Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (Apo2L/TRAIL) selectively induces apoptosis in transformed cells. Normal cells and certain tumor cells can evade Apo2L/TRAIL induced cell death, but the determinants of Apo2L/TRAIL sensitivity are poorly understood. To better understand the factors that contribute to Apo2L/TRAIL resistance, we characterized two colon carcinoma lines with pronounced differences in Apo2L/TRAIL sensitivity. Colo205 cells are highly sensitive to Apo2L/TRAIL whereas Colo320 cells are unresponsive. Components of the DISC (death inducing signaling complex) could be immunoprecipitated from both cell lines in response to Apo2L/TRAIL. Sensitizing agents including a proteasome inhibitor conferred Apo2L/TRAIL sensitivity in Colo320 cells, indicating that the apoptotic machinery was intact and functional. We specifically suppressed the expression of Bcl-2, FLIP or XIAP in Colo320 cells. Downregulation of either FLIP or XIAP but not Bcl-2 restored sensitivity of Colo320 cells to Apo2L/TRAIL. Moreover, stable knockdown of XIAP expression in Colo320 subcutaneous tumors resulted in suppression of tumor growth and sensitivity to Apo2L/TRAIL in vivo. Our results indicate that only a specific subset of anti-apoptotic proteins can confer resistance to Apo2L/TRAIL in Colo320 cells. Elucidation of the factors that contribute to Apo2L/TRAIL resistance in tumor cells may provide insight into combination therapies with Apo2L/TRAIL in a clinical setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apo2L/TRAIL is a type II transmembrane protein of the TNF superfamily that was identified based on sequence homology to TNF and Fas ligand (FasL) [1, 2]. Early functional studies indicated that Apo2L/TRAIL has a potent ability to trigger apoptosis in a variety of tumor cell lines but not normal cells, highlighting its potential as a candidate therapeutic in cancer [3, 4]. Apo2L/TRAIL induces apoptosis by binding to two death receptors, DR4 (TR-1) and DR5 (TR-2), that contain cytoplasmic death domains (DD) and can initiate an apoptosis signaling cascade [5–7]. Two isoforms of DR5 have been described [8]. Apo2L/TRAIL also binds three additional receptors, DcR1, DcR2 and OPG, that do not trigger apoptosis upon ligand engagement [9–11]. Ligand binding to death receptors DR4 and DR5 results in recruitment of the adaptor protein Fas-associated death domain (FADD) by the cytoplasmic death domain, followed by the recruitment and activation of the initiator caspases 8 and 10. FLICE inhibitory protein (FLIP) is also recruited to the receptor complex and can act as an inhibitor of caspase 8 when present at high levels [12, 13]. Recruitment of FADD, caspase 8, caspase 10 and FLIP to the death receptor forms the death inducing signaling complex, or DISC. In some cells, activation of caspase 8 is sufficient for subsequent activation of the effector caspases 3 and 7, which cleave intracellular substrates to execute cellular apoptosis [14, 15]. This is often referred to as the extrinsic pathway of cell death activation. In other cells, signal amplification via the mitochondrial or intrinsic pathway is necessary for apoptosis. The intrinsic pathway is primarily activated by DNA-damaging agents, but is also coupled to the extrinsic pathway by caspase 8 or 10 induced cleavage of the pro-apoptotic Bcl-2 family member Bid. Truncated Bid interacts with additional pro-apoptotic proteins Bax and Bak, resulting in the release of cytochrome c and Smac/DIABLO from the mitochondria [16, 17]. Cytochrome c, together with Apaf-1, activates caspase 9, which can further activate caspases 3 and 7, resulting in signal amplification and cellular apoptosis. Smac/DIABLO promotes apoptosis by binding to and neutralizing the inhibitor of apoptosis proteins, or IAPs, which can sequester caspase 3 [18]. Thus, cellular apoptosis is regulated by the functions of multiple pro- and anti-apoptotic proteins and by the coordination of both extrinsic and intrinsic pathways.
Malignant cells commonly have defects in cell death control and apoptosis [19]. Although many tumor cells display marked sensitivity to Apo2L/TRAIL, a spectrum of sensitivity ranging from highly sensitive to completely resistant has been observed. Over-expression of anti-apoptotic factors is one mechanism by which cells can acquire resistance to Apo2L/TRAIL and is known to occur in many cancers [20]. Thus, one potential mechanism to overcome tumor resistance to Apo2L/TRAIL could be by suppressing anti-apoptotic factors.
