Abstract
Our study aimed to compare death signalling pathways triggered by lupulone in TRAIL-sensitive human colon cancer cells (SW480) and in their derived TRAIL-resistant metastatic cells (SW620). Lupulone (40 μg/ml) up-regulated expression of TRAIL DR4/DR5 death receptors at the cell surface of both cell lines, even in the absence of exogenous TRAIL ligand. Cell death induced by lupulone was inhibited in SW480 and SW620 cells exposed to blocking anti-DR4/DR5 antibodies. In SW480 cells, lupulone triggered cell death through a cross-talk between TRAIL-DR4/DR5 and the mitochondrial (intrinsic) pathways involving caspase-8 activation and Bid protein cleavage. As a consequence mitochondrial cytochrome c was released into the cytosol and activation of caspases-9 and -3 was observed. In the metastatic SW620 cells, lupulone restored the sensibility of these cells to TRAIL ligand and activated the extrinsic apoptotic pathway via DR4/DR5 death receptors and the involvement of the caspase-8/caspase-3 cascade. The demonstration that lupulone is able to activate TRAIL-death signalling pathways even in TRAIL resistant cancer cells highlights the potential of this natural compound for cancer prevention and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most predominant cause of cancer-related death [1]. Actually chemotherapy like 5-fluorouracil (5-FU), oxaliplatin (FOLFOX) or irinotecan (CPT-11) has a limited efficacy in advanced colorectal cancer and new approaches for colon cancer treatment are urgently required [2, 3]. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a potential anticancer agent because of its capacity to kill selectively cancer cells without toxic effects on normal cells [4, 5]. TRAIL induces apoptosis by binding to apoptotic TRAIL receptors DR4 and DR5. It was shown that the activation of these receptors lead to formation of homo or heterocomplexes for recruitment and activation of Fas-associated death domain (FADD), followed by activation of initiator caspases (caspases-8, -9 and -10) and effector caspase-3 that is ultimately responsible for apoptosis [6, 7].
Two main signalling pathways are generally involved in the process leading to apoptosis, either the intrinsic mitochondria-dependent pathway and/or the extrinsic death receptor pathway [8]. Furthermore a cross-talk exists between both pathways. Indeed, caspase-8 activation by death receptors can either activate directly caspase-3 and/or cleave Bid protein, a member of the Bcl2 family, to generate the formation of a 15 kDa truncated Bid (tBid) protein. The truncated tBid protein can then disrupt mitochondrial membrane potential leading to the release of cytochrome c into the cytosol and activation of caspase-9 and caspase-3 [8–10]. Mitochondrial dysfunction may also activate the formation of intracellular reactive oxygen species (ROS) that can damage proteins, DNA and other cellular organites involved in cell death [11, 12].
The bitter acids of hops (Humulus lupulus L.) consist of alpha-acids or humulone and beta-acids or lupulone. Lupulone is a mixture of isomers n-, co- and ad-lupulones [13, 14]. We reported previously that lupulone induces apoptosis in SW620 colon-derived metastatic cells by activating death receptors signalling pathway and antagonizes tumor development in a preclinical model of colon carcinogenesis [15]. In addition, lupulone was shown to inhibit angiogenesis in vitro and in vivo [16]. In order to get more insight into the intimate mechanisms involved in the pro-apoptotic effects of lupulone we aimed to compare the death signalling pathways triggered by lupulone in TRAIL-sensitive human colon cancer cells (SW480) and in their derived TRAIL-resistant metastatic cells (SW620) [17].
Materials and methods
Lupulone
Lupulone was obtained from an industrial by-product that contains high amounts of beta-acids (Brasseries Kronenbourg, Strasbourg, France) and was isolated following the procedure described previously [15].
Cell culture
SW620 and SW480 cells were obtained from the European Collection of Animals Cell Culture (Salisbury, UK). They were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mM glucose and supplemented with 10% heat-inactivated (56°C) horse serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 1% non-essential amino acids (Invitrogen Corp., Cergy Pontoise, France) and kept at 37°C in a humidified atmosphere with 5% CO2. For experiments, after trypsinization (0.5% trypsin/2.6 mM ethylenediamine tetraacetic acid), cells were seeded at 1 × 106 cells in culture dishes (100 mm internal diameter) or at 2 × 105 cells in culture dishes (25 mm internal diameter). The culture medium was DMEM supplemented with 3% heat-inactivated horse serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 μg/ml transferrin, 5 ng/ml selenium, 10 μg/ml insulin and 1% non-essential amino acids (Invitrogen Corp., France).
Cell death analysis by flow cytometry
Cell death was analysed by flow cytometry as follows. 1 × 106 SW480 or SW620 cells were seeded in culture dishes and harvested by trypsinization at different time points (15, 24 and 48 h) after initial treatment with lupulone (40 μg/ml). Cells were centrifuged and fixed with 1 ml methanol:PBS (9:1 v/v), washed twice in phosphate buffer saline (PBS) and re-suspended in 200 μl PBS containing 0.25 μg/ml RNase A and 0,1 mg/ml propidium iodide (Sigma Aldrich, Munchen, Germany). After incubation in the dark at 37°C for 30 min, the fluorescence of 10,000 cells was analyzed by flow cytometry and CellQuest software (FACScan, BD Biosciences, Erembodegem, Belgium).
