induction of anti-apoptosis or both. Our data tested on cell cycle distribution demonstrated that treatment with exogenous TNC resulted in the enrichment of pancreatic cancer cells in S-phase (proliferative fraction) with a concomitant decrease in number of cells in G1 phase. At the same time, PD98058 and PDTC can block this effect (Fig. 5b). Altogether, our findings demonstrate that TNC can promote cell division and reduce cell apoptosis.
Discussion
TNC is a large hexameric extracellular glycoprotein. It is not detected in most healthy adult tissues, but is expressed upon tissue injury. TNC has been proven to play a pathophysiological role in tumorigenesis and tumor progression. However, the effect of TNC on cancer progression is cell type-dependent, which might constitute its determinant value for the progression of individual cancer. High TNC expression correlates with low survival cancers such as glioma, lung carcinoma and osteosarcoma, but a previous study reports a positive correlation between TNC expression and better prognosis in breast cancer patients [10]. In the present study, we used immunohistochemistry and western blotting analysis to investigate the expression of TNC and apoptotic related proteins in human pancreatic cancer tissues and found an overexpression of TNC protein in cancer tissues, indicating its important role in the tumorigenesis of pancreatic cancers. We suggested that overexpression of TNC protein could serve as a diagnostic marker of pancreatic cancer. Moreover, TNC is mainly secreted by PSCs and tumor cell and existed in tumor ECM, we found the overexpression of ERK1/2, p-ERK1/2, Bcl-xL, Bcl-2 proteins in tumors, indicating that TNC may act as an ECM secreted factor involved in the apoptotic process in pancreatic cancer. Further analysis regarding the function of TNC, ERK1/2 proteins was performed to detect a possible underlying mechanism for TNC in regulating tumor cell apoptosis.
Another observation of the present study was the TNC effect on modulating tumor cell apoptosis and chemo-resistance in pancreatic cancer cell. Because TNC overexpression correlates with poor tumor differentiation in vivo, we focused on the poorly differentiated cell line PANC-1 to test cell apoptosis function. It was shown that TNC inhibited serum starvation-induced apoptosis in PANC-1 and rescued cells from gemcitabine-induced apoptosis. Apoptosis influences cell survival and controls cell proliferation and differentiation. Suppression of apoptotic programs contributes to tumor initiation and reduces treatment sensitivity [25]. The present study indicated that TNC regulated cell apoptosis and promoted the tumor progression in pancreatic cancer. Moreover, gemcitabine is a nucleoside analog used as first-line chemotherapy for pancreatic cancer. Active phosphorylated gemcitabine metabolites induce apoptosis by incorporation into DNA and by the inhibition of ribonucleotidereductase, an enzyme that catalyzes the conversion of ribonucleotides to deoxyribonucleotidase [26, 27]. It was demonstrated in the study that TNC reduced gemcitabine sensitivity through the inhibition of cell apoptotic activation in pancreatic cancer. Consequently, TNC expression in tumors might indicate the chemoresistance response and cancer development. In addition, it was showed that both exogenous and endogenous TNC affected cancer cell apoptosis. The finding suggested that targeting TNC may be a potentially useful strategy for improving pancreatic cancer therapy effect. But for cell proliferation, we found that only the exogenous TNC accelerated cell growth. This is possibly because TNC affects cell growth in an environment containing extrinsic TNC in pancreatic cancer.