In this study we examined the mechanisms of resistance to Apo2L/TRAIL exhibited by the colon adenocarcinoma line Colo320. We found that Colo320 cells express death receptors on their surface and can engage a functional DISC in response to Apo2L/TRAIL, yet fail to undergo apoptosis in the presence of ligand. We identified pharmacological agents that were able to sensitize Colo320 cells to Apo2L/TRAIL, in particular protein synthesis and proteasome inhibitors. These agents may modulate the levels of anti-apoptotic factors that could contribute to Colo320 cell resistance. To better understand this we generated specific siRNAs against anti-apoptotic proteins and found that suppression of either XIAP or FLIP expression readily sensitized Colo320 cells to Apo2L/TRAIL. Furthermore, we demonstrate that stable suppression of XIAP in Colo320 subcutaneous tumors is sufficient to inhibit tumor growth and confer sensitivity to Apo2L/TRAIL mediated anti-tumor activity in vivo. Our data support the notion that combinations of Apo2L/TRAIL with specific inhibitors to anti-apoptotic factors such as FLIP or XIAP could augment the effects of Apo2L/TRAIL in a clinical setting.
Materials and methods
Cell lines, antibodies, and reagents
Colo320 and Colo205 cells (ATCC, Manassas, VA) were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FCS (HyClone Laboratories, Logan, UT), L-glutamine and antibiotics. FACS antibodies: Apo2L/TRAIL R1-M271, Apo2L/TRAIL R2-M413, Apo2L/TRAIL R3-M430 and Apo2L/TRAIL R4-M444 were produced at Amgen Washington and have been described [21]. Immunoblotting antibodies: Bcl-XL, caspase 3, caspase 8, DFF45/DFF35, FADD, PARP, Bid (Cell Signaling, Beverly, MA); Bax, cIAP2, Smac/DIABLO, survivin, XIAP (R&D Systems, Minneapolis, MN); Bcl-2, cIAP1 (BD PharMingen, San Diego, CA); caspase 10, clone 4C1 (MBL International, Woburn, MA); FLIP, clone NF6 (Alexis Biochemicals, San Diego, CA); DR4, DR5, DcR2 (Cayman Chemical, Ann Arbor, MI). Recombinant human Apo2L/TRAIL was produced at Genentech, Inc (So. San Francisco, CA). Flag-Apo2L/TRAIL and anti-Flag antibody M2 were produced at Amgen Washington. MG-132 was from Calbiochem (La Jolla, CA), cycloheximide was from Sigma (St. Louis, MO) and resazurin was from R&D Systems (Minneapolis, MN). SiRNAs with the following sense sequences were designed and synthesized by Qiagen (Valencia, CA). XIAP siRNA 5′-CGAGAGAUUUGGAAAGAUAdTdT-3′ FLIP siRNA 5′-CCUUGUUUCGGACUAUAGAdTdT-3′; Non-silencing siRNA 5′-UUCUUCGAACGUGUCACGUdTdT-3′—AlexaFluor488. Bcl-2 is a validated siRNA (Qiagen, Valencia, CA, cat(1022536).
Viability assay
Cells were plated at 2 × 104 cells/well. Cells treated with MG-132 were pre-treated for 30 min before the addition of Apo2L/TRAIL. After 24 hours, viability was assessed using ATPLite 1step Assay (Perkin-Elmer, Boston, MA) according to manufacturer’s protocols. For Fig. 5, viability was monitored using a resazurin-based assay. Briefly, cells were plated at 1 × 103 cells/well in media +/− 500 ng/ml doxycycline for 5 days followed by 100 ng/ml Apo2L/TRAIL. After 48 h, resazurin was added to 10%. Absorbance was read at 544/590 2 h post addition.
Caspase 3 activity assay
Cells were plated at 1.8 × 104 cells/well and treated with Apo2L/TRAIL at indicated amounts. After 5 h, caspase 3 activity was measured using the Caspase Glo 3/7 assay (Promega, Madison, WI) according to manufacturer’s protocols.
