Abstract
Autophagy refers to the process by which lysosomes degrade intracellular components. Three basic forms of it, macro-, micro-, and chaperon-mediated autophagy, exist in cells. Several studies have shown that dysregulation of macroautophagy compromises the viability of neurons. Recent evidence indicates that chaperone-mediated autophagy plays a role in direct degradation of neuronal transcription factor MEF2D, a protein known to promote neuronal survival. Disruption of this regulatory pathway by α-synuclein leads to neuronal stress, which may underlie neuronal loss in Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Parkinson’s disease
Parkinson’s disease (PD) is the most common neurodegenerative disorder affecting movement. The disease is estimated to have a prevalence rate of 1% at age 65, affecting millions of people worldwide. PD is characterized by the degenerative loss of pigmented dopaminergic neurons in the substantial nigra pars compacta (SNc) of the brain. The other pathological hallmark at cellular level is the presence of eosinophilic cytoplasmic inclusions named Lewy bodies in the affected DA neurons, which are thought to represent aggresomes composed of the protein α-synuclein and other proteins such as ubiquitin [1].
PD is progressive due to the gradual loss of DA neurons in SNc over an extended period of time. Current therapies aimed at ameliorating the symptoms by restoring dopaminergic signaling fail to alter the progression of the disease. Substantial efforts have been made to understand the etiology of PD and to illustrate the mechanisms that underlie the loss of DA neurons. About 5–10% PD cases are clearly hereditary, which can be linked to alteration of specific genes [2]. These include genes encoding α-synuclein, PINK1, DJ-1, Parkin, and LRRK2. Dissecting how these genetic changes impact DA neurons has greatly advanced our understanding of the molecular basis of pathological changes in PD. However, most of the cases of PD are sporadic, indicating that their precise causes are not known. Epidemiological studies have identified the link between the exposure to certain environmental factors including toxicants and the incidence of PD [3], supporting a role of neurotoxicants in the pathogenic process of PD.
Our current understanding of how these etiological factors contribute to loss of DA neurons is still incomplete. One widely accepted model is that genetic and environmental factors converge on cellular targets to interrupt the homeostasis and cause oxidative stress. One such key target is mitochondria. In support of this, accumulating experimental evidence indicates that several genes mutated in familial PD and toxins may exert pathogenic effects via modulating mitochondrial function. For example, it is now clear that several PD-associated genes encode proteins which are localized to mitochondria under various conditions [4]. These include α-synuclein, Parkin, PINK1, DJ-1, and LRRK2. Similarly, several toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, or paraquat inhibit mitochondria and complex I [5], causing parkinsonism in human or animals. Thus, these findings support a prominent role for mitochondrial dysfunction as one of proximal sites of action for PD related etiological factors.
Basic autophagic machinery
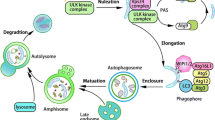
Autophagy refers to the process by which lysosomes degrade intracellular constituents, including both organelles and soluble proteins [6]. This process is traditionally regarded as a cellular response to stress typically related to nutrient deprivation, toxin exposure, infection, or oxidative stress. In contrast to the ubiquitin-proteasome pathway which degrades mostly short-lived proteins, autophagy is primarily involved in breaking down proteins with long-half lives and damaged organelles. Some basic form of this machinery is highly conserved from yeast to mammals. Depending on how lysosomes receive the cargo, autophagy can be further classified into three types, namely macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA).
Macroautophagy, often referred to as autophagy, involves multiple steps beginning with induction and ending with the release of the degraded products to the cytosol. It is characterized by the sequestration of cytosolic components in an autophagic vacuole or autophagosome, which fuses with lysosomal membrane to deliver its contents for degradation. This double-membrane vacuole is the morphological hallmark of autophagy. The process is highly inducible in response to stress signals and regulated at various junctures by a large number of autophagy-related (ATG) genes, which involves controlling the activity of mTOR (mammalian target of rapamycin), a suppressor of macroautophagy. Microautophagy is less well studied than macroautophagy. Unlike macroautophagy, microautophagy does not involve the formation of double-membrane vacuoles. Rather, the defining feature of microautophagy is the invagination or exvagination of lysosomal membrane to sequester cytosolic components. Mitophagy is a term describing the selective sequestration and removal of mitochondria by either macro- or microautophagy [7].