Detection of TRAIL receptors DR4, DR5, DcR1 and DcR2
Cells were treated with lupulone (40 μg/ml) and harvested by trypsinization at 24 and 48 h. Cell pellets were washed with PBS and incubated with FITC-conjugated mouse anti-human TRAIL receptors, DR4, DR5, DcR1 and DcR2 (Alexis Biochemicals, Lausen, Switzerland) or FITC-conjugated mouse IgG1 monoclonal isotype control antibody (BD Biosciences, Belgium) for 30 min at 4°C in the dark. After washing with PBS, cells were re-suspended in PBS and the fluorescence (515 nm) of 10,000 events per sample were analyzed by FACScan and CellQuest Software (BD Biosciences, Belgium).
TRAIL and lupulone combinations and effects of DR4-, DR5-blocking antibodies
Cells (2 × 105) were seeded on culture dishes (25 mm diameter) and were incubated with TRAIL (50 ng/ml) (Alexis Biochemicals, Switzerland) or with lupulone (10 μg/ml) or with a combination TRAIL + lupulone. Cells were harvested by trypsinization after 24 and 48 h, centrifuged and fixed with 1 ml methanol:PBS (9:1 v/v). After washing with PBS, cell pellets were re-suspended in 200 μl PBS containing 0.25 μg/ml RNase A and 0.1 mg/ml propidium iodide (Sigma Aldrich, Germany). After incubation in the dark at 37°C for 30 min, the fluorescence of 10,000 cells was analyzed by flow cytometry and CellQuest software (FACScan, BD Biosciences, Belgium). For experiments with DR4, DR5 blocking antibodies, cells were pre-treated with lupulone or TRAIL, 24 h before the addition of human blocking anti-DR4 or anti-DR5 antibodies (250 ng/ml) (Alexis Biochemicals, Switzerland) and cells were harvested after 24 h.
Measurements of caspase-8, caspase-3 and caspase-9 activities
After incubation with lupulone (40 μg/ml), cells were harvested, washed in PBS and stored at −80°C. Caspase-8 and -3 activities were determined by using caspase-8 and caspase-3 Assay Colorimetric Kits (Sigma Aldrich, Germany).The hydrolysis of the peptide substrate Ac-IETD-pNA by caspase-8 or of the peptide substrate Ac-DEVD-pNA by caspase-3 results of the release of a pNA moiety. The concentration of pNA was calculated from the absorbance values at 405 nm and calibration curve of defined pNA solutions. Values were expressed as nmol pNA/mg of total protein. Another kit was used for the determination of caspase-9 activity using the APOPCYTO Caspase-9 Colorimetric Assay Kit (MBL International Corporation, Nagoya, Japan). This assay was similar to the procedure described above, but the enzyme substrate was LEHD-pNA. Data were expressed as nmol pNA/mg of total protein.
Effect of caspase-8 inhibition on cell death induced by lupulone
Cells (2 × 105) were seeded on culture dishes (25 mm diameter) and were pre-treated with the specific caspase-8 inhibitor, Z-IETD-FMK at 50 μM (R&D systems, Abingdon, UK) during 2 h 30 min before the incubation with lupulone (40 μg/ml) during 24 and 48 h. Cells were harvested by trypsinization, centrifuged and fixed with 1 ml methanol:PBS (9:1 v/v). After washing with PBS, cell pellets were re-suspended in 200 μl PBS containing 0.25 μg/ml RNase A and 0.1 mg/ml propidium iodide (Sigma Aldrich, Germany). After incubation in the dark at 37°C for 30 min, the fluorescence of 10,000 cells was analyzed by flow cytometry and CellQuest software (FACScan, BD Biosciences, Belgium).
Western blot analysis of Bid protein expression
Cells (5 × 106) exposed to lupulone, were harvested at different time points, washed with PBS and incubated for 30 min at 4°C in a lysate buffer (Tris–HCl 50 mM pH 7.5, NaCl 150 mM, EDTA 5 mM, DTT 1 mM, Triton X-100 1%). After an ultra-centrifugation for 30 min at 16,000g at 4°C, the protein content was measured with the Lowry method. Proteins were submitted to a 15% SDS-polyacrylamide gel electrophoresis for 2 h 30 min at 65 V. Then proteins were transferred to nitrocellulose membranes for 2 h 30 min at 80 V (BioRad Laboratories, Marnes-la-Coquette, France), blocked with bovine serum albumin (BSA) 3%-Tween 20 0.1% (Tris–HCl 10 mM pH 7.5, NaCl 0.1%) for 1 h and incubated overnight at 4°C with the primary monoclonal antibodies: rabbit anti-human Bid at 1:500 (BD Biosciences, Belgium) or mouse anti-human beta-actin at 1:2,000 (Chemicon Int., Hampshire, UK) after stripping for 30 min at 50°C with β-mercaptoethanol 100 mM, SDS 2% and Tris–HCl 62.5 mM pH 6.7. The membranes were washed and incubated with 0.02 μg/ml HRP-conjugated goat anti-rabbit IgG (Calbiochem, Merck Biosciences, Darmstadt, Germany) or with 0.02 μg/ml HRP-conjugated goat anti-mouse IgG (Pierce, Perbio Science, Brebières, France) for 1 h and visualized by Super Signal West Pico Chemiluminescent Substrate System (Pierce, France). Intensity of bands was analyzed by Gel Doc 2000 and One 1-D Analysis Software (BioRad Laboratories, France).