Flow cytometry
Cells were incubated in blocking buffer (10% FBS/5% goat serum/FACS buffer), washed in FACS buffer (1% FBS/PBS), and resuspended in 10 μg/ml primary antibody or isotype control for 45 min at 4°C. After washing, cells were resuspended in 10 μg/ml biotinylated secondary for 45 min at 4°C, washed again and incubated with 10 μg/ml streptavidin-phycoerythrin (BD Pharmingen, San Diego, CA) for 45 min at 4°C. Cells were analyzed on a FACScaliber flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
DISC immunoprecipitation
1.8 × 107 cells were plated for each condition. After 24 h, 12.5 μg FlagApo2L/TRAIL was pre-complexed with 25 μg M2 antibody and added to cells for 10 min. No FlagApo2L/TRAIL was added to unstimulated controls. Lysates from stimulated cells were cleared by centrifugation and immunoprecipitated with protein G sepharose (Sigma, St. Louis, MO). Lysates from non-stimulated controls were cleared by centrifugation and then 0.625 μg FlagApo2L/TRAIL pre-complexed with 1.25 μg M2 was added, followed by incubation with protein G sepharose. Samples were washed 5× in lysis buffer and analyzed by Western blot. Anti-caspase 8 monoclonal (Alexis Biochemicals, San Diego, CA) and anti-FADD monoclonal (BD Transduction Laboratories, San Diego, CA) antibodies were used for DISC IPs.
siRNA transfections
8 × 104 cells/well were plated and transfected with 1 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and 40 pmol of gene specific primer or control siRNA according to the manufacturer’s instructions. After 48 h, 200 ng/ml Apo2L/TRAIL was added for 24 h. Cells were harvested and analyzed by Western blot.
shRNA lentiviral vectors
The lentiviral vector backbone has been described [22]. It was modified to incorporate the T-Rex tetracycline repressor gene (Invitrogen Corp., Carlsbad CA) upstream of an EMCV-IRES neomycin-resistance gene (Invitrogen Corp., Carlsbad CA). The shRNA expression components were incorporated as a Gateway cassette between the RRE and Ψ sites of the vector backbone.
Gene-specific shRNA triggers were designed and cloned into the pENTR/hH1/TO T-Rex expression vector according to manufacturer’s protocols (Invitrogen Corp., Carlsbad CA). The sense strand of each shRNA trigger is as follows: shXIAP-1, 5′-GCGGTGCTTTAGTTGTCATGC-3′; shXIAP-2, 5′-GCTGTAGATAGATGGCAATAT-3′; shXIAP-4, 5′-GGTTCAGTTTCAAGGACATTA-3′; shCTRL, 5′-GTCTCCACGCGCAGTACATTA-3′. Lentiviral vector stocks were generated according to manufacturer’s protocols (Invitrogen Corp., Carlsbad CA). Crude culture supernatants were harvested and concentrated ∼100-fold by ultracentrifugation at 100,000× g for 2 h prior to aliquoting in PBS + 0.5% BSA for storage at −80°C. Vector stocks were assayed for activity on 293 cells, and titers of 107–109 TU/ml were typically attained. Colo320 cells were transduced with specific lentiviral vectors by incubating 106 cells with 107 TU of vector in a single well of a 12 well tissue culture dish in 500 μl of RPMI containing 10 μg/ml DEAE Dextran for 6 h. Two days post transduction cells were selected in media containing 500 μg/ml G418 (Invitrogen Corp., Carlsbad CA).
Subcutaneous xenografts
Female CB17 SCID mice (Charles River Laboratory, Wilmington, MA) aged 8–10 weeks were injected subcutaneously with 2 × 106 parental Colo320, Colo320-shControl or Colo320-shXIAP-4 cells. For parental tumors, mice were treated with vehicle or Apo2L/TRAIL (60 mg/kg daily for 5 days) when tumors reached approximately 180 mm3. For Colo320-shControl or Colo320-shXIAP-4 tumor bearing mice, drinking water was replaced with 2 mg/ml doxycycline in 5% sucrose when tumors were ∼100 mm3. Five days later, mice were treated with vehicle or Apo2L/TRAIL (60 mg/kg daily for 5 days). Tumor dimensions were measured by a digital caliper and tumor volumes were calculated as [length × (width)2]/2. All experimental procedures were under the Amgen animal use guidelines and approved by the Amgen Institutional Animal Care and Use Committee.