Unlike macro- and microautophagy, CMA does not involve membrane vacuoles. Rather, it is regulated by direct protein interaction. Two key regulators, chaperone protein heat-shock cognate 70 (Hsc70) and lysosomal membrane receptor Lamp2A, control the CMA process [8]. Hsc70, in a complex with other co-chaperone proteins, binds to cytosolic proteins containing a pentapeptide targeting motif KFERQ and delivers substrate proteins to the surface of lysosomes [9]. Binding of this chaperone-substrate complex to Lamp2A leads to the translocation of substrates cross the lysosomal membrane. Both the level and availability of Lamp2A are regulated by stress signals [10, 11], making Lamp2A one of the major modes by which CMA activity is controlled.
Autophagy and Parkinson’s disease
Emerging evidence supports the view that dysregulation of autophagy may contribute to the pathogenic process in PD [12]. Several genetic risk factors associated with PD have been connected to different forms of autophagy. This includes evidence demonstrating that both macroautophagy and CMA regulate α-synuclein [13, 14]. Furthermore, the CMA process can be disrupted by the α-synuclein A53T mutant. Similarly, it has been shown that the ubiquitin C-terminal hydrolase L1 (UCH-L1) mutant I94M interrupts the CMA process by binding to Lamp2A with abnormally high affinity [15]. Parkin has been shown to be re-localized to mitochondria following mitochondrial damage, which promotes mitophagy [16]. LRRK2 mutants have been recently found to induce the increase or accumulation of autophagic vacuoles [17]. Loss of PINK1 function has been proposed to affect autophagy [18].
Strong experimental and epidemiological data exist to support an etiological role for neurotoxins in PD. There are hints that many of these toxins may also alter the process of autophagy. For example, mitochondrial complex I inhibitor rotenone can inhibit capacity of autophagy [19]. 6-OHDA up-regulates LC3 in nigral DA neurons [20]. Paraquat induces the accumulation of autophagic vacuoles [21]. There is limited information on how these toxins may affect CMA. Our preliminary studies show that limited exposure to 6-OHDA activates CMA by increasing the levels of Lamp2A (Qian Yang’s unpublished data).
Transcription factor MEF2D, neuronal survival, and autophagy
Myocyte enhancer factor 2 (MEF2) was initially identified as a nuclear factor important for muscle cell differentiation [22]. Subsequent studies identified four vertebrate members of MEF2, A–D. Sequence analysis shows that MEF2s share a highly homologous N-terminus spanning the first 86 amino acid residues. This region can be further divided into two sub-domains. Amino acid residues 1–56 are termed MADS domain due to the homology to transcription factors MCM1, Agamous, Deficiens, and Serum response factor. Amino acid residues 57–86 are conserved among the four MEF2 isoforms and therefore, termed as MEF2 domain. The N-terminus is responsible for MEF2 hetero- or homo-dimerization and binding to an A/T rich cis-DNA element. The C-terminus of MEF2 is more diverse in its sequence and is required for MEF2-mediated transcription activation. MEF2 activity is highly regulated in cells. This regulation is mainly achieved through either direct interaction of the N-terminus of MEF2s with other co-factors or posttranslational modifications such as phosphorylation at the C-terminus.
Although the cellular function for MEF2s was first studied in muscle cells, the roles of MEF2s have now been described in increasing numbers of cellular systems including neurons, immune cells, vascular cells, and adipocytes to name a few. One of the exciting advances is the understanding of the critical roles that MEF2s play in several cellular processes in neurons [23]. Accumulating evidence suggests that different MEF2 isoforms are involved in neuronal development, drug addictions, synaptic plasticity, and survival.
MEF2 and neuronal survival
The first piece of evidence revealing the MEF2s’ role in neuronal survival was provided by a study investigating the mechanisms of neuronal activity-dependent survival. Using cultured primary cerebellar granule neurons (CGNs) as a model, the authors investigated the function of MEF2s in mediating neuronal activity [24]. This study found that neuronal activity activated MEF2 by p38-mediated phosphorylation. Using a dominant negative approach, it was shown that inhibition of MEF2s blocked neuronal activity-induced survival of CGNs, leading to their apoptosis. Subsequent studies verified the role of MEF2s in neuronal survival. For example, MEF2C was required for mediating the survival effect of neurotrophic factor BDNF in cortical neurons [25]. Thus, it appears that various isoforms of MEF2s may mediate the survival of different types of neurons in several experimental paradigms.