Moreover, we identified the regulatory effects of TNC on suppressing the cell apoptosis in pancreatic cancer through activation of ERK1/2/NF-κB/p65 signals and inducing the drug resistance. In general, NF-κB plays a vital role in blocking apoptosis through various mechanisms, of which induction of antiapoptotic protein expression is more important. The center of the cell’s decision in response to an apoptotic signal is the Bcl-2 family of apoptotic regulators, including anti-apoptotic proteins such as Bcl-xL, Bcl-2 and pro-apoptotic proteins, such as Bax, Bak and Bad [28]. The activated Bcl-xL, Bcl-2 functions in an anti-apoptotic manner, maintaining mitochondrial integrity and preventing cytochrome c release from mitochondria to inhibit the caspases activation [29]. Caspases initiate and execute cell death by inactivating anti-apoptotic proteins and shutting down DNA replication and repair. The previous studies have proven that TNC activates ERK1/2 to operate cellular functions and ERK/NF-κB signals modulate tumor cell apoptosis in some tumors [20–23].TNC can affect the cell behaviors in a variety of ways because of its interaction with different cell surface receptors or ECM molecules, but ERK/NF-κB signals play critical role in inducing apoptosis resistance. Following NF-κB activation by phosphorylated ERK1/2, NF-κB/p65 translocates to the nucleus and is response for Bcl-2 transcription. Simultaneously, ERK1/2 functions as the direct Bcl-2 kinases. The activated ERK co-localizes with Bcl-2 in the mitochondria and phosphorylates Bcl-2 at serine 70 to regulate its anti-apoptotic activity [30]. We revealed that TNC initiated NF-κB/p65 activation through phosphorylation of ERK1/2, and NF-κB/p65 nucleus translocation to promote Bcl-xL/Bcl-2 protein expression. The latter protein reduced caspase activity to block cell apoptosis. We also found that TNC mediated gemcitabine chemoresistance via the regulation of ERK1/2/NF-κB/p65 signal function, which resulted in the inhibition of gemcitabine-induced apoptosis. Hence, we indicated that TNC might be a special predictor of gemcitabine chemo-resistance. However, the suppression of the ERK1/2/NF-κB/p65 signals might enhance the gemcitabine sensitization of pancreatic cancer with highly expressed TNC protein.
Additionally, because exogenous TNC promoted pancreatic tumor cell proliferation, we detected the TNC’s effects on regulating the cell cycle function [31]. Our study found that TNC significantly enhanced G1/S transition. This effect was blocked by the inhibitor of ERK1/2/NF-κB/p65 molecule. It has been reported that NF-κB is the mediator of cell growth and proliferation, and NF-κB increases the expression of cyclin D1 to enhance transition from G1 to S phase. Thus, we assumed that TNC/ERK1/2/NF-κB/p65 molecule might promote cell growth of pancreatic cancer by manipulating cell cycle progression. However, the mechanism about the TNC’s regulated functions needs further study to be better understood.
In conclusion, our data indicated TNC in cellular matrix induces an activation of ERK1/2/NF-κB/p65 signaling cascade and thereby mediates resistance to apoptosis in pancreatic cancer. TNC could serve as a diagnostic marker and predictor of gemcitabine response and potentially as a target for chemotherapy of pancreatic cancer.
Materials and methods
Patients and sample preparation
Fresh tissues of pancreatic adenocarcinoma and paracarcinoma were obtained from 25 patients who received radical pancreatectomy for pancreatic adenocarcinoma without radiation or chemotherapy before the operation. The diagnosis was confirmed according to the standard of American Joint Committee on Cancer (AJCC, 2010). Histological slides were reviewed by two experienced pathologists blinded to the clinical data. All the patients provided written informed consent, and the study was approved by China Anti-Cancer Association (CACA). Each pancreatic cancer surgical specimen was cut into pieces 1 cm3 in size, promptly fixed with 100 g/L formaldehyde solution and embedded in paraffin for further study.
Immunohistochemistry and scoring method
Paraffin sections of tumor and paracarcinoma tissues from all 25 surgical specimens were cut to 4 μm thickness, then de-paraffinized with xylene, and rehydrated with graded ethanol. Primary 1:50 diluted anti-TNC (mouse monoclonal antibody, Santa Cruz, TX, USA) was used. The sections were incubated with biotinylated secondary antibody and then washed. The chromogen 3, 3′-diaminutesobenzidine tetrachloride (DAB) (Serva, Heidelberg, Germany) was applied as a substrate. The sections were counterstained with Harris’s haematoxylin for nuclei. For the detection of ERK1/2, p-ERK1/2, Bcl-2, Bcl-xL, tumor and Para carcinoma tissues from 15 patients were chosen randomly. Primary antibodies of 1:100 diluted anti-ERK1/2(rabbit antibody, Proteintech Group, IL, USA), 1:500 diluted anti-p-ERK1/2 (rabbit monoclonal antibody, Cell Signaling Technology, Inc., MA, USA), 1:100 diluted anti-Bcl-2 (rabbit antibody, Proteintech Group), 1:500 diluted anti-Bcl-xL (rabbit monoclonal antibody, Cell Signaling Technology, Inc., MA, USA) were used.
The expression of TNC was scored according to the extent and intensity of the staining.