Mitochondrial membrane potential
As described previously [15] cells were cultured with lupulone and harvested by trypsinization at 24 and 48 h. Mitochondrial membrane potential was measured by the MitoProbeTM DiOC2(3) assay kit (Invitrogen Corp., France). After trypsinization, cells were washed once in PBS and incubated with DiOC2(3) at 37°C for 30 min in the dark. Cells were washed and re-suspended in PBS for flow cytometric analysis (10,000 events). Cells stained with DiOC2(3) can be visualised with excitation at 488 nm and green (FL-1, 515 nm) or red (FL-3, >600 nm) emissions filters according to the manufacturer’s instructions. This method can quantify cells with depolarized mitochondria. Histograms, resulting from flow cytometric measurements, were analyzed by the CellQuest Software (FACScan, BD Biosciences, Belgium).
Oxidative stress production and effect of ROS inhibition
ROS production was performed by treating cells with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, mixed isomers, Invitrogen Corp., France). Cells were collected by trypsinization and resuspended in pre-warmed PBS containing 8 μM CM-H2DCFDA and incubated for 30 min in the dark.
H2O2 (0.3%, v/v) was used for positive control and was added to culture medium 30 min before trypsinization. Green fluorescence resulting from the oxidation of CM-H2DCFDA by free radicals was detected by flow cytometry using FL-1 filter (530 nm).
For ROS inhibition assays, cells were pre-treated with vitamin C (50 μM) for 3 h and exposed to lupulone (40 μg/ml) for 24 and 48 h. Analyses of SubG0/G1 cell population distribution were performed after labelling cells with propidium iodide and assays were carried out as described previously.
Measure of cytochrome c release
Cells (1 × 106/dish) were cultured in the presence or absence of lupulone, harvested by scrapping, washed twice in PBS and stored at −80°C. Mitochondrial and cytoplasmic fractions were prepared using the Active Motif’s Mitochondrial Extract Kit (Active Motif Europe, Rixensart, Belgium). Cytochrome c present in mitochondrial and cytoplasmic fractions was detected by the sandwich ELISA method, using the Function ELISATM Cytochrome c Kit (Active Motif Europe, Belgium). Colorimetric detection was assessed by reading absorbance at 450 nm and results were adjusted to the total protein content. Values were expressed as ng cyt c/mg of total protein.
Statistical analysis
All experiments were performed at least three times. Data are reported as mean ± SE. Statistical differences between control and treated groups were evaluated using the Student’s t-test or the Student–Neuman–Keuls multiple comparison test. Differences between groups are considered significant at P < 0.05.
Results
Cell death induction by lupulone
Propidium iodide allows to characterize cell distribution in each phase of the cell cycle like G0/G1, S or G2/M, by measurement of cellular DNA content. Induction of cell death causes DNA degradation and dead cells exhibit a DNA content lower than 2n. These cells are detected by flow cytometry in the SubG0/G1 region. This approach allows to determine the amount of dead or dying cells in a population but gives no information on the cell death process (apoptosis or necrosis) [18, 19]. Figure 1 shows that the subG0/G1 population increased progressively at 15, 24 and 48 h after treatment with lupulone (40 μg/ml) in both cell lines. At 48 h, the population of apoptotic cells was enhanced by 16% in SW480 cells and by 31% in SW620 cells over the non-treated cells indicating a higher sensibility of the SW620 cells to lupulone treatment.
Effects of lupulone on SW620 and SW40 cell death. Cells were treated with DMSO 0.1% ± lupulone (40 μg/ml) for 24 or 48 h. At each time point, cells were stained with propidium iodide and analysed by flow cytometry as described in section “Materials and methods”. (a) Cell death is expressed as the percent of stained cells (M1) corresponding to the subG0/G1 region containing the hypodiploids bodies. (b) Data in the table are the mean value ± SE of at least three separate experiments after 48 h of treatment. Lupulone treatment versus controls: *P < 0.05
Expression of DR4 and DR5 death receptors in lupulone-treated SW480 cells
The two DR4 and DR5 death receptors of TRAIL are involved in apoptotic signal transduction [20, 21]. We have previously reported that lupulone induced DR4 and DR5 expression in the metastatic SW620 cells [15]. As shown in Fig. 2, treatment of SW480 cells with lupulone increased significantly DR4 and DR5 death receptor expression with a prominent effect on DR5, as was shown previously for SW620 cells. DR5 expression was significantly enhanced in 70% of SW480 cells treated with lupulone after 15 h and this level was maintained throughout the 48 h treatment period, while the increase of DR4 expression was delayed in time and was enhanced in SW480 cells only after 48 h of treatment. A weak over-expression (of about 10%) of the anti-apoptotic DcR1 and DcR2 receptors was also observed in treated SW480 cells as was also shown previously for SW620 cells [15].
Analysis of TRAIL receptor expression in SW480 cells. Cells were treated with DMSO 0.1% ± lupulone (40 μg/ml) for 15, 24 or 48 h. At each time point, cells were harvested and stained with FITC-conjugated monoclonal antibodies against the four types of TRAIL receptors (DR4, DR5, DcR1 and DcR2). Increased green fluorescence was measured by flow cytometry and data are represented by histograms as the percent of cells expressing TRAIL receptors. Data are the mean value ± SE of at the least three separate experiments. Lupulone treatment versus controls: *P < 0.05
Combined treatment with lupulone and TRAIL
The human colon cancer cell line SW480 is known to be TRAIL-sensitive while its derived metastatic cell line SW620 is TRAIL-resistant [22]. We investigated the effects of exogenous recombinant human TRAIL alone, or in combination with lupulone. TRAIL used as a single drug was ineffective on SW620 cells, whereas the combined treatment TRAIL + lupulone enhanced significantly cell death after 48 h of treatment in both cell lines (55% for SW480 cells and 74% for SW620 cells) (Fig. 3a).
