Abstract
It is still enigmatic under which circumstances cellular demise induces an immune response or rather remains immunologically silent. Moreover, the question remains open under which circumstances apoptotic, autophagic or necrotic cells are immunogenic or tolerogenic. Although apoptosis appears to be morphologically homogenous, recent evidence suggests that the pre-apoptotic surface-exposure of calreticulin may dictate the immune response to tumor cells that succumb to anticancer treatments. Moreover, the release of high-mobility group box 1 (HMGB1) during late apoptosis and secondary necrosis contributes to efficient antigen presentation and cytotoxic T-cell activation because HMGB1 can bind to Toll like receptor 4 on dendritic cells, thereby stimulating optimal antigen processing. Cell death accompanied by autophagy also may facilitate cross priming events. Apoptosis, necrosis and autophagy are closely intertwined processes. Often, cells manifest autophagy before they undergo apoptosis or necrosis, and apoptosis is generally followed by secondary necrosis. Whereas apoptosis and necrosis irreversibly lead to cell death, autophagy can clear cells from stress factors and thus facilitate cellular survival. We surmise that the response to cellular stress like chemotherapy or ionizing irradiation, dictates the immunological response to dying cells and that this immune response in turn determines the clinical outcome of anticancer therapies. The purpose of this review is to summarize recent insights into the immunogenicity of dying tumor cells as a function of the cell death modality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depending on the lethal stimulus, tumor cells can die by distinct cell death mechanisms including apoptosis and necrosis. Cellular stress or oncogenic transformation can also cause the induction of mitotic catastrophe, cellular senescence and/or autophagy, which in many instances accompanies early apoptosis. A panoply of noxious agents including anti-cancer chemotherapeutics can induce cell death. Most chemotherapeutic agents kill tumor cells through a morphologically homogeneous apoptotic pathway. Among these, only a few agents have the capacity to stimulate immunogenic cell death. EL4 thymoma, Glasgow osteosarcomas, and CT26 colon cancer cells treated with oxaliplatin, as well as CT26 colon cancers and MCA205 fibrosarcomas treated with anthracyclins, respond far better to chemotherapy when they are implanted into immunocompetent mice rather than into immunodeficient, athymic (nu/nu) hosts [1–3]. Local radiotherapy of TS/A breast cancers is also more efficient in immunocompetent than in immunodeficient mice [1]. In conclusion, the outcome of treatment with anthracyclins, oxaliplatin and radiotherapy depends on the active contribution of the host immune system [4, 5].
Whether tumor cell death is immunogenic or not depends to a large extent on the death-initiating stimulus, yet is not a simple correlate of cell death. Thus some, but not all cell death inducers cause the exposure of immunogenic factors on the cell surface or the release of immunogenic signals into the extracellular space. In addition, the same anticancer agent can cause the exposure/release of immunogenic signals from some tumor but not for others, due to the fact that this exposure/release requires the intervention of specific signal transduction pathways [6, 7].
Anticancer chemo- and radiotherapies induce cell death in rapidly proliferating tumor cells, as well as in cells of the hematopoietic system including the immune system. Due to its role during normal development and physiological cellular turnover, the main cell death modality, apoptosis, has been thought to be intrinsically non-immunogenic or tolerogenic. Nevertheless, the dogma that apoptosis is non-immunogenic while necrosis is automatically pro-inflammatory and immunogenic, does not withstand experimental verification. Tumor vaccination studies in mice showed that some apoptosis-inducing treatments caused immune-dependent tumor regression whereas others did not [1, 8], pointing to a hitherto unsuspected heterogeneity in the biochemical pathways leading to apoptotic cell death. DNA-damaging agents as they are used in cancer therapy can induce apoptosis. In addition, such agents can induce an irreversible arrest of the cell cycle termed “senescence”. Recently, cellular senescence has been shown to lead to the release of a broad spectrum of cytokines and immunologically relevant factors [9–11], thereby suggesting yet another scenario in which cancer treatment may influence and perhaps ignite anti-cancer immune responses.
Cell death modalities triggered by anti-tumor therapy
Tumor cell death can be induced by a panoply of distinct triggers including hypoxia, shortage of nutrients, absence of essential growth factors or conventional anticancer treatments (that is radiotherapy and chemotherapy). Cancer cells can die through different mechanisms and this cell death can be accompanied by distinct morphological changes, depending on the precise cause of death. The cell death modalities can be classified according to phenomenological and ultrastructural changes [12, 13] into type 1, 2 and 3 cell deaths that are apoptosis, autophagic cell death and necrosis, respectively (Fig. 1).
Cell death modalities. Cell death can be classified in apoptotic (type 1), necrotic (type 2) and autophagic (type 3), mostly based on morphological criteria. Autophagy is characterized by the formation of autophagic vesicles. It is an important eukaryotic response to cellular stress and in many cases lead to adaptation and survival of the cell. Apoptosis, which typically shows nuclear fragmentation and apoptotic blebbing, is often accompanied by autophagy, especially at earlier stages. At later stages, apoptotic cells can acquire features of necrosis (then termed secondary necrosis), namely swelling and membrane rupture. The network of cell death modalities is closely intertwined and facilitates efficient removal of cells destined to die
Apoptosis
Apoptotic cell death is morphologically defined by chromatin condensation (pyknosis), nuclear fragmentation (karyorrhexis), shrinkage of the cytoplasm and formation of apoptotic bodies [14]. Apoptosis is usually, but not exclusively, associated with caspase activation [15, 16] and mitochondrial membrane permeabilization [17, 18]. Caspases are the major proteases responsible for the proteolytical cleavage of numerous substrates during this process. Most of the aforementioned morphological changes are direct or indirect consequences of the controlled activation of caspases and other hydrolases that catalyze the rapid degradation of cellular substructures.