An extracellular staining reaction in the stroma around the tumor cells was considered positive. The extent of positive staining was scored by the percentage of the positively stained area. Stained area in each region of interest: 0 for a percentage <5 %, 1 for 5–25 %, 2 for 25–50 %, 3 for 50–75 %, and 4 for ≥75 %. The intensity of staining was scored as 0, 1, 2 and 3 for the representation of negative (no staining), mild (weak but detectable above control), moderate (distinct) and intense (strong). The percentage of positively stained area and intensity of staining were multiplied to produce a weighted score [11, 32]. The scoring was performed by two independent evaluators without the knowledge of the patients’ pathological and clinical characteristics.
Cell culture and transfection
The PANC1, Capan-2 and SW1990 cell lines were purchased from Cancer Institute & Hospital of the Chinese Academy of Medical Sciences (Shanghai, China). SW1990 and Capan-2 were cultured in RPMI-1640 medium (Biological Industries, Kibbutz BeitHaemek, Israel), containing 10 % fetal bovine serum (Biological Industries), l-glutaminutese (2 mM), 100 U/ml of penicillin and 100 mg/ml streptomycin. The PANC-1 cell lines were cultured in RPMI-DMEM medium (Biological Industries), containing 10 % fetal bovine serum and 100 U/ml penicillin/streptomycin. All cells were grown in an incubator with a constant temperature of 37 °C and 5 % CO2. Small interfering RNA (siRNA) targeting h TNC were synthesized by GenePharma (Shanghai, China). The selected target sequence for TNC was used: 5′-GCTGGGATGCCCTCAAACT-3′. The sequence 5′-ATTCTCCGAACGTGTCAC-3′ was used as a negative control. SiRNA targeting h ERK1/2 (No.6560) was synthesized by Cell Signal Technology (Cell Signaling Technology, Inc.).Cells were transfected with the siRNA using lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. To optimize efficiency, cells were transfected with siRNA twice with an interval of 24 h. 2 × 105 PANC-1 cells were transfected with 5 ml siRNA duplex following the manufacturer’s instructions. For determining transfection efficiency, RT-PCR and DNA gel electrophoresis methods were used to test the h TNC expression level, RT-PCR and western blot methods were used to test the h ERK1/2’s expression level.
TNC coating method
TNC was purchased from Millipore (Millipore, Billerica, MA, USA). Coating was allowed to be absorbed overnight at 4 °C, and wells were then washed with PBS to remove unbound proteins and blocked at 37 °C for 30 min by the addition of 0.2 % heat-denatured (85 °C for 12 min) BSA (Millipore) in PBS. Finally, plates were washed twice with sterile PBS and sterilized by UV exposure for 30 min.
TNC coating was performed at a final concentration of 1 mg/cm2 in 96-well plates for cell viability and cytotoxicity analysis and 24-well plates for cell immunofluorescence testing. For immunoblotting and flow cytometry analysis, TNC was coated on the 6-well plates with a concentration of 0.5 mg/cm2. Coverslips used for immunofluorescence experiments were placed into 24-well plates before the TNC protein was coated. Uncoated plates, blocked with BSA, are referred to in this manuscript as “uncoated”.
Cell viability measurement
Cells were seeded in 96-multi-well plates in RPMI-DMEM medium containing 10 % FBS, with a density of 1 × 104 cells/well, and allowed to attach to the medium for 8 h before different treatments were applied. Cells were counted using Cell Counting Kit-8 (Beyotime, Jiangsu, China) following the manufacturer’s protocol, and the absorbance was measured at 450 nm. For cytotoxicity assay, the cytotoxicity of gemcitabine (Gem, Eli Lilly, IN, USA) was tested by CCK-8 assay. The cell viability was measured using ELISA and optical density (OD) values were read at 450 nm. The survival rate was calculated with the formula [(As−Ab)/(Ac−Ab)] × 100 % (As stands for the absorbance of experimental groups; Ab stands for the absorbance of the blank group, which contains complete medium without cells; Ac represents the control group, which contains cells with no treatments.) The 50 % inhibitory concentration of cell growth (IC50) was calculated by non-linear least squares curve fitting [33].