Effects of lupulone and TRAIL combinations. SW480 (TRAIL-sensitive) and SW620 (TRAIL-resistant) cells were treated with DMSO 0.1% ± TRAIL (50 ng/ml) or ±lupulone (10 μg/ml) or ±TRAIL + lupulone for 24 or 48 h. At each time point, cells were harvested and stained with propidium iodide and analysed by flow cytometry. (a) Effect of combination TRAIL + lupulone on cell death. The number of hypodiploid cells present in the subG0/G1 region is represented as histograms after 24 and 48 h of various treatments. (b) Specific inhibition of DR4/DR5 with human blocking anti-DR4, anti-DR5 antibodies (@DR4/DR5) reduced lupulone-induced cell death. SW480 and SW620 cells were pre-treated with TRAIL or lupulone 24 h before the addition of @DR4/DR5 (250 ng/ml). Data are presented as cytometer histograms and are the mean value ± SE of at the least three separate experiments. Treated cells versus controls: *P < 0.05
Effects of DR4 and DR5-blocking antibodies
To confirm the involvement of TRAIL signalling pathway in lupulone-induced cell death, TRAIL apoptotic receptors was blocked with specific blocking anti-DR4 and anti-DR5 antibodies. Indeed, up-regulation of DR4 and DR5 at the same time induced the formation of homocomplexes or heterocomplexes with activation of FADD and also caspase-8 [6, 7]. Moreover, SW620 cells were TRAIL-resistant and did not express DR4 and DR5 at the cell surface under basal conditions [15, 17]. For these reasons, a pre-treatment with lupulone or TRAIL was performed, in order to assess the effects of DR4 and DR5 blocking antibodies after up-regulation by lupulone.
The inactivation of DR4 and DR5 death receptors caused a significant reduction in the amount of hypodiploid cells in SW480 and SW620 cells treated for 24 h with lupulone (Fig. 3b). A 50% reduction of cell death was also observed in SW480 cells treated with TRAIL and exposed to the blocking antibodies. These data indicate that in both SW480 and SW620 cells, lupulone-triggered cell death through an activation of the TRAIL DR4/DR5 receptor pathway.
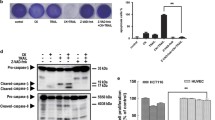
Effect of caspase-8 inhibition on cell death and Bid cleavage
In most cases, activation of the extrinsic death receptor pathway targets the activation of caspase-8. This pathway may involve a direct activation of caspase-3 consequently to caspase-8 activation or may initiate a cross-talk with the intrinsic (mitochondrial) apoptotic pathway through Bid protein cleavage by active caspase-8 leading to mitochondria dysfunction and release of cytochrome c into the cytosol [23–25]. A significant increase of caspase-8 activity reaching its maximum value at 24 h for SW620 cells and at 36 h for SW480 cells was observed after treatment with lupulone (Fig. 4a). Inhibition of caspase-8 induced a significant reduction in the amount of dying cells at 24 h (70%) and at 48 h (55%) in both cell lines (Fig. 4b). Moreover, Bid protein cleavage was detected 48 h after lupulone treatment in both cell lines (Fig. 5a). These data suggest that both in SW480 cells and SW620 cells, lupulone-triggered cell death mainly by caspase-8 activation.
Caspase-8 activity and effects of caspase-8 inhibition. Cells were exposed to DMSO 0.1% ± lupulone (40 μg/ml). (a) Effect of lupulone on caspase-8 activity. At each time point cells were harvested for measurement of caspase-8 activity by colorimetric test. TRAIL (50 ng/ml) was used as a positive control. Data are indicated as nmol of pNA released/mg of total protein and are the mean value ± SE of at the least three independent experiments. Lupulone treatment versus controls: *P < 0.05. (b) Effect of caspase-8 inhibition on cell death. SW480 and SW620 cells were pre-treated with specific caspase-8 Z-IETD-FMK inhibitor, for 2 h 30 min before lupulone treatment. Data are presented as cytometer histograms and are the mean value ± SE of at the least three separate experiments. Treated cells versus controls: *P < 0.05
Bid cleavage and effect of lupulone on mitochondrial membrane permeabilization (ΔΨm). (a) Determination of Bid protein cleavage was analysed by Western blotting. A band of 24 kDa corresponding to nature form of Bid protein was detected. Attenuation of this band indicated a cleavage of Bid protein. β-Actin was used as an internal control. Data are representative of at least three independent experiments. (b) ΔΨm determination. Cells were treated with DMSO 0.1% ± lupulones (40 μg/ml) for 24, 48 or 62 h. At each time point cells were harvested and stained with DiOC2(3) probe for mitochondrial membrane potential (ΔΨm). Columns represent the percent of cells with reduced red fluorescent corresponding to the percent of cells with reduced ΔΨm. Data are the mean value ± SE of at the least three independent experiments. Lupulone treatment versus controls: *P < 0.05
Mitochondria membrane permeabilization
Alterations of mitochondria membrane potential and permeability cause mitochondria dysfunction which is directly involved in the intrinsic apoptotic pathway [26–28]. We assessed mitochondria membrane potential changes by flow cytometry after staining cells with DiOC2(3) reagent. This reagent accumulates into mitochondria. The intensity of DiOC2(3) staining decreases in the mitochondria of cells treated with agents disrupting mitochondrial membrane integrity leading to the collapse of membrane potential (ΔΨm). As shown in Fig. 5b, a strong reduction of ΔΨm was observed after a 48 h treatment with lupulone (40 μg/ml) in both cell lines.