Two major pathways can lead to the activation of the apoptotic program. The intrinsic pathway is under the strict control by members of the Bcl-2 protein family. These proteins contain at least one Bcl-2 homology (BH) domain and can be subdivided into pro- and anti-apoptotic members. So-called “BH3 only” proteins are always proapoptotic and act as stress sensors. DNA damage can cause the transcriptional activation of some BH3-only proteins such as Puma and Noxa, whose expression is governed by p53 [19]. Moreover, some BH3-only proteins can be activated by posttranslational modifications, as this has been demonstrated for Bad, Bim and Bmf [20, 21]. BH3-only proteins can neutralize the antiapoptotic action of some Bcl-2 family proteins (such as Bcl-2, Bcl-XL or Mcl-1) and/or stimulate the proapoptotic activity of multidomain proteins from the Bcl-2 family (such as Bak and Bax) [22]. Once activated, Bax and/or Bak form supramolecular complexes within intracellular membranes and cause mitochondrial outer membrane permeabilization (MOMP), thus releasing proapoptotic mitochondrial intermembrane space proteins including cytochrome c [23] into the cytosol. Cytochrome c triggers the activation caspase-9 within the apoptosome, hence setting of the caspase activation cascade.
The extrinsic pathway is involved in the clearance of tumor cells by the immune system [24]. This pathway depends on the binding of a series of specific ligands (such as tumor necrosis factor, TNF) to death receptors of the TNF receptor family, causing their trimerisation. The subsequent recruitment of adapter molecules like TRADD or FADD enables the binding and autoproteolytic activation of pro-caspase-8, which in turn leads either to a direct activation of effector caspases such as caspase-3 and -7 or rather stimulates an indirect pathway, namely by triggering MOMP with subsequent cytochrome c release, apoptosome activation and caspase-9-dependent caspase-3 and -7 activation [25].
Cells that undergo physiological apoptosis are rapidly and specifically recognized and engulfed by phagocytic cells [26] like macrophages, immature dendritic cells (DCs), endothelial cells or fibroblasts. Phagocytosis by macrophages is associated with the release of anti-inflammatory mediators like transforming growth factor-β (TGF-β) [27], prostaglandin E2 [28] or platelet-activating factors [29], in apparent accord with the hypothesis that apoptosis is immunologically silent due to the avoidance or even suppression of local inflammation [30]. However, this appealing hypothesis does not apply to all experimental situations. Recent studies have revealed that treatment of tumor cells with anthracyclins [31], oxaliplatin or ionizing irradiation [1, 32, 33], but not with other apoptosis inducing drugs (such as mitomycin C, etoposide or staurosporin) could induce a potent immune response in vivo when dying cells were injected into immunocompetent mice. This implies that some types of apoptosis are immunogenic while others are not [8, 34–36]. In other words, the apparent morphological homogeneity of apoptosis can hide a certain degree of biochemical heterogeneity that in turn influences the immunogenicity of cell death.
Autophagy
Autophagy is an important eukaryotic response to cellular stress like protracted nutrient deprivation, hypoxia or infection. Macroautophagy, hereafter referred to as autophagy, involves the sequestration of cellular material within characteristic double- or multi-membraned autophagosomes and its subsequent degradation upon fusion of the autophagosomes with lysosomes [37]. Initiation of autophagy upon growth factor deprivation involves reduced signaling via class I phosphatidylinositol-3-kinase, resulting in the inactivation of Akt/PKB and mTOR [38]. Conversely, enhanced signaling through the class III PI3K/Vps34 complex, which contains Beclin-1 (or Atg6), can initiate autophagy [39]. Autophagy serves as a major turnover mechanism to eliminate supernumerary or damaged organelles, intracellular pathogens, aggregate-prone proteins and superfluous portions of cytoplasm. By promoting catabolic reactions, autophagy generates new metabolic substrates that meet the bioenergetic needs of cells and allow for adaptive protein synthesis. Autophagy promotes survival by adapting cells to stress conditions. Nevertheless, persistent autophagy, which depletes the cell of organelles and critical proteins, reportedly can lead to a caspase independent form of cell death [40].
The tumor suppressor p53 has been identified as a central node in stress- and nutritional-response networks as it exerts pleiotropic effects on metabolism, anti-oxidant defense, genomic stability, proliferation, senescence and cell death [41]. DNA damage, which is typically induced by chemotherapeutic agents, can result in p53-dependent autophagy [42]. Moreover, the re-expression of p53 in p53-deficient cancer cells has been shown to cause senescence and apoptosis [43], as well as autophagy [44]. Although the activation of p53-dependent genes can induce autophagy, the removal of a cytoplasmic pool of p53 stimulates autophagy through transcription-independent mechanisms [45], underscoring the central role of p53 in the regulation of the cellular catabolism.
During autophagy, cells usually fail to manifest signs of apoptosis such as chromatin condensation, although an initial massive autophagic vacuolization may precede or accompany apoptosis in some circumstances [46]. However, these morphological observations cannot distinguish whether cell death is simply accompanied by autophagy or whether it is truly executed by autophagy. Reportedly, in some settings autophagy acts as a molecular backup mechanism to execute cell death when apoptosis is inhibited. However, when the adaptive functions of autophagy are blocked during nutrient starvation, cells undergo accelerated death by apoptosis [46, 47]. In other settings, autophagy deficiency can stimulate necrotic cell death [48]. Inhibition of autophagy also compromises the clearance of dying cells [49], which exacerbates local inflammation and may favor tumor growth.
Apart from its role as innate defense mechanism against invading pathogens [50] autophagy and digestion of endogenously synthesized cytosolic proteins enables their processing for MHC II presentation [51, 52], thus connecting autophagy with adaptive immunity. Additional implications for autophagy in activating an immune response have recently been discovered in a comparative study in which apoptosis-incompetent Bax−/−Bak−/− mouse embryonic fibroblasts (MEF) were compared with apoptosis-competent wild type MEF, after treatment with etoposide (which usually induces non-immunogenic apoptosis, see above). Bax−/−Bak−/− MEF demonstrate massive autophagy in response to this two cell death inducer and were found to be superior in facilitating crosspriming of CD8+ and CD4+ T cells in vivo. Since this gain in immunogenicity was lost after depletion of the essential autophagy mediator Atg5, autophagy likewise enhances the immunogenicity of etoposide- or staurosporin-induced cell death [53].