Immunohistochemistry
Cross sections of each tumor were collected in 10% neutral buffered formalin, fixed and embedded in paraffin. XIAP antigen was retrieved in a pressure cooker using Biocare Diva Decloaker (Concord, CA) and blocked with CAS block (Zymed-Invitrogen Corp., Carlsbad, CA) for 10 min at room temperature. Cleaved caspase 3 antigen was retrieved by microwave in Biogenex Citra (San Ramon, CA) to boiling for 7 min. and blocked with CAS block (Zymed-Invitrogen, Carlsbad, CA) for 10 min at room temperature. Anti-XIAP (MBL International, Woburn, MA) or anti-caspase 3 (Cell Signaling Technology, Beverly, MA) was incubated overnight at 4°C followed by quenching with Dako Peroxidase Blocking Reagent (Glostrup, Denmark) for 25 min. Secondary antibody incubation was with Dako Envision Mouse (Glostrup, Denmark) for XIAP or HRP-goat anti-rabbit (Jackson Immunoresearch Labs, West Grove, PA) for caspase 3, followed by DAB detection after 5 min.
Statistical analysis
Experiments were repeated three times unless otherwise indicated. Data were presented as mean +/− SEM. To adjust to the initial tumor size in individual mice (3 days before treatment), the fold changes were calculated at each time point and used for the analysis. The method of ANOVA with repeated measures was employed for comparing tumor volumes between treatment groups across all post treatment time points. A p-value less than 0.05 for group effect indicates a significant difference in tumor sizes between the groups. The analysis was performed using SAS® software package (SAS Institute Inc., Cary, NC).
Results
Colo320 cells are resistant to Apo2L/TRAIL mediated apoptosis despite surface expression of death receptors
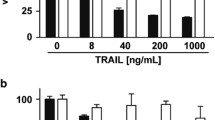
As a model for better understanding the cellular events that mediate resistance, we examined the effects of Apo2L/TRAIL on two colon carcinoma cell lines, Colo205 and Colo320. Whereas treatment of Colo205 cells with Apo2L/TRAIL resulted in a dose-dependent loss of cell viability and a corresponding increase in caspase 3 activity, no effect on cell viability or caspase 3 activity was observed in Colo320 cells (Fig. 1A, B). Cleavage of the caspase 3 substrates PARP and DFF45/DFF35 was also observed in Colo205 cells (Fig. 1C). In Colo320 cells, initial processing of caspase 3 was seen in response to Apo2L/TRAIL, but this failed to fully activate caspase 3, as cleavage of caspase 3 substrates was not detected (Fig. 1C). We also examined the activity of Apo2L/TRAIL on tumor xenografts derived from Colo205 and Colo320 cells. Whereas growth of Colo205 tumors was inhibited by Apo2L/TRAIL, we observed no effect on Colo320 tumors (Fig. 1D). This indicated that, similar to our in vitro observations, Colo320 tumors were also unresponsive to Apo2L/TRAIL.
Differential sensitivity of Colo205 and Colo320 cells to Apo2L/TRAIL in vitro and in vivo. A, Colo205 and Colo320 cells were treated with indicated amounts of Apo2L/TRAIL and analyzed for viability 24 h later using the ATPLite 1step assay. B, Colo205 and Colo320 cells were treated with indicated amounts of Apo2L/TRAIL and analyzed 5 hours later by caspase 3/7 activity assay. C, Colo205 and Colo320 cells were treated with PBS or 200 ng/ml Apo2L/TRAIL for 5 h. Lysates were analyzed by Western blotting using antibodies against the indicated proteins. D, Colo205 (10/group) or Colo320 (8/group) subcutaneous tumors were allowed to grow to approximately 180 mm3 in the flank of SCID mice and were treated with either vehicle or Apo2L/TRAIL (60 mg/kg i.p.) on days 0–4. Tumor volumes were monitored over the treatment period and for an additional week after treatment was finished. E, Surface Apo2L/TRAIL receptor expression levels were analyzed by flow cytometry on Colo205 and Colo320 cells. Horizontal and vertical axes indicate the fluorescence intensity and relative number of cells, respectively. Dotted histograms are isotype controls; black histograms are antibodies against specific receptors
To determine if resistance to Apo2L/TRAIL was mediated by receptor expression, we compared the surface expression levels of DR4, DR5, DcR1 and DcR2. Both DR4 and DR5 were expressed at comparable levels on Colo205 and Colo320 cells as judged by flow cytometry (Fig. 1E). We saw greater expression of DcR1 on Colo205 cells over Colo320 cells, and greater expression of DcR2 on Colo320 cells over Colo205 cells. Whereas DcR1 lacks an intracellular domain, DcR2 contains a non-functional truncated death domain. The biological roles of signaling through DcR1 and DcR2 have been poorly understood. However, it was recently reported that DcR2 is preferentially co-recruited to DR5 within the DISC, where it inhibits initiator caspase activation [23]. DcR1 appears to act as a competitor for Apo2L/TRAIL binding. Whether the elevated levels of DcR2 in Colo320 cells results in impaired DISC assembly and initiator caspase activation was not addressed in this study. Apo2L/TRAIL binding to both cell lines was also found to be equivalent (data not shown).