Oxidative stress production
Mitochondria are a major source of ROS and perturbations of mitochondria functions may favour the formation of ROS [29, 30]. Therefore we measured ROS production by flow cytometry using the fluorescent probe CM-H2DCFDA [31]. Green fluorescence generated by the reaction between the fluorescent probe and ROS was analysed in both SW480 and SW620 cells. As shown in Fig. 6a, a significant increase in the percentage of ROS-producing cells was observed for lupulone treated SW480 cells after 48 h (20%) and 62 h (30%). In contrast, no induction of ROS production was observed in SW620 cells treated with lupulone.
ROS production by lupulone and ROS inhibition by vitamin C. Cells were treated with DMSO 0.1% ± lupulones (40 μg/ml) for 24, 48 h or 62 h. At each time point cells were harvested and stained with CM-H2DCFDA. H2O2 (0.3% v/v) was used as positive control, cells with increased green fluorescence produced ROS. (a) The percentage of cells with increased green fluorescence is shown on the right figure and FACS histograms are showing data at 62 h. Columns are the mean value ± SE of at the least three separate experiments. Lupulone treatment versus controls: *P < 0.05. (b) Histogram present data of vitamin C effects on ROS production in SW480 cells. Columns are the mean value ± SE of at the least three separate experiments. Columns not sharing the same superscript differ significantly: *P < 0.05. (c) Effect of ROS inhibition on cell death induced by lupulone in SW480 cells. Cells were harvested, stained with propidium iodide and analysed by flow cytometry. Data are presented as cytometer histograms and are the mean value ± SE of at the least three separate experiments. Columns not sharing the same superscript differ significantly: *P < 0.05
Effect of ROS inhibition on cell death
Antioxidants, like vitamin C (ascorbic acid), are preventing free radical induced cell damage [32]. Vitamin C reduced significantly the production of oxidative stress induced by lupulone in SW480 cells (Fig. 6b). However, the amount of hypodiploid cells was only marginally reduced by vitamin C inlupulone treated cells (Fig. 6c). This suggested that in SW480 cells, the enhanced ROS production caused by lupulone did not play a major role in the apoptotic process.
Cytochrome c release
Cytochrome c is often released following mitochondria membrane perturbation and plays a major role in cell death [33] by initiating caspase-9 activation in combination with other pro-apoptotic factors [34]. Release of cytochrome c was measured by the ELISA method in cytosolic and mitochondria fractions. In SW620 cells, the concentration of cytochrome c remained unchanged in cytosolic and mitochondria fractions in the presence or absence of lupulone (Fig. 7a). In contrast, lupulone induced significantly the release of cytochrome c into the cytosol of SW480 cells after 48 h of treatment (43 ng of cyt c/mg protein in lupulone treated cells versus 27 ng of cyt c/mg protein in non-treated cells) (Fig. 7b).
Detection of mitochondrial (mito.) and cytosolic (cyto.) cytochrome c (cyt c) in SW480 and SW620 cells. Cells were exposed to DMSO 0.1% ± lupulone (40 μg/ml) for 48 and 62 h. The mean values obtained at each time point are shown on the histograms. Data are indicated as ng cyt c/mg of total protein. (a) SW620 cells: no changes were observed. (b) Lupulone induced the release of cyt c into the cytosol of SW480 cells. Columns are the mean value ± SE of at the least three separate experiments. Lupulone treatment versus controls: *P < 0.05
Caspase-9 and caspase-3 activities
Since the release of cytochrome c in the cytosol may activate caspase-9 and caspase-3 cascade, we determined the activities of these two enzymes by measuring the colorimetric reaction produced during the cleavage of their specific substrates, LEHD-pNA for caspase-9, and Ac-DEVD-pNA for caspase-3 [35]. Caspase-9 and caspase-3 were both activated in SW480 cells treated with lupulone after 48 h (Fig. 8). In contrast, caspase-9 activity was not significantly increased in SW620 cells exposed to lupulone, but an important stimulation of caspase-3 occured in these cells.
Effects of lupulone on caspase-9 and caspase-3 activities. Cells were treated with DMSO 0.1% ± lupulone (40 μg/ml) for 48 and 62 h. The measurement of caspase activities were performed by colorimetric method using specific substrates as described in section “Materials and methods”. Data are indicated as nmol pNA released/mg of total protein. Activities of caspase-9 (casp-9) and caspase-3 (casp-3) (a) in SW620 cells and (b) in SW480 cells. Data are presented as the mean value ± SE of at the least three independent experiments. Lupulone treatment versus controls: *P < 0.05
Discussion
The present study demonstrates that two different apoptotic signalling pathways are triggered by lupulone in either the human colon cancer SW480 cells or in their derived metastatic SW620 cells. In SW480 cells, lupulone initiated cell death mainly through a cross-talk between TRAIL-death receptors and the intrinsic pathway via an activation of caspase-8 and the cleavage of Bid protein and mitochondrial membrane permeabilization. Such dysfunctions favoured the release of cytochrome c from inner mitochondria space into the cytosol leading finally to the activation of caspase-9 and of caspase-3 proteases (Fig. 9a). In contrast, in the metastatic SW620 cells which normally do not express TRAIL-death receptors DR4/DR5 and are resistant to TRAIL treatment, lupulone was able to induce the expression of DR4/DR5 at the cell surface and restore the sensibility of these cells to TRAIL. In these cells, the main apoptotic pathway activated by lupulone was the extrinsic pathway involving activation of TRAIL death receptors, caspase-8 and caspase-3 (Fig. 9b).