Necrosis
Necrosis, named type 3 cell death, is morphologically characterized by an increase in cell volume (oncosis) leading to the early rupture of the plasma membrane. This process is accompanied by dilatation and final dismantling of cytoplasmic organelles, in particular mitochondria [12, 54]. Necrosis often is the unregulated consequence of non-physiological stress or massive, acute cell injury. On the contrary, programmed necrosis can occur as a result of the activation of specific signal transduction cascades, even during development [55] and in adult tissue homeostasis [56]. One particular form of programmed necrosis, necroptosis, is induced by TNF-receptor signaling and involves the obligatory activation of the RIP-1 kinase [57]. In these settings, the RIP-1 kinase can inhibit ATP/ADP exchange by a direct interaction with the adenine nucleotide translocase (ANT), thereby causing mitochondrial dysfunction and cell death [58]. Thus mitochondrial alterations may constitute a rate-limiting step of necrotic cell death, at least in some instances [13].
The cell’s decision to die from necrosis or apoptosis is dictated at least in part by the abundance of intracellular energy stores. Indeed, whereas apoptosis requires a minimal amount of intracellular ATP, necrosis is generally accompanied by its near-to-complete depletion [59]. The inhibition of caspases or the elimination of essential caspase activators such as APAF-1 [60], can switch the morphological appearance of cell death from apoptosis to autophagy or necrosis [15, 60, 61]. Thus, the same upstream signal can produce different types of cell death as a function of the activation or inhibition of catabolic enzymes in the cell, underlining the close relation between cell death modalities. In contrast to apoptotic cells, whose remains are engulfed completely by phagocytes, necrotic cells are internalized by a macropinocytotic mechanism, meaning that only parts of the cell are taken up by phagocytes [62].
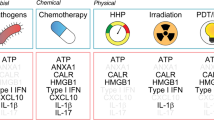
Unlike apoptosis, which only under certain circumstances exhibits an immunogenic response, necrosis is considered to be immunologically harmful at all times, because of the sudden release of proinflammatory mediators [63]. Necrotic cell death often causes the release of proinflammatory cytokines, such as interleukin-8 (IL-8), IL-10, tumor necrosis factor-α (TNF-α) [64] or of terminal mediators of inflammation like HMGB1 [65, 66] (Fig. 2).
Immunogenic determinants of tumor cell death. During early apoptosis calreticulin (CRT) is exposed on the surface. This CRT exposure is followed by that of phosphatidylserine (PS). High mobility group box 1 (HMGB1) is released during late stages of apoptosis. During necrosis (or secondary necrosis, if following apoptosis), heat shock proteins are exposed and released. Plasma membrane ruptures can also lead to the release of interleukin 8 (IL-8) and IL-10. Additionally, tumor necrosis factor α (TNF-α), HMGB1, major histocompatibility complex class I related A (MIC-A) as well as RNA and DNA are released from necrotic cells to trigger an immune response
Mitotic catastrophe
Mitotic catastrophe represents a type of cell death that occurs during mitosis and that is often preceded by micronucleation and multinucleation events. Mitotic catastrophe results from a combination of cellular damage and deficient cell cycle checkpoints like the DNA structure and the spindle assembly checkpoints [67]. Failure to arrest the cell cycle before or at mitosis triggers an attempt of aberrant chromosome segregation, which culminates in the activation of the apoptotic pathway and ultimately leads to cellular demise. Cell death occurring during the metaphase/anaphase transition is often characterized by the activation of caspase-2. Inhibition of cell death resulting from mitotic catastrophe, leads to asymmetrical division resulting in the generation of aneuploid daughter cells [68]. Thus, mitotic catastrophe may be viewed as a mechanism that protects against unwarranted (and possibly oncogenic) aneuploidization [13, 67]. The possible immunogenic potential of cells undergoing mitotic catastrophe has not been investigated yet.
Cellular senescence
Senescence was first described as a permanent state of proliferative arrest occurring in cells after extended culture in vitro [69]. Telomere erosion has been found to be one of the causes of replicative senescence during extended cell culture. Several other stress-inducing factors including DNA damage, exposure to reactive oxygen species (ROS), chemotherapeutic drugs, and aberrant oncogenic signaling initiate a similar process of senescence. Cellular senescence limits the proliferative capacity of damaged cells due to a cell cycle arrest in the G1 phase, in response to stress that puts cells at risk of malignant transformation [70]. Senescent cells develop a flattened, enlarged morphology and exhibit specific molecular senescence-associated markers like senescence associated β-galactosidase, heterochromatin foci and lipofuscin granules [71, 72]. Cellular senescence can be induced by stimuli as diverse as telomere shortening, DNA damage, oxidative stress, chemotherapeutic drugs, and expression of certain activated oncogenes [70, 73]. In spite of the diversity of these stimulatory signals, only a few senescence-inducing signal transduction pathway, mainly involving p53 and pRB have been characterized [74–76]. Under normal conditions in the healthy cell, p53 is constitutively degraded through mouse double minute 2 (MDM2) mediated proteasomal targeting. Suppression of MDM2 activity upon mitogenic stress or DNA damage leads to the stabilization of functional p53, which arrests the cell cycle by upregulating the cyclin-dependent kinase inhibitor p21. In a second pathway, the retinoblastoma protein pRB can be activated by p16 upon cellular stress or DNA damage and then binds to members of the E2F family of transcription factors, thus avoiding cell cycle progression [77, 78]. The two pathways manifest ample crosstalk in the control of cellular senescence, and can also overlap with death pathways like apoptosis and necrosis [79].