Differential expression of pro- and anti-apoptotic regulatory proteins in Colo205 and Colo320 cell lysates and DISC immunoprecipitations
To address whether pro- and anti-apoptotic factors were differentially expressed in Colo205 and Colo320 cells, we analyzed whole cell lysates from resting cells (Fig. 2A). No significant difference in the expression of IAP family proteins was observed, including survivin, XIAP, and cIAP2. cIAP1 levels were somewhat elevated in Colo320 cells relative to Colo205 cells. Levels of Smac were also equivalent. Among the Bcl-2 family members analyzed, Bcl-XL was decreased in Colo320 cells as compared to Colo205 cells. Strikingly, there was no detectable Bcl-2 expression in Colo205 cells relative to Colo320 cells raising the possibility that Bcl-2 might be contributing to Apo2L/TRAIL resistance in Colo320 cells. It is possible that other Bcl-2 family members may also be differentially expressed. Interestingly, we also found that FADD, caspase 8 and caspase 10 levels were reduced in Colo320 cells relative to Colo205 cells, whereas FLIP levels were equivalent (Fig. 2A).
Expression of apoptotic pathway components in resting Colo205 and Colo320 cells and in DISC immunoprecipitations. A, Cell lysates were harvested and immunoblots probed with specific antibodies against the indicated proteins. C205 corresponds to Colo205 cells; C320 corresponds to Colo320 cells. B, DISC components from Colo205 and Colo320 extracts were immunoprecipitated and immunoblotted for the presence of DR5, caspase 8, FADD, FLIP and caspase 10. The presence (+) or absence (−) of Apo2L/TRAIL is as indicated. Arrows indicate full length and cleaved caspase-8 and -10
FADD, caspase 8 and 10, and FLIP, along with death receptors, comprise the DISC. Ligand engagement of death receptors leads to DISC assembly, thereby facilitating caspase 8 activation and subsequent activation of caspases 3 and 7. Given that several DISC components were reduced in Colo320 cells, we wanted to compare DISC recruitment in response to Apo2L/TRAIL. In these experiments, cells were briefly exposed to Flag-Apo2L/TRAIL and the ligand-receptor complex was immunoprecipitated using the anti-Flag antibody M2. To examine DISC formation in unstimulated cells, Flag-Apo2L/TRAIL was added directly to cell lysates, followed by immunoprecipitation with M2 antibody. Both Colo205 and Colo320 cells were able to form a complete DISC in response to Apo2L/TRAIL (Fig. 2B). However, significant differences in the relative amounts of DISC components were observed. Relative to Colo205, levels of both isoforms of DR5, caspase 8, caspase 10 and FADD were reduced in the Colo320 DISC, although FLIP levels were equivalent. This altered stoichiometry was consistent with our observations in whole cell lysates, where FLIP levels were equivalent in both cell types, but caspase 8, caspase 10 and FADD levels were lower in Colo320 cells (Fig. 2A). Thus, the FLIP to caspase 8 ratio is higher in the Colo320 DISC and this could affect the degree of caspase 8 activation [24, 25]. Increased FLIP levels can limit caspase 8 recruitment to the DISC and reduce activation, whereas knockdown of FLIP promotes initiator caspase activation in response to Apo2L/TRAIL [26].