Scheme of the different apoptotic signalling pathways activated by lupulone in SW480 and SW620 cells. (a) In SW480 cells (TRAIL-sensitive), lupulone caused activation of extrinsic and intrinsic pathway involving DR4/DR5 receptors, caspase-8 activation, cleavage of Bid (tBID) protein, mitochondria perturbation, ROS production, cytochrome c release, and caspase-9 and -3 activations. (b) In SW620 cells (TRAIL-resistant), lupulone activated mainly the extrinsic pathway, involving DR4/DR5 receptors activation and a direct caspase-3 activation by caspase-8. Despite Bid protein cleavage, the loss of mitochondria membrane potential was insufficient to cause cytochrome c release and caspase-9 activation
Our data showing that, the combination of TRAIL ligand and lupulone enhanced cell death in both cancer cell lines, which are either sensitive or resistant to TRAIL treatment, may provide a high interest. Indeed numerous studies are focused on the discovery of new drugs targeting the TRAIL-death receptors with the aim to either potentiate TRAIL effect, or to increase DR4 and DR5 receptor expressions [36, 37]. Activation of DR4 or DR5 receptors was shown to selectively induce apoptosis in a variety of tumour and transformed cells but not in most normal cells [38]. At present, the development of specific antibodies directed against DR4 or DR5 may represent a promising approach in cancer therapy. Thus, recent clinical trials showed that patients with advanced cancers treated with monoclonal antibody (HGS-ETR2) activating DR5 expression responded well to this novel apoptosis-inducing agent, particularly in combination with conventional chemotherapy [39]. Furthermore, TRAIL is produced by different cells of the immune system as macrophages or natural killer cells and induces apoptosis in cancer cells [40]. Some cases of TRAIL-resistance have been described in colon cancer cell lines [3, 9], and also in cancer patients [41]. TRAIL-resistance can occur at several steps of the signalling pathway like DR4-DR5/DcR1-DcR2 receptor ratios, caspase-8 mutations, the expression of FLICE-inhibitory protein (FLIP) which is a caspase-8 inhibitor, or through the expression of X-linked inhibitor of apoptosis protein (XIAP), a human member of the inhibitor of apoptosis (IAP) protein family [41]. Here, we show that lupulone may be a new drug able to activate specifically DR4/DR5 receptors in TRAIL-resistant and TRAIL-sensitive cancer cells. Moreover, inhibition of cell death, induced by lupulone with specific anti-DR4 and anti-DR5 blocking antibodies, indicated that lupulone activates TRAIL-death signalling pathway in both cell lines. This assumption is sustained, by the observed increase of caspase-8 activity by 36 and 48 h after lupulone treatment, and by the reduction of lupulone-induced cell death after a specific inhibition of caspase-8.
Caspase-8 has two preferred substrates, one is caspase-3, which after activation may directly cause cell death and the other substrate is Bid protein, a member of the pro-apoptotic Bcl-2 family. Bid protein cleavage disrupts mitochondria, and favours the release of apoptotic factors like cytochrome c [10]. Bid protein is the link in the cross-talk between the extrinsic and the intrinsic apoptotic pathways. Lupulone increased caspase-8 activity leading to the cleavage of Bid protein and the stimulation of caspase-3 activity in both SW480 and SW620 cells. However caspase-3 exhibited an earlier and more potent increase in the metastatic SW620 cells as compared to SW480 cells. These data suggested that, in SW620 cells, the main apoptotic pathway triggered by lupulone might be the extrinsic (death receptor) pathway.
We found that the mitochondrial membrane potential was strongly reduced after lupulone treatment in both cell lines, indicating a potential role of mitochondria in lupulone-triggered apoptosis. To get more insight into the process of mitochondria disruption, we measured intracellular ROS formation, which may play a critical role in apoptosis [11, 42]. Indeed, in human colon cancer cells, intrinsic ROS production may induce cell death by activating the release of cytochrome c and may also lead to cellular damages and provide a way to kill cancer cells [43, 44]. Surprisingly, we found that only the SW480 cells produced ROS after lupulone treatment. Furthermore, SW480 cells but not SW620 cells treated with lupulone evidenced a significant release of cytochrome c into the cytosol associated with an activation caspase-9. Interestingly, vitamin C which exhibit protective effects on mitochondria [32], reduced significantly ROS production induced by lupulone but did not prevent lupulone-triggered cell death. These observations confirm that, in SW480 cells, both intrinsic and extrinsic pathways are activated by lupulone and that ROS production was not a major player in the apoptotic process.
Our study indicates that lupulone induces apoptosis by two different mechanisms in SW480 cells and in their derived metastatic SW620 cells. In SW480 cells, the extrinsic and intrinsic apoptotic pathways are both activated by lupulone, the intrinsic (mitochondrial) pathway being the prominent pathway. Lupulone activated DR4 and DR5 death receptors, caspase-8 and induced Bid protein cleavage, which is involved in mitochondria membrane disruption, cytochrome c release, caspase-9 activation, leading finally to apoptosis via the effector caspase-3.