Western blotting analysis
The extracted proteins from tissues and whole cell from cell lines were prepared in lysis buffer(50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 % glycerol, 1 %TritonX-100, 25 mM NaF, and 2 mM NaVO4) supplemented with 1× protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany) and phosphatase inhibitor cocktails I and II (Roche Molecular Biochemicals). In addition, the cell nuclear protein and cytosolic protein were separately extracted using the Nucleoprotein Extraction Kit (Keygen Biotech). To extract proteins from pancreatic cancer tissues and paracarcinoma tissues, the specimens were first cut into small pieces and then lysed with 1 ml RIPA (Sangon Biotech, Shanghai, China) per 100 mg tissue. After being homogenized manually with disperser, the lysate was subjected to centrifugation at 16,000g. Proteins from supernatants were quantified by the Bradford method (Axygen, USA) and added into an equal volume of 1× loading buffer. Next, 20 μg of the proteins mixed with loading buffer was separated by SDS-PAGE. The protein blots were detected by the primary antibodies as follows: 1:100 diluted mouse monoclonal anti-TNC, 1:2000 diluted rabbit anti-ERK1/2,1:2000 diluted rabbit monoclonal anti-p-ERK1/2, 1:1000 diluted rabbit anti-Caspase-3, 1:1000 diluted rabbit anti-PARP (Cell Signaling Technology, Inc.), 1:1000 diluted rabbit polyclonal anti-NF-κB/p65, 1:1000 diluted anti-Bcl-2, 1:1000 diluted anti-Bcl-xL, 1:5000 diluted mouse anti-β-actin. The corresponding secondary antibodies were used at 1:10,000. Protein bands were detected using an ECL detection system (Millipore) and then imaged on a gel imaging system (Odyssey, LI-COR Biosciences). The digital images of the western blot bands were quantified by Meta Morph software (MDS Analytical Technologies) after the background subtraction.
Real time PCR detection
Total mRNA from PANC-1 cells for each treatment was isolated by Trizol (TianGen biotech, Beijing, China) and reverse-transcribed to cDNA by Trans Script First-Strand cDNA Synthesis (TianGen biotech). Real time RT-PCR was performed using Trans Strat Top Green qPCR Super Mix (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol and tested by the DA7600 Real-time Nucleic Acid Amplification Fluorescence Detection System (Da An Gene, Guangdong, China) in 25 μl reaction volumes. For an equal control, mRNA of β-actin from human was tested at the same time. The 2−ΔΔCt method was used to calculate the relative mRNA fold changes. The statistical results were averaged from three independent experiments. After each test, melting curve was checked to ensure that a single amplification product was obtained. The following primers were used in the real-time PCR and were synthesized by Sangon (Shanghai, China) β-actin 5′-TGCGTGACATTAAGGAGAAG-3′ (forward) and 5′-GCTCGTAGCTCTTCTCCA-3′ (backward), h ERK 5′-TCCCAAATGCTGACTCCA-3′ (forward) and 5′-CGGGTCGTAATACTGCTCC-3′ (backward), h TNC 5′-GTCACCGTGTCAACCTGATG-3′ (forward) and 5′-GCCTGCCTTCAAGATTTCTG-3′ (backward). The conditions for h ERK PCR were 39 cycles of 95 °C for 10 s for denaturation, 60 °C for 15 s for annealing and 72 °C for 45 s for extension. The conditions for h TNC PCR and β-actin PCR were 45 cycles of 95 °C for 10 s for denaturation, 56 °C for 5 s for annealing and 72 °C for 10 s for extension.
Flow cytometry analysis
PANC-1 cells were seeded (1 × 106cells/well) in a 6-well plate. Following different treatments, cells were harvested and stained with propidium iodide (PI) and FITC Annexin V (AnV) (FITC Annexin V Apoptosis Detection Kit I, BD Biosciences, NJ, USA) followed by flow cytometry (BD-FACS CantoTM II). Percentage of cell population in apoptosis was calculated using Mod Fit LT software. For cell-cycle analysis, cells were synchronized with serum free media. Seventy-two hours later, 1 × 106 cells/well were seeded in 6-well plate coated with TNC in complete medium and gave different treatment. After 48 h of treatment, floating and attached cells were collected and fixed in 70 % ethanol overnight at 4 °C. The cells were then stained with PI, using 10 μl PI (5 mg/ml)/RNase (Keygen, Nanjing, China) staining buffer at room temperature in the dark for 1 h. Stained cells were analyzed with flow cytometry to calculate the cell population percentages in various phases of cell cycle using Mod Fit LT software. At least 2 × 104 cells were counted in each test.