Although IL-8 and GROa have well characterized tumor promoting activities, recent results suggest that these chemokines can participate in senescence through an action on the chemokine receptor CXCR2. Thus, senescent cells can activate a self-amplifying secretory program in which CXCR2-binding chemokines reinforce growth arrest [9]. Another secreted factor, insulin growth factor binding protein 7 (IGFBP7), also induces cellular senescence in melanocytes that contain activating mutations in the BRAF oncogene [11].
The first oncogene shown to trigger senescence was a tumor-derived allele of H-RAS [80]. Recent reports suggest that RAS-induced senescence involves a DNA damage response induced by replication stress [70]. Thus, senescence may counter the tumor-promoting effects of hyperproliferative mutations, acting as a cell-intrinsic mechanism of tumor suppression [81]. Although the physiological relevance of oncogene-induced senescence has been debated, recent reports indicate that this process acts as a potent barrier against tumorigenesis [82]. Expression of oncogenic BRAFV600E induces senescence in cultured fibroblasts or melanocytes. A genome-wide RNA interference (RNAi) screen led to the identification of IGFBP7, a secreted protein that is required for the induction of senescence in these cells [11]. The synthesis and secretion of IGFBP7 in turn can trigger apoptosis in cells that have progressed to melanoma, showing how a feedback loop of secreted factors initiates a cell death program in oncogene transformed cells. Whether such secreted factors also affect anti-cancer immunosurveillance and later anti-cancer immune responses elicited by chemotherapy remains on open conundrum.
Immunogenic effectors and their influence on the immune system
Calreticulin
Calreticulin (CRT) is a Ca2+-binding chaperone that is usually located in the lumen of the endoplasmic reticulum (ER). In interplay with the ER-resident disulfide isomerase ERp57, CRT facilitates proper folding of most ER-chaperoned proteins. In addition, CRT has been implicated in cell removal by binding and activating CD91 (also called LDL-receptor related protein, LRP) on engulfing cells [83].
Calreticulin was found to be exposed on the outer leaflet of the cells during the early phase of cell death upon treatment with anthracyclins. The translocation of CRT is induced upon treatment of tumor cells with anthracyclins, oxaliplatin and ionizing irradiation. Unlike, other cell death inducers targeting ER (like thapsigargin, tunicamiycin and brefeldin), mitochondria (arsenite, betulinic acid and C2 ceramide) or DNA (Hoechst 33342, camptothecin, etoposide and mitomycin C) fail to induce CRT exposure and immunogenic cell death [1]. The translocation and exposure of CRT dictates the immunogenicity of tumor cell death, presumably because surface-exposed CRT facilitates the engulfment of dying tumor cells by DC [1, 32, 33].
In patients with acute myeloid leukemia (AML), CRT has been found to translocate to the surface of circulating tumor cells in response to intravenous injection of anthracyclins. However, this CRT translocation was only observed in malignant myeloblasts of some but not all treated patients [84]. Therefore, the existence of resistance mechanisms has to be postulated, meaning that some tumors but not others can be stimulated to expose CRT on the cell surface. The human neuroblastoma cell line (SH-SY5Y) is intrinsically incapable to expose CRT in response to anthracyclin treatment. However, this defect can be overcome by depleting ER Ca2+. Treatment with thapsigargin (which blocks the ER Ca2+ pump SERCA and hence induces ER Ca2+ depletion) or transgenic expression of the Ca2+-permeable ER channel reticulon-1C restored the ability to expose CRT in SH-SY5Y cells, strongly suggesting that a Ca2+-related signaling event is necessary for CRT-exposure [7].
Anthracyclin-induced CRT exposure is accompanied by ER stress such as the phosphorylation of the eukaryotic initiation factor 2α (eIF2α), which signals an immediate arrest in protein synthesis. Inhibition of the eIF2α-specific phosphatase, which consists of the general protein phosphatase 1 (PP1) and its regulatory subunit growth arrest and DNA damage inducible gene 34 (GADD34), by means of chemical inhibitors induced CRT translocation, which was accompanied by the hyperphosphorylation of eIF2α [1]. The phosphorylation of eIF2α therefore seems to be a prerequisite for the exposure of CRT from the ER at the cell surface, as it occurs after treatment of tumor cells with anthracyclins or ionizing irradiation. In addition, caspase signaling is required for efficient CRT exposure. Treatment with the pan-caspase inhibitor Z-VAD-fmk or transfection of cells with the caspase inhibitor p35 (a Baculovirus-derived inhibitor of apoptosis protein, IAP) abolished the immunogenic effect of anthracyclin treatment [31] and inhibited CRT surface exposure [1]. This result implies that one biochemical hallmark of apoptosis—caspase activation—is closely linked to the immunogenicity of cell death.
When CRT moves to the cell surface at a pre-apoptotic stage (that is before phosphatidylserine expose and before plasma membrane permeabilization), it does not travel alone. We found that the co-translocation of CRT with the ER-resident disulfide isomerase ERp57 is obligatory for the immunogenic outcome of anthracyclin treatment. The knockdown or knockout of ERp57 inhibits the anthracyclin-induced translocation of CRT, and conversely, knockdown or knockout of CRT inhibits the anthracyclin-induced surface exposure of ERp57, indicating that the interaction of both proteins is obligatory for their co-translocation [6]. CT26 tumors that lack ERp57 (and hence are unable to expose CRT) are resistant to anthracyclin chemotherapy in immunocompetent hosts in conditions in which isogenic control cell lines do respond to chemotherapy. The failure of tumor cells with ERp57 knockdown to elicit immune responses and to respond to chemotherapy can be overcome by exogenous supply of recombinant CRT protein. Thus, intratumoral injection of CRT can reestablish the response of ERp57-deficient tumor cells to anthracyclin therapy [6]. These observations indicate that tumors that possess an intrinsic defect in the CRT-translocation machinery become resistant to anthracyclin chemotherapy due to their incapacity to elicit an anti-cancer immune response.