The implication of the intrinsic apoptotic pathway and differences in the growth rate of the two cell lines may explain the different sensitivity to lupulone observed between SW480 and SW620 cells. Indeed, SW620 cells have a higher invasive capacity and have a higher turn-over rate than SW480 cells [45]. Moreover, in SW620 cells, lupulone activated mainly the extrinsic (death receptor pathway) with a direct activation of caspase-3 by caspase-8. Even if a Bid protein cleavage occurred with a loss of mitochondria membrane potential, these events were not sufficient to activate cytochrome c release and caspase-9 activation, and therefore do not play a critical role in the apoptotic effects triggered by lupulone in SW620 cells. These observations are in correlation with our previous findings on Bcl-2 protein family in SW620 cells [15]. Anti-apoptotic proteins like Bcl2, BclxL or Mcl1 are able to stabilize mitochondrial membrane after a disruption of mitochondria, while Bax protein, a pro-apoptotic protein of the Bcl2 family, is a key factor in mitochondria integrity and cytochrome c release [8, 46]. The Bax/Bcl2 ratio is an important parameter for mitochondria involvement in the apoptotic process [47, 48]. In our previous report, we observed that Bax/Bcl2 ratio remained unaffected in lupulone-treated and untreated SW620 cells, indicating that these two proteins are not involved in lupulone-triggered apoptosis in SW620 cells [15]. The implication of Bax protein in mitochondrial dysfunction observed in SW480 cells treated with lupulone will be investigated, since Bax may be an important factor in the activation of the intrinsic apoptotic pathway observed in these cells.
Our study demonstrating that lupulone up-regulated DR4 and DR5 receptors in TRAIL-sensitive cells (SW480) but also in TRAIL-resistant cells (SW620) may be of high potential interest for cancer chemoprevention and therapy. It remains to be determined whether lupulone is acting similarly in both cell lines by activating DR4/DR5 gene expression, or/and by favoring protein translocation from cytoplasm to the cell membrane.
In conclusion, the hop beta-acid lupulone appears to be a promising anti-cancer agent. Lupulone induces apoptosis through an activation of TRAIL-signalling pathway in the SW480 human colon cancer cell line (TRAIL-sensitive) and also in its derived TRAIL-resistant metastatic cells line. These observations suggest the use of lupulone in cancer chemoprevention but also in chemotherapy.
References
Janakiram NB, Rao CV (2008) Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin 29:1–20
Galligan L, Longley DB, McEwan M et al (2005) Chemotheray and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors and c-FLIP. Mol Cancer Ther 4:2026–2036. doi:10.1158/1535-7163.MCT-05-0262
Voelkel-Johnson C, Hannun YA, El-Zawahry A (2005) Resistance to TRAIL is associated with defects in ceramide signaling that can be overcome by exogenous C6-ceramide without requiring down-regulation of cellular FLICE inhibitory protein. Mol Cancer Ther 4:1320–1327. doi:10.1158/1535-7163.MCT-05-0086
Kelley SK, Harris LA, Xie D et al (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics and safety. J Pharmacol Exp Ther 299:31–38
Buchsbaum DJ, Forero-Torres A, Lobuglio AF (2007) TRAIL-receptor antibodies as a potential cancer treatment. Future Oncol 3:405–409. doi:10.2217/14796694.3.4.405
Kischkel FC, Lawrence DA, Chuntharapai A et al (2000) Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611–620. doi:10.1016/S1074-7613(00)80212-5
Suliman A, Lam A, Datta R et al (2001) Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene 20:2122–2133. doi:10.1038/sj.onc.1204282
Falschlehner C, Emmerich CH, Gerlach B, Walczak H (2007) TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39:1462–1475. doi:10.1016/j.biocel.2007.02.007
Sandra F, Esposi MD, Ndebele K et al (2005) Tumor necrosis factor-related apoptosis-inducing ligand alters mitochondrial membrane lipids. Cancer Res 65:8286–8297. doi:10.1158/0008-5472.CAN-04-1913
Vasilevskaya IA, O’Dwyer PJ (2005) 17-Allylamino-17-demethoxygeldanamycin overcomes TRAIL resistance in colon cancer cell lines. Biochem Pharmacol 70:580–589. doi:10.1016/j.bcp.2005.05.018
Izeradjene K, Douglas L, Tillman DM, Delaney AB, Houghton JA (2005) Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res 65:7436–7445. doi:10.1158/0008-5472.CAN-04-2628
Kulkarni AC, Kuppusamy P, Parinandi N (2007) Oxygen, the lead actor in the pathophysiologic drama enactment of the trinity of normoxia, hypoxia and hyperoxia in disease and therapy. Antioxid Redox Signal 9:1717–1730. doi:10.1089/ars.2007.1724
Verzele M, Potter MDE (1978) High-performance liquid chromatography of hop bitter substances. J Chromatogr A 166:320–326. doi:10.1016/S0021-9673(00)92283-0
Malowicki MG, Shellhammer TH (2005) Isomerization and degradation kinetics of hop (Humulus lupulus) acids in a model wort-boiling system. J Agric Food Chem 53:4434–4439. doi:10.1021/jf0481296
Lamy V, Roussi S, Chaabi M et al (2007) Chemopreventive effects of lupulone, a hop acid, on human colon cancer-derived metastatic SW620 cells and in rat model of colon carcinogenesis. Carcinogenesis 28:1575–1581. doi:10.1093/carcin/bgm080
Siegel L, Miternique-Grosse A, Griffon C et al (2008) Antiangiogenic properties of lupulone a bitter acid of hop cones. Anticancer Res 28:289–294
Vaculová A, Hofmanová J, Soucek K, Kozubík A (2006) Different modulation of TRAIL-induced apoptosis by inhibition of pro-survival pathways in TRAIL-sensitive and TRAIL-resistant colon cancer cells. FEBS Lett 580:6565–6569. doi:10.1016/j.febslet.2006.11.004