Immunofluorescent staining
PANC-1 cells were seeded on coverslips and cultured for 24 h. Then, different treatments were performed. Cells adhering to coverslips were washed with PBS, fixed with 4 % formaldehyde in PBS buffer for 15 min at room temperature, washed three times with TBS (50 mM Tris, 138 mM NaCl, 2.7 mM KCl, pH 7.6), permeabilized with Triton X-100 (0.25 % v/v in TBS) for 5 min, then washed twice and blocked for 1 h in blocking solution (TBS containing 10 % goat serum and 0.1 %Triton X-100). Then, incubation with 1:50 diluted rabbit polyclonal anti- NF-κB/p65 was performed in the blocking solution at 4 °C in a humidified chamber overnight. After washing three times with TBS, slides were incubated with goat anti-rabbit IgG-FITC (Santa Cruz) at room temperature in blocking solution for 1 h. Coverslips were then washed again three times before being counterstained with 1:10,000 dilute DAPI for 5 min, briefly washed with TBS, covered with anti-fade mounting medium (Vectashield, Loerrach, Germany) and placed onto microscope slides. Slides were examined under a Zeiss Axiovert fluorescence microscope (Carl Zeiss AG, Jena, Germany). Negative controls were performed omitting the primary antibodies.
NF-κB/p65 activity assay
The nuclear translocation of NF-κB/p65 subunit was detected by immunoblotting or luciferase assay. To prepare nuclear and cytoplasm extracts, cells were washed in ice cold PBS and resuspended in buffer A (every ml bufferA added 1 μl DTT, 5 μl 100 Mm PMSF, 1 μl protease inhibitor). Cells were incubated for 15 min on ice, and then buffer B was added and the cells were incubated for 1 additional minute. The lysate was centrifuged at 16,000g for 5 min. The supernatant (cytoplasm protein) was collected, and the precipitate was re-suspended with buffer C (every ml buffer C added 1 μl DTT, 5 μl 100 Mm PMSF, 1 μl protease inhibitor). Aliquots were then incubated on ice for 40 min with intermittent vibration and centrifugation at 16,000g for 20 min, and then supernatants were collected (nuclear protein). Immunoblotting was performed to test the presence of NF-κB/p65 subunits in the nucleus and cytoplasm using anti-NF-κB/p65antibody.
For luciferase assays, cells were transiently transfected with pNF-κB/p65-Luc plasmids (Beyotime) and Psv-β-galactosidase control vector (Promega, WI, USA) using Lipofectamine 2000. Luciferase and β-galactosidase activities were measured using an Orion luminometer, and cellular protein contents were determined using the luciferase reporter gene assay kit (Beyotime) and β-galactosidase assay kit (Beyotime). Luciferase activities were normalized versus lysate protein contents, and β-galactosidase activity was used as an internal control to determine transduction efficiency.
Statistical analysis
Statistical analyses were performed with SPSS 16.1 software using two-way analysis of variance in Fig. 1, and other numerical data are reported as the mean ± SD. Two-tailed Student’s t test was used to assess the difference of proteins expression, IC50 values and cell percentage in cell cycle. A value of p < 0.05 was used as the criterion for statistical significance.