The presence of a CRT specific receptor on the surface of dying tumor cells is essential for the immunogenicity of cell death because neutralizing CRT-specific antibodies or siRNA-mediated CRT silencing suppress the efficacy of anti-cancer vaccines based on dying tumor cells. Conversely, such vaccination effects could be restored by supplying recombinant CRT [1, 32, 33]. It has been shown that phagocytosis of dying cells by J774 macrophages can be abolished by blocking the CD91 receptor with a specific antibody or by saturating it with recombinant receptor associated protein (RAP) [83]. Other proteins like thrombospondin as well as the complement factor C1q have also been suggested to bind to CRT and to function as molecular bridges between CRT and CD91 [85]. CRT reportedly binds to other surface receptors like the scavenger receptor A (SR-A) or the scavenger receptor expressed on endothelia cells (SREC-I) [86]. Therefore, the exact nature of the CRT receptor involved in the engulfment of dying tumor cells by immature DC remains to be elucidated.
Heat shock proteins
Inducible heat shock proteins (HSP) constitute a class of chaperones that can be induced by multiple different stressors [87]. Under non-lethal stress conditions, HSP function to protect cells by refolding damaged proteins or by redirecting them to proteasomal degradation. HSP70 and HSP90 can translocate from intracellular compartments to the cell surface and hence can participate in the activation of the immune system during necrosis [88]. The recognition of HSP exposed by tumor cells can be mediated by TLR4, which facilitates intracellular antigen processing and presentation [89]. Scavenger receptors may also participate in the recognition of HSP and might stimulate DC maturation. Interestingly the α-isoform of HSP90 shows similarities to CRT with regard to its ER-localization and function as a chaperon, suggesting a general mechanism that may account for the exposure of ER proteins during immunogenic treatments. The exposure of HSP has been closely related to necrosis [90] and apoptosis inhibitory effects have been assigned to HSP overexpression [91]. Intracellular HSP70 can block apoptosis at multiple levels including through the inhibition of Apaf-1, apoptosis inducing factor (AIF), p53, JNK or Bax [92–94]. HSP90 also exerts antiapoptotic functions by interacting with Apaf-1, by inactivating Bad and by activating NFκB [95]. Recently, surface-exposed HSP90 has been shown to contribute to the immunogenicity of human myeloma cell death elicited by the proteasome inhibitor bortezomib. This surface HSP90 may stimulate DC maturation [96]. These results suggest that the presence of HSPs at the surface of dying tumor cells facilitates their recognition by DC and/or stimulate the maturation of DC.
High-mobility group box 1
Cells that undergo necrosis release HMGB1, which has proinflammatory properties [65]. It has been thought for long that HMGB1 release would be a specific marker of necrosis. Nonetheless, apoptotic and autophagic cells [97] may also release HMGB1, at least under certain circumstances. Recently, the redox status of HMGB1 has been discovered to be important for its immunological potential during apoptotic release [98]. In healthy cells, HMGB1 binds to chromatin and influences transcription and other nuclear functions. HMGB1 can either be actively secreted from inflammatory cells or passively released from necrotic cells [66]. The release of HMGB1 from the nucleus of dying tumor cells to their cytoplasm and subsequently to the extracellular space during later stages of apoptosis constitutes a crucial step in the activation of antigen presenting cells [2]. HMGB1 has been shown to bind to at least three different surface receptors expressed on DC, namely the receptor for advanced glycosylation (RAGE), TLR2 and TLR4 [99, 100]. The binding of HMGB1 to TLR4 can facilitate the processing and presentation of tumor derived antigens by inhibiting fusion of phagosomes with lysosomes, thereby preventing the precocious degradation of tumor antigens and enabling their traffic towards the dedicated antigen-presenting compartment [2]. Neutralization or knockdown of HMGB1 or knockout of TLR4 abolishes the capacity of dying tumor cells to elicit anticancer immune responses both in vitro and in vivo. CT26 colon cancers, TS/A mammary cancers, EL4 thymomas or Glasgow osteosarcomas failed to respond to anti-cancer chemotherapies or radiotherapies when they were implanted in tlr4 −/− mice, in conditions in which they readily responded to therapy in TLR4-sufficient wild type mice [2].
The intracellular adapter molecule MyD88 is involved in TLR signaling by mediating a signaling cascade that can be separated from TRIF-dependent signals. It could be shown that MyD88 (but not TRIF) is important for the perception of immunogenic cell death. Oxaliplatin, which proved to block the growth of Glasgow osteosarcomas established in WT mice (as well as trif −/− mice) failed to induce an anti-tumor effect in myD88 −/− hosts. Thus, a TLR4/MyD88 dependent pathway participates in the chemotherapy-induced anti-cancer immune response. The relevance of this signaling has been underlined by studies with breast cancer patients bearing a loss-of-function allele of TLR4 that reduces the affinity of TLR4 for HMGB1. Patients bearing the loss-of-function alleles of TLR4 relapsed more rapidly after local radiotherapy and systemic anthracyclin therapy than patients bearing the normal allele of TLR4 [2, 3].
NKG2D-ligands
In response to oncogenes or DNA damaging agents, cells manifest a stereotyped DNA damage response. This response can lead to the expression of ligands for the stimulatory immune receptor expressed by natural killer (NK) cells and T cells, NKG2D, such as MHC class I polypeptide-related sequence A (MICA) or the retinoic acid early transcript 1 (RAE1) [101]. During the DNA damage response, DNA double strand breaks elicit the recruitment and enzymatic activation the protein ataxia telangiectasia mutated (ATM), a kinase, followed by the ATM-mediated activation of check point kinases (such as Chk1 or Chk2) and the activating phosphorylation of the tumor suppressor protein p53. Pre-neoplastic lesions and in situ carcinomas often harbor activated, phosphorylated ATM, CHK1, and p53, coupled to an increase in senescence and apoptosis. In contrast, advanced cancers tend to suppress or lose this DNA damage response. NK, NKT and cytotoxic T cells efficiently destroy cancer cells that express NKG2D [102]. In addition, DNA damage can stimulate the expression of Fas/CD95 and TNF-related apoptosis-inducing ligand (TRAIL) receptors on cancer cells, sensitizing them to FasL or TRAIL-mediated lysis [103].