MEF2D and Parkinson’s disease
As discussed above, Parkinson’s disease is characterized by the loss of dopaminergic neurons in SNc. The link between MEF2D and Parkinson’s disease came from the initial observation that cyclin dependent kinase 5 (Cdk5) directly phosphorylates MEF2D at Ser444 [26]. Phosphorylation of MEF2D by Cdk5 at Ser444 led to an inhibition of MEF2D transcription activity. Subsequent studies showed that phosphorylation of MEF2D at Ser444 makes MEF2D susceptible to the specific degradation by caspases [27]. This would lead to a severe reduction in the levels of MEF2D protein, inhibiting MEF2Ds effect on neuronal survival. In cultured neurons, inhibition of MEF2D correlated with Cdk5-mediated neuronal death following toxic stress.
Several lines of evidence support the notion of dysregulation of Cdk5 in Parkinson’s disease. Earlier studies showed that Cdk5 and its regulator p35 co-localize with Lewy bodies [28, 29]. Moreover, striatal excitotoxic lesions with toxin quinolinic acid were found to result in the apoptosis of DA neurons in SNc [30]. This correlated well with increased expression of Cdk5 in the apoptotic neurons. Cdk5 was later shown to mediate the MPTP-induced loss of DA neurons in a mouse model of PD [31]. More recently, the link between Cdk5, MEF2D, and loss of DA neurons was established in the MPTP model of PD [32]. It was shown that MPTP-induced DA neuronal loss and behavioral outcomes were dependent on Cdk5 activator p35. MPTP treatment led to phosphorylation of MEF2D at Ser444 in vivo. Importantly, a MEF2D mutant which is resistant to Cdk5 phosphorylation protected DA neurons from MPTP-induced toxicity in mice. Thus, Cdk5-mediated modulation of MEF2D accounts, at least in part, for MPTP-triggered loss of SNcDA neurons in vivo.
MEF2D and chaperone-mediated autophagy
Many studies have provided ample evidence supporting an important role of macroautophagy in Parkinson’s disease. But relatively little is known about the role of CMA in the pathogenic process of PD. A recent study by us revealed a previously unrecognized connection between CMA and MEF2D. Yang et al. [33] showed that MEF2D is translocated to the cytoplasm under basal condition. MEF2D was shown to interact with CMA regulator Hsc70 via its N-terminal domain and be delivered to lysosomes for degradation. Purified MEF2D bound to and was translocated into lysosomes in a co-incubation assay. Inhibiting CMA function led to accumulation of MEF2D in the cytoplasm while enhancing CMA activity reduces it. Thus, MEF2D qualifies as a substrate of CMA. Maintaining adequate CMA activity is apparently critical for MEF2D function since inhibition of lysosomal function reduces MEF2-dependent transcriptional activity.
CMA-mediated degradation of MEF2D can be interrupted by α-synuclein. Yang et al. [33] showed that over-expression of either wild type or mutants of α-synuclein reduces the binding between Hsc70 and MEF2D, results in accumulation of MEF2D in the cytoplasm, and inhibits MEF2 activity. Consistent with these findings, MEF2D levels were significantly higher in the brains of PD patients than in controls. These findings suggest that interfering with the normal CMA-mediated turnover of survival factor MEF2D by α-synuclein may underlie its toxicity and the pathogenesis of PD. Indeed, over-expression of a form of MEF2D that preferentially localizes to the nucleus could attenuate α-synuclein-induced loss of neuronal viability. Overall, this work presents evidence that identifies a direct link between CMA and neuronal survival machinery, supports the role of CMA as being critical for neuronal survival, and reveals the deregulation of CMA-MEF2D in PD (Fig. 1). Therefore, it may lead not only to better understanding of the causes of PD but also to future studies on the role of autophagy in other neurodegenerative diseases.