References
Siegel Rebecca, Naishadham Deepa, Jemal Ahmedin (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Stathis Anastasios, Moore MJ (2010) Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 7:163–172
Schneider G, Siveke JT, Eckel F, Schmid RM (2005) Pancreatic cancer: basic and clinical aspects. Gastroenterology 128:1606–1625
Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS (2004) Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29:179–187
Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128:907–921
Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA (1993) The pancreas: biology, pathobiology and disease. Raven, New York
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M et al (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mousemodel of pancreatic cancer. Science 324:1457–1461
Omary MB, Lugea A, Lowe AW, Pandol SJ (2007) The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 117:50–59
Orend G, Chiquet-Ehrismann R (2006) Tenascin-C induced signaling in cancer. Cancer Lett 244:143–163
Orend G, Saupe F, Schwenzer A, Midwood K (2014) The extracellular matrix and cancer: regulation of tumor cell biology by tenascin-C. iConcept, Hong Kong
Juuti A, Nordling S, Louhimo J, Lundin J, Haglund C (2004) Tenascin C expression is upregulated in pancreatic cancer and correlates with differentiation. J Clin Pathol 57:1151–1155
Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P (2006) Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol 208:673–685
Jones PL, Jones FS (2000) Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol 19:581–596
Paron I, Berchtold S, Vörös J, Shamarla M, Erkan M, Höfler H, Esposito I (2011) Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS One 6:e21684
Fryer BH, Field J (2005) Rho, rac, pak and angiogenesisold roles and newly identified responsibilities in endothelial cells. Cancer Lett 229:13–23
Jones PL, Crack J, Rabinovitch M (1997) Regulation of Tenascin-C, a vascular smooth muscle cell survival factor that interacts with the αvβ3 integrin to promote epidermal growth. J Cell Biol 139:279–293
Chiquet-Ehrismann R, Chiquet M (2003) Tenascins: regulation and putative functions during pathological stress. J Pathol 200:488–499
Mettouchi A, Cabon F, Montreau N, Dejong V, Vernier P, Gherzi R, Mercier G, Binétruy B (1997) The c-Jun-induced transformation process involves complex regulation of tenascin-C expression. Mol Cell Biol 17:3202–3209
Iyer AK, Tran KT, Griffith L, Wells A (2008) Cell surface restriction of EGFR by a tenascin cytotactin-encoded egf-like repeat is preferential for motility-related signaling. J Cell Physiol 214:504–512
Kim SO, Kim MR (2013) Gingerol prevents disassembly of cell junctions and activities of MMPs in invasive human pancreas cancer cells through ERK1/2/ NF-κB/Snail signal transduction pathway. Evid Based Complement Alternat Med 2013:761852
Rengifo-Cam W, Umar S, Sarkar S, Singh P (2007) Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-κB. Cancer Res 67:7266–7274
Cheng Y, Diao DM, Zhang H, Song YC, Dang CX (2013) Proliferation enhanced by NGF-NTRK1 signaling makes pancreatic cancer cells more sensitive to 2DG-induced apoptosis. Int J Med Sci 10:634–640
Armstrong MB, Bian X, Liu Y, Subramanian C, Ratanaproeksa AB, Shao F, Yu VC, Kwok RP, Opipari AW, Castle VP (2006) Signaling from p53 to NF-kappaB determines the chemotherapy responsiveness of neuroblastoma. Neoplasia 8:964–974
Tod J, Jenei V, Thomas G, Fine D (2013) Tumor-stromal interactions in pancreatic cancer. Pancreatology 13:1–7
Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21:485–495
Gilbert JA, Salavaggione OE, Ji Y, Pelleymounter LL, Eckloff BW, Wieben ED, Ames MM, Weinshilboum RM (2006) Gemcitabine pharmacogenomics: cytidine deaminase and deoxycytidylate deaminase gene resequencing and functional genomics. Clin Cancer Res 12:1794–1803
Bergman AM, Pinedo HM, Talianidis I, Veerman G, Loves WJ, van der Wilt CL, Peters GJ (2003) Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer 88:1963–1970
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiter of cellsurvival. Science 281:1322–1326
Westphal S, Kalthoff H (2003) Apoptosis: targets in pancreatic cancer. Mol Cancer 7:2–6
Ruvolo PP, Deng X, May WS (2001) Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 15:515–522
Chen W, Li Z, Bai L, Lin Y (2011) NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci (Landmark Ed) 16:1172–1185
Lu CD, Altieri DC, Tanigawa N (1998) Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 58:1808–1812
Giovannetti E, Mey V, Danesi R, Mosca I, Del Tacca M (2004) Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res 10:2936–2943
Acknowledgments
This study was funded by National Natural Fund (31370861) and Tianjin Basic Research Plan Project (13JCZDJC31300). The authors declare that they have no conflict of interest. We thank Tianjin Medical University Cancer Institute and Hospital for providing human pancreatic adenocarcinoma samples and for all expert discussions. We thank Dwayne Stupack (University of California, San Diego, USA) for giving helpful advice during the course of our study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, M., He, X., Wei, W. et al. Tenascin-C induces resistance to apoptosis in pancreatic cancer cell through activation of ERK/NF-κB pathway. Apoptosis 20, 843–857 (2015). https://doi.org/10.1007/s10495-015-1106-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1106-4