The senescence of tumor cell, which can be triggered in a p53 dependent fashion in cells undergoing DNA damage, may also elicit signals to the innate immune system Senescence has been associated with the up regulation of inflammatory cytokines such as MCP-1, IL-1ß, IL-15 or TLR4, which in turn stimulate an innate immune response that facilitates the clearance of senescent tumor cells [43].
Nucleotide release
During apoptotic and necrotic cell death, degrading cellular corpses release nucleotides, RNA and DNA, which may exert immunostimulatory effects. RNA which is released during cell death can interact with TLR3 on the surface of DC [104], double-stranded DNA can stimulate macrophages and DC [105]. Nucleotides may stimulate the maturation of DC accompanied by an activation of the NF-κB signaling [106, 107]. Multiple pattern recognition receptors (PRR) expressed on the surface by antigen-presenting cells are necessary for mediating these reactions [108]. However, the contribution of tumor-derived polynucleotides, oligonucleotides, nucleotides and nucleosides and their PRR to the anti-cancer immune response has to be further investigated [109].
Inflammatory cytokines
Dying tumor cells can release proinflammatory cytokines that can be instrumental in eliciting an immune response. Necrotic cell death is assumed to be the cell death modality that is associated with the indiscriminate release of soluble intracellular constituents to the extracellular medium [110]. Indeed, necrotic cells are able to act on fibroblasts, macrophages and DCs, activating NF-κB and inducing the expression of genes that are involved in inflammatory responses and tissue repair [90] including the cytokine-induced neutrophil chemoattractant (KC) and macrophage inflammatory protein-2, metalloproteinase 3 and vascular endothelial growth factor, TNF-α, IL-8, IL-10, and IL-6 [111]. These general proinflammatory features seem to be absent from apoptotic cells [62]. The induction of an immune response by a general and unspecific release of multiple immunogenic factors therefore seems to be a unique feature of necrosis.
Conclusion
The innate and cognate immune responses elicited by immunogenic chemotherapy and ionizing irradiation are required for an optimal outcome of anti-cancer treatments. During the course of immunogenic cell death intracellular factors are exposed on the cell surface. These changes in the composition of the cell surface, as well as the release of soluble immunogenic signals determine the outcome of therapy. In some cases, apoptosis may become fully immunogenic. The exposure of CRT together with ERp57 occurs early during apoptosis and depends on caspase activation. The release of soluble factors that accompanies later stages of cell death (namely secondary necrosis) facilitates the efficient activation of the immune system and thereby the clearance of tumor cells from the organism. The order of events and its spatiotemporal appearance during the course of tumor cell death seems to constitute the key that can unlock the immune system (Fig. 3a, b). As discussed here, the immune response against dying tumor cells can play a major role in determining therapeutic success. If tumor cell death occurs in a potentially immunogenic fashion and if the immune system is capable of perceiving this immunogenicity, a potent innate and cognate immune response raised against dying cancer cells can contribute to the control and elimination of residual cancer (stem) cells. It is tempting to speculate that such an anticancer immune response constitutes a conditio sine qua non for the long-term success of tumor therapy.
Key events of immunogenic tumor cell death. A temporal sequence of events headed by calreticulin (CRT) exposure during early apoptosis and followed by high-mobility group box 1 (HMGB1)-release during later stages of cell death facilitates efficient dendritic cell (DC) activation and maturation. HMGB1 uses toll like receptor 4 (TLR4) to bind to DC. In contrast, the CRT receptor is still elusive. The presentation of tumor antigens by mature DC finally leads to CD4+ and CD8+ T-cell activation
References
Obeid M, Tesniere A, Ghiringhelli F et al (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61. doi:10.1038/nm1523
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 220:47–59. doi:10.1111/j.1600-065X.2007.00573.x
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059. doi:10.1038/nm1622
Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G (2008) The anticancer immune response: indispensable for therapeutic success? J Clin Invest 118:1991–2001. doi:10.1172/JCI35180
Zitvogel L, Kroemer G (2008) The immune response against dying tumor cells: avoid disaster, achieve cure. Cell Death Differ 15:1–2. doi:10.1038/sj.cdd.4402267
Panaretakis T, Joza N, Modjtahedi N et al (2008) The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 15:1499–1509. doi:10.1038/cdd.2008.67
Tufi R, Panaretakis T, Bianchi K et al (2008) Reduction of endoplasmic reticulum Ca2+ levels favors plasma membrane surface exposure of calreticulin. Cell Death Differ 15:274–282. doi:10.1038/sj.cdd.4402275
Zitvogel L, Casares N, Pequignot MO, Chaput N, Albert ML, Kroemer G (2004) Immune response against dying tumor cells. Adv Immunol 84:131–179. doi:10.1016/S0065-2776(04)84004-5
Acosta JC, O’Loghlen A, Banito A et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133:1006–1018. doi:10.1016/j.cell.2008.03.038
Kuilman T, Michaloglou C, Vredeveld LC et al (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133:1019–1031. doi:10.1016/j.cell.2008.03.039
Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132:363–374. doi:10.1016/j.cell.2007.12.032
Kroemer G, El-Deiry WS, Golstein P et al (2005) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 12(Suppl. 2):1463–1467. doi:10.1038/sj.cdd.4401724
Galluzzi L, Maiuri MC, Vitale I et al (2007) Cell death modalities: classification and pathophysiological implications. Cell Death Differ 14:1237–1243. doi:10.1038/sj.cdd.4402148
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kroemer G, Martin SJ (2005) Caspase-independent cell death. Nat Med 11:725–730. doi:10.1038/nm1263
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163. doi:10.1152/physrev.00013.2006
Ferri KF, Kroemer G (2001) Mitochondria—the suicide organelles. Bioessays 23:111–115. doi:10.1002/1521-1878(200102)23:2<111::AID-BIES1016>3.0.CO;2-Y