Nicoletti I, Migliorati G, Pagliacci MC et al (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279. doi:10.1016/0022-1759(91)90198-O
Sasaki K, Yamagata T, Mitani K (2008) Histone deacetylase inhibitors trichostatin A and valproic acid circumvent apoptosis in human leukemic cells expressing the RUNX1 chimera. Cancer Sci 99:414–422. doi:10.1111/j.1349-7006.2007.00699.x
Henson ES, Johnston JB, Gibson SB (2008) The role of TRAIL death receptors in the treatment of hematological malignancies. Leuk Lymphoma 49:27–35. doi:10.1080/10428190701713655
Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK (2007) Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal 2:10. doi:10.1186/1750-2187-2-10
Jin Z, McDonald ERIII, Dicker DT, El-Deiry WS (2004) Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem 279:35829–35839. doi:10.1074/jbc.M405538200
Basu A, Castle VP, Bouziane M, Bhalla K, Haldar S (2006) Crosstalk between extrinsic and intrinsic cell death pathways in pancreatic cancer: synergistic action of estrogen metabolite and ligands of death receptor family. Cancer Res 66:4309–4318. doi:10.1158/0008-5472.CAN-05-2657
Segal M, Niazi S, Simons MP, Galati SA, Zangrilli JG (2007) Bid activation during induction of extrinsic and intrinsic apoptosis in eosinophils. Immunol Cell Biol 85:518–524. doi:10.1038/sj.icb.7100075
Chawla-Sarkar M, Bae SI, Reu FJ et al (2004) Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ 11:915–923. doi:10.1038/sj.cdd.4401416
Lemasters JJ, Qian T, Bradham CA et al (1999) Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr 31:305–319. doi:10.1023/A:1005419617371
Denecker G, Vercammen D, Steemans M et al (2001) Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell Death Differ 8:829–840. doi:10.1038/sj.cdd.4400883
Verma M, Singh SK, Bhusman S et al (2008) In vitro cytotoxic potential of Polyalthia longifolia on human cancer cell lines and induction of apoptosis through mitochondrial-dependent pathway in HL-60 cells. Chem Biol Interact 171:45–56. doi:10.1016/j.cbi.2007.08.010
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922. doi:10.1007/s10495-007-0756-2
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141. doi:10.1016/S0300-9084(02)01369-X
Korystov YN, Shaposhnikova VV, Korystova AF, Emel’yanov MO (2007) Detection of reactive oxygen species induced by radiation in cells using the dichlorofluorescein assay. Radiat Res 168:226–232. doi:10.1667/RR0925.1
KC S, Carcamo JM, Golde DW (2005) Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 19:1657–1667. doi:10.1096/fj.05-4107com
Garrido C, Galluzzi L, Brunet M et al (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433. doi:10.1038/sj.cdd.4401950
Marfè G, Morgante E, Di Stefano C et al (2008) Sorbitol-induced apoptosis of human leukemia is mediated by caspase activation and cytochrome c release. Arch Toxicol 82:371–377
Martinsson P, Ekelund S, Nygren P, Larsson R (2002) The combination of the antitumoural pyridyl cyanoguanidine CHS 828 and etoposide in vitro—from cytotoxic synergy to complete inhibition of apoptosis. Br J Pharmacol 137:568–573. doi:10.1038/sj.bjp.0704888
Vermot-Desroches C, Sergent E, Bonnin B, Widjdenes J (2005) Characterization of monoclonal antibodies directed against TRAIL or TRAIL receptors. Cell Immunol 236:86–91. doi:10.1016/j.cellimm.2005.08.012
Ishii M, Iwai M, Harada Y et al (2007) Soluble TRAIL gene and actinomycin D synergistically suppressed multiple metastasis of TRAIL-resistant colon cancer in the liver. Cancer Lett 245:134–143. doi:10.1016/j.canlet.2005.12.040
Mérino D, Lalaoui N, Morizot A et al (2006) Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol 26:7046–7055. doi:10.1128/MCB.00520-06
Plummer R, Attard G, Pacey S et al (2007) Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 13:6187–6194. doi:10.1158/1078-0432.CCR-07-0950
Herbeuval JP, Lambert C, Sabido O et al (2003) Macrophages from cancer patients: analysis of TRAIL, TRAIL receptors, and colon tumor cell apoptosis. J Natl Cancer Inst 95:611–621
Van Geelen CM, de Vries EG, de Jong S (2004) Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat 7:345–358. doi:10.1016/j.drup.2004.11.002
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–1483. doi:10.1146/annurev.pharmtox.47.120505.105122
Hsu WH, Hsieh YS, Kuo HC et al (2007) Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol 81:719–728. doi:10.1007/s00204-006-0169-y
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110. doi:10.1016/j.drup.2004.01.004
De Vries JE, Dinjens WN, de Buyne GK et al (1995) In vivo and in vitro invasion in relation to phenotypic characteristics of human colorectal carcinoma cells. Br J Cancer 71:271–277
Armstrong JS (2006) Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioassays 28:253–260. doi:10.1002/bies.20370
Matsumoto H, Wada T, Fukunaga K et al (2004) Bax to Bcl-2 Ratio and Ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancer. Jpn J Clin Oncol 34:124–130. doi:10.1093/jjco/hyh026
Agarwal MK, Agarwal ML, Athar M et al (2004) Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle 3:205–211
Acknowledgements
Virginie Lamy is supported by a fellowship and fundings provided by the Conseil Régional d’Alsace, France. Authors would like to thank Dr J.-F. Doriat (Brasseries Kronenbourg, Strasbourg, France) for supplying the industrial by-product containing high amounts of beta-acids.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lamy, V., Roussi, S., Chaabi, M. et al. Lupulone, a hop bitter acid, activates different death pathways involving apoptotic TRAIL-receptors, in human colon tumor cells and in their derived metastatic cells. Apoptosis 13, 1232–1242 (2008). https://doi.org/10.1007/s10495-008-0250-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0250-5