Concluding remarks
The findings that CMA degrades survival factor MEF2D in neurons indicate that CMA directly modulates the survival machinery. Maintaining the proper turnover of MEF2D under basal conditions is important for neuronal homeostasis. The finding that this pathway is sensitive to the toxic effect of α-synuclein highlights the possibility that dysregulation of CMA-MEF2D may underlie the pathogenesis of PD. Mechanistically, it is important to determine how accumulation of MEF2D in the cytoplasm may hinder neuronal homeostasis and whether other MEF2 isoforms can also serve as CMA substrates. In addition, it is likely that CMA may regulate additional proteins involved in neuronal survival and death. Their identification should provide new insights into the pathogenic process of PD. Finally, it would be important to demonstrate that CMA activity is altered in animal models of PD and in brain tissues of PD patients. Such findings shall encourage us to develop assays to monitor CMA activity in cells and model animals, and devise effective approaches to alter CMA in cells and model animals as a therapeutic strategy.
References
Cookson MR (2009) Alpha-synuclein and neuronal cell death. Mol Neurodegener 4:9
Abeliovich A, Flint Beal M (2006) Parkinsonism genes: culprits and clues. J Neurochem 99:1062–1072
Hatcher JM, Pennell KD, Miller GW (2008) Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci 29:322–329
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609
Przedborski S, Ischiropoulos H (2005) Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal 7:685–693
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253
Majeski AE, Dice JF (2004) Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol 36:2435–2444
Chiang HL, Dice JF (1988) Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem 263:6797–6805
Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1:570–583
Kiffin R, Christian C, Knecht E, Cuervo AM (2004) Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15:4829–4840
Rubinsztein DC, DiFiglia M, Heintz N et al (2005) Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 1:11–22
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Kabuta T, Furuta A, Aoki S, Furuta K, Wada K (2008) Aberrant interaction between Parkinson disease-associated mutant UCH-L1 and the lysosomal receptor for chaperone-mediated autophagy. J Biol Chem 283:23731–23738
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803
Plowey ED, Cherra SJ 3rd, Liu YJ, Chu CT (2008) Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem 105:1048–1056
Chu CT (2009) Tickled PINK1: mitochondrial homeostasis and autophagy in recessive Parkinsonism. Biochim Biophys Acta 1802:20–28
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2007) Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci 120:4155–4166
Dagda RK, Zhu J, Kulich SM, Chu CT (2008) Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson’s disease. Autophagy 4:770–782
Gonzalez-Polo RA, Niso-Santano M, Ortiz-Ortiz MA et al (2007) Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol Sci 97:448–458
Yu YT, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B (1992) Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev 6:1783–1798
Mao Z, Wang X (2006) Expression, function, and regulation of transcription factor MEF2 in neurons. In: Thiel G (ed) Transcription factors in the nervous system, chapter 14. Wiley-VCH, pp 285–305
Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME (1999) Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286:785–790
Liu L, Cavanaugh JE, Wang Y, Sakagami H, Mao Z, Xia Z (2003) ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc Natl Acad Sci USA 100:8532–8537
Gong X, Tang X, Wiedmann M et al (2003) Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38:33–46
Tang X, Wang X, Gong X et al (2005) Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J Neurosci 25:4823–4834
Brion JP, Couck AM (1995) Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol 147:1465–1476
Nakamura S, Kawamoto Y, Nakano S, Akiguchi I, Kimura J (1997) p35nck5a and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson’s disease. Acta Neuropathol 94:153–157
Henchcliffe C, Burke RE (1997) Increased expression of cyclin-dependent kinase 5 in induced apoptotic neuron death in rat substantia nigra. Neurosci Lett 230:41–44
Smith PD, Crocker SJ, Jackson-Lewis V et al (2003) Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 100:13650–13655
Smith PD, Mount MP, Shree R et al (2006) Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci 26:440–447
Yang Q, She H, Gearing M et al (2009) Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323:124–127
Acknowledgments
We thank Brian Ciliax for his critical comments. This work was supported by NIH grants to Z. M. (AG023695, NS048254, ES015317, ES016731-0002) and Robert Woodruff Health Sciences Center Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Q., Mao, Z. Dysregulation of autophagy and Parkinson’s disease: the MEF2D link. Apoptosis 15, 1410–1414 (2010). https://doi.org/10.1007/s10495-010-0475-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0475-y