Marzo I, Susin SA, Petit PX et al (1998) Caspases disrupt mitochondrial membrane barrier function. FEBS Lett 427:198–202. doi:10.1016/S0014-5793(98)00424-4
Oda E, Ohki R, Murasawa H et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058. doi:10.1126/science.288.5468.1053
Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ (2003) Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem 278:18811–18816. doi:10.1074/jbc.M301010200
Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ (2004) Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem 279:8837–8847. doi:10.1074/jbc.M311578200
Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15:1481–1486. doi:10.1101/gad.897601
Wei MC, Lindsten T, Mootha VK et al (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14:2060–2071
Schulze-Osthoff K, Krammer PH, Droge W (1994) Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J 13:4587–4596
Los M, Van de Craen M, Penning LC et al (1995) Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 375:81–83. doi:10.1038/375081a0
Savill J, Dransfield I, Gregory C, Haslett C (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975. doi:10.1038/nri957
Chen W, Frank ME, Jin W, Wahl SM (2001) TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14:715–725. doi:10.1016/S1074-7613(01)00147-9
Fournier T, Fadok V, Henson PM (1997) Tumor necrosis factor-alpha inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Biol Chem 272:31065–31072. doi:10.1074/jbc.272.49.31065
Savill J, Fadok V (2000) Corpse clearance defines the meaning of cell death. Nature 407:784–788. doi:10.1038/35037722
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I (1997) Immunosuppressive effects of apoptotic cells. Nature 390:350–351. doi:10.1038/37022
Casares N, Pequignot MO, Tesniere A et al (2005) Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 202:1691–1701. doi:10.1084/jem.20050915
Obeid M, Panaretakis T, Joza N et al (2007) Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 14:1848–1850. doi:10.1038/sj.cdd.4402201
Obeid M, Panaretakis T, Tesniere A et al (2007) Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res 67:7941–7944. doi:10.1158/0008-5472.CAN-07-1622
Albert ML, Pearce SF, Francisco LM et al (1998) Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188:1359–1368. doi:10.1084/jem.188.7.1359
Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86–89. doi:10.1038/32183
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252. doi:10.1038/32588
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752. doi:10.1038/nrm2239
Boya P, Gonzalez-Polo RA, Casares N et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040. doi:10.1128/MCB.25.3.1025-1040.2005
Furuya N, Yu J, Byfield M, Pattingre S, Levine B (2005) The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 1:46–52
Amaravadi RK, Thompson CB (2007) The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res 13:7271–7279. doi:10.1158/1078-0432.CCR-07-1595
Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8:275–283. doi:10.1038/nrm2147
Feng Z, Zhang H, Levine AJ, Jin S (2005) The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA 102:8204–8209. doi:10.1073/pnas.0502857102
Xue W, Zender L, Miething C et al (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660. doi:10.1038/nature05529
Amaravadi RK, Yu D, Lum JJ et al (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 117:326–336. doi:10.1172/JCI28833
Tasdemir E, Maiuri MC, Galluzzi L et al (2008) Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10:676–687. doi:10.1038/ncb1730
Gonzalez-Polo RA, Boya P, Pauleau AL et al (2005) The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci 118:3091–3102. doi:10.1242/jcs.02447
Shimizu S, Kanaseki T, Mizushima N et al (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6:1221–1228. doi:10.1038/ncb1192
Degenhardt K, Mathew R, Beaudoin B et al (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10:51–64. doi:10.1016/j.ccr.2006.06.001
Qu X, Zou Z, Sun Q et al (2007) Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128:931–946. doi:10.1016/j.cell.2006.12.044
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766. doi:10.1016/j.cell.2004.11.038
Paludan C, Schmid D, Landthaler M et al (2005) Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307:593–596. doi:10.1126/science.1104904
Dengjel J, Schoor O, Fischer R et al (2005) Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA 102:7922–7927. doi:10.1073/pnas.0501190102
Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML (2009) Autophagy within the antigen donor cell facilitates efficient antigen cross-priming. Cell Death Differ (accepted)
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–669. doi:10.1016/j.ceb.2004.09.011
Roach HI, Clarke NM (2000) Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br 82:601–613. doi:10.1302/0301-620X.82B4.9846
Barkla DH, Gibson PR (1999) The fate of epithelial cells in the human large intestine. Pathology 31:230–238. doi:10.1080/003130299105043
Holler N, Zaru R, Micheau O et al (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495. doi:10.1038/82732
Temkin V, Huang Q, Liu H, Osada H, Pope RM (2006) Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol 26:2215–2225. doi:10.1128/MCB.26.6.2215-2225.2006
Nicotera P, Leist M, Ferrando-May E (1998) Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett 102–103:139–142. doi:10.1016/S0378-4274(98)00298-7
Golstein P, Kroemer G (2005) Redundant cell death mechanisms as relics and backups. Cell Death Differ 12(Suppl. 2):1490–1496. doi:10.1038/sj.cdd.4401607
Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol 9:967–970. doi:10.1016/S0960-9822(99)80425-4
Krysko DV, Denecker G, Festjens N et al (2006) Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ 13:2011–2022. doi:10.1038/sj.cdd.4401900
Vakkila J, Lotze MT (2004) Inflammation and necrosis promote tumour growth. Nat Rev Immunol 4:641–648. doi:10.1038/nri1415
Fadok VA, Bratton DL, Guthrie L, Henson PM (2001) Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol 166:6847–6854
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251. doi:10.1126/science.285.5425.248
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837. doi:10.1038/sj.onc.1207528
Kops GJ, Weaver BA, Cleveland DW (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5:773–785. doi:10.1038/nrc1714
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636. doi:10.1016/0014-4827(65)90211-9