Apo2L/TRAIL resistance of Colo320 cells can be overcome by chemical sensitization
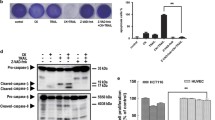
To determine whether Colo320 cells could be rendered responsive to Apo2L/TRAIL, we combined Apo2L/TRAIL with various pharmacological agents. The presence of either the protein synthesis inhibitor cycloheximide or the proteasome inhibitor MG132 in combination with Apo2L/TRAIL partially sensitized Colo320 cells to undergo apoptosis (Fig. 3A, B). Apo2L/TRAIL in combination with cycloheximide or MG132 increased caspase 8, caspase 3 and PARP cleavage as compared to either agent alone. In addition, cleavage of the Bcl-2 family member Bid was observed, indicating that the intrinsic pathway was recruited. Although Colo320 cells were sensitized to Apo2L/TRAIL with cycloheximide or MG132, they were still not as sensitive as Colo205 cells, based on the proportion of viable cells remaining after treatment (Fig. 3A, B). Less than 5% of Colo205 cells remained viable after treatment with Apo2L/TRAIL alone or with either combination. In contrast, 38% and 42% of Colo320 cells remained after treatment with Apo2L/TRAIL and cycloheximide or Apo2L/TRAIL and MG132, respectively.
Colo320 cells are sensitized to Apo2L/TRAIL mediated apoptosis by cycloheximide and MG132. A, Colo205 and Colo320 cells were treated for 5 h with cycloheximide (CHX) (10 mg/ml), Apo2L/TRAIL (200 ng/ml), or a combination of CHX + Apo2L/TRAIL. Lysates were analyzed by Western blotting using antibodies against the indicated proteins. Viability was monitored using the ATPLite assay. Percent viable cells for each condition are listed at the bottom. B, Colo205 and Colo320 cells were treated for 24 h with MG-132 (1 μM), Apo2L/TRAIL (200 ng/ml), or a combination of MG-132 + Apo2L/TRAIL. Cells exposed to MG-132 were pre-treated with the proteasome inhibitor for 30 minutes before the addition of Apo2L/TRAIL. Lysates were analyzed by Western blotting using antibodies against the indicated proteins. Viability was monitored using the ATPLite assay. Percent viable cells for each condition are listed at the bottom. C, Colo320 cells were incubated for 24 h with (+) and without (−) MG-132 (1 μM) or cycloheximide (10 μg/ml). All cells were treated with Apo2L/TRAIL followed by a standard DISC IP as described and immunoblotted with antibodies against the indicated proteins
We wanted to identify which anti-apoptotic molecules were specifically modulated in response to these pharmacological agents. In cycloheximide treated Colo205 and Colo320 cells, FLIPL expression was significantly reduced as compared to untreated cells, whereas FLIPS levels remained largely unchanged (Fig. 3A). FLIP has been demonstrated to have a high turnover rate and be strongly downregulated in the presence of cycloheximide [27]. MG132 treatment in both cell types also resulted in slight decreases in the expression of FLIPL, FLIPS and XIAP. It is possible that altered expression of additional proteins in response to cycloheximide or MG132 also contributed to sensitizing Colo320 cells to Apo2L/TRAIL mediated apoptosis. Under conditions where apoptosis was induced in both cell types, FLIPL, FLIPS, Bcl-2 and XIAP levels were also significantly reduced (Fig. 3A). This is likely a result of caspase mediated FLIP, Bcl-2 and XIAP cleavage as cells undergo apoptosis, as FLIP, Bcl-2 and XIAP are known caspase substrates [28, 29]. Collectively, these data indicated that modulation of protein turnover, either by blocking protein synthesis or protein degradation, was able to induce sensitivity to Apo2L/TRAIL in Colo320 cells.
We also examined the DISC from Colo320 cells treated with Apo2L/TRAIL and either cycloheximide or MG132 (Fig. 3C). FLIPL was no longer detectable in the DISC IPs from Apo2L/TRAIL and cycloheximide treated Colo320 cells, consistent with our observations in whole cell lysates (Fig. 3A). In the DISC IPs from Apo2L/TRAIL and MG132 treated Colo320 cells, caspase 8 was elevated, indicating that MG132 might be stabilizing caspase 8. The net result was that caspase 8 levels were specifically increased in the DISC relative to FLIPL, either by increased FLIPL degradation with cycloheximide or by increased caspase 8 stabilization with MG132.
Downregulation of XIAP or FLIP sensitizes Colo320 cells to Apo2L/TRAIL induced apoptosis
The data in Fig. 3 suggested that FLIPL and XIAP may be important for resistance of Colo320 cells to Apo2L/TRAIL induced apoptosis, as reduction of these proteins by cycloheximide or proteasome inhibitor treatment augmented Apo2L/TRAIL mediated apoptosis. However, since metabolic inhibitors modulate the expression of many proteins, multiple factors may be contributing to the observed sensitization. To determine whether specific downregulation of XIAP or FLIP could confer Apo2L/TRAIL sensitization, we suppressed expression of XIAP or FLIP in Colo320 cells using RNA interference technology. Transfection of cells with siRNA triggers specific for XIAP or FLIP resulted in reduced expression of these proteins and cleavage of caspase 3 and its substrate PARP upon addition of Apo2L/TRAIL (Fig. 4A, B). Thus, downregulation of XIAP or FLIP alone directly restored Apo2L/TRAIL sensitivity in Colo320 cells.
Knockdown of XIAP or FLIP but not Bcl-2 promotes Apo2L/TRAIL mediated caspase 3 activity in Colo320 cells. Colo320 cells were transfected with no siRNA, a non-silencing control siRNA, or gene specific siRNA: (A) XIAP specific, (B) FLIP specific, (C) Bcl-2 specific. 48 h later, transfected cells were stimulated with Apo2L/TRAIL (200 ng/ml) for 24 h. Lysates were analyzed by Western blotting using antibodies against the indicated proteins. (D) Colo320 cells transfected with no siRNA, a non-silencing control siRNA or XIAP specific siRNA were harvested 48 h after transfection and analyzed by Western blot using antibodies against the indicated proteins
As significantly higher Bcl-2 expression was originally noted in Colo320 vs. Colo205 cells, we also tested a Bcl-2 specific siRNA. Moreover, administration of Apo2L/TRAIL with sensitizing agents to Colo320 cells induced Bid cleavage, suggesting a requirement for intrinsic pathway activation for Apo2L/TRAIL mediated apoptosis. Upon transfection of a Bcl-2 specific siRNA into Colo320 cells, Bcl-2 gene expression was downregulated (Fig. 4C). However, no caspase 3 cleavage was observed in the presence of Apo2L/TRAIL, indicating that Bcl-2 inhibition could not reverse Apo2L/TRAIL resistance in Colo320 cells, although inhibition of either XIAP or FLIP alone could. Importantly, we did not observe any non-specific downregulation of cIAP1, cIAP2 or survivin in the presence of the XIAP siRNA in Colo320 cells (Fig. 4D).
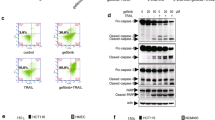
Inducible silencing of XIAP in Colo320 cells and xenograft tumors promotes Apo2L/TRAIL mediated apoptosis and suppresses tumor growth
To investigate the importance of XIAP more directly in determining sensitivity to Apo2L/TRAIL, we generated stable Colo320 cell lines in which XIAP was conditionally suppressed. Three different constructs with unique XIAP specific short hairpin RNA (shRNA) triggers (shXIAP-1, -3, -4) were designed and cloned into a doxycycline-inducible lentiviral vector. A scrambled shRNA trigger was used as a negative control (shCtrl). These were transduced into Colo320 cells and after selection for stable transductants using a G418-resistance marker, the pooled cells were tested for their ability to suppress XIAP expression in vitro. In the presence of doxycycline, XIAP expression was suppressed in all 3 Colo320-shXIAP cell lines, but not in Colo320-shCtrl cells (Fig. 5A). In the absence of doxycycline, XIAP protein expression was equivalent in Colo320-shCtrl and Colo320-shXIAP-1, -3 and -4 cells, suggesting that this inducible system was tightly regulated. When cells were treated with Apo2L/TRAIL in the presence of doxycycline, we observed cleavage of caspase 3 and the caspase 3 substrates PARP and DFF45/DFF35 specifically in Colo320-shXIAP cells, but not Colo320-shCtrl cells. This indicated that induced suppression of XIAP was capable of restoring sensitivity to Apo2L/TRAIL mediated apoptosis in