Campisi J (2007) d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740. doi:10.1038/nrm2233
Itahana K, Campisi J, Dimri GP (2007) Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 371:21–31. doi:10.1007/978-1-59745-361-5_3
Kurz T, Terman A, Brunk UT (2007) Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys 462:220–230. doi:10.1016/j.abb.2007.01.013
van Heemst D, den Reijer PM, Westendorp RG (2007) Ageing or cancer: a review on the role of caretakers and gatekeepers. Eur J Cancer 43:2144–2152. doi:10.1016/j.ejca.2007.07.011
Di Leonardo A, Linke SP, Clarkin K, Wahl GM (1994) DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 8:2540–2551. doi:10.1101/gad.8.21.2540
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14:501–513. doi:10.1016/S1097-2765(04)00256-4
Vicencio JM, Galluzzi L, Tajeddine N et al (2008) Senescence, apoptosis or autophagy? When a damaged cell must decide its path—a mini-review. Gerontology 54:92–99. doi:10.1159/000129697
Helmbold H, Deppert W, Bohn W (2006) Regulation of cellular senescence by Rb2/p130. Oncogene 25:5257–5262. doi:10.1038/sj.onc.1209613
Vaziri H, Benchimol S (1999) Alternative pathways for the extension of cellular life span: inactivation of p53/pRb and expression of telomerase. Oncogene 18:7676–7680. doi:10.1038/sj.onc.1203016
Kapic A, Helmbold H, Reimer R, Klotzsche O, Deppert W, Bohn W (2006) Cooperation between p53 and p130(Rb2) in induction of cellular senescence. Cell Death Differ 13:324–334. doi:10.1038/sj.cdd.4401756
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602. doi:10.1016/S0092-8674(00)81902-9
Lowe SW, Cepero E, Evan G (2004) Intrinsic tumour suppression. Nature 432:307–315. doi:10.1038/nature03098
Narita M, Lowe SW (2005) Senescence comes of age. Nat Med 11:920–922. doi:10.1038/nm0905-920
Gardai SJ, McPhillips KA, Frasch SC et al (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334. doi:10.1016/j.cell.2005.08.032
Chaput N, De Botton S, Obeid M et al (2007) Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference. J Mol Med 85:1069–1076. doi:10.1007/s00109-007-0214-1
Ogden CA, de Cathelineau A, Hoffmann PR et al (2001) C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med 194:781–795. doi:10.1084/jem.194.6.781
Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV (2004) SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem 279:51250–51257. doi:10.1074/jbc.M406202200
Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C (2007) Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 81:15–27. doi:10.1189/jlb.0306167
Saito K, Dai Y, Ohtsuka K (2005) Enhanced expression of heat shock proteins in gradually dying cells and their release from necrotically dead cells. Exp Cell Res 310:229–236. doi:10.1016/j.yexcr.2005.07.014
Asea A, Kraeft SK, Kurt-Jones EA et al (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442. doi:10.1038/74697
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546. doi:10.1093/intimm/12.11.1539
Jaattela M (1995) Over-expression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer 60:689–693. doi:10.1002/ijc.2910600520
Wadhwa R, Yaguchi T, Hasan MK, Mitsui Y, Reddel RR, Kaul SC (2002) Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp Cell Res 274:246–253. doi:10.1006/excr.2002.5468
Ravagnan L, Gurbuxani S, Susin SA et al (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3:839–843. doi:10.1038/ncb0901-839
Lee JS, Lee JJ, Seo JS (2005) HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem 280:6634–6641. doi:10.1074/jbc.M412393200
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85. doi:10.1038/43466
Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV (2007) Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 109:4839–4845. doi:10.1182/blood-2006-10-054221
Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A (2008) Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ 16:175–183
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29:21–32. doi:10.1016/j.immuni.2008.05.013
Tesniere A, Apetoh L, Ghiringhelli F et al (2008) Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 20:504–511. doi:10.1016/j.coi.2008.05.007
Haynes NM, van der Most RG, Lake RA, Smyth MJ (2008) Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol 20:545–557. doi:10.1016/j.coi.2008.05.008
Gasser S, Orsulic S, Brown EJ, Raulet DH (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436:1186–1190. doi:10.1038/nature03884
Guerra N, Tan YX, Joncker NT et al (2008) NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28:571–580. doi:10.1016/j.immuni.2008.02.016
Bauer S, Groh V, Wu J et al (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727–729. doi:10.1126/science.285.5428.727
Kariko K, Ni H, Capodici J, Lamphier M, Weissman D (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279:12542–12550. doi:10.1074/jbc.M310175200
Ishii KJ, Suzuki K, Coban C et al (2001) Genomic DNA released by dying cells induces the maturation of APCs. J Immunol 167:2602–2607
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K (2005) Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10:459–469. doi:10.1007/s10495-005-1875-2
Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K (1997) Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol 139:1635–1643. doi:10.1083/jcb.139.7.1635
Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A (2006) Pentraxins as a key component of innate immunity. Curr Opin Immunol 18:10–15. doi:10.1016/j.coi.2005.11.009
Kepp O, Tesniere A, Zitvogel L, Kroemer G (2009) The immunogenicity of tumor cell death. Curr Opin Oncol (accepted)
Matzinger P (2002) The danger model: a renewed sense of self. Science 296:301–305. doi:10.1126/science.1071059
Li M, Carpio DF, Zheng Y et al (2001) An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol 166:7128–7135
Acknowledgments
G.K. is supported by a special grant from Ligue contre le Cancer (équipe labellisée) as well as by grants from European Commission (Active p53, AICR, RIGHT, Trans-Death, Death-Train, ChemoRes) and by Institut National contre le Cancer (INCa). O.K. receives a long term fellowship from EMBO, A.T is supported by INSERM. F.S. is supported by Fondation pour la recherché Medical, L.S. is supported by Death Train Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kepp, O., Tesniere, A., Schlemmer, F. et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 14, 364–375 (2009). https://doi.org/10.1007/s10495-008-0303-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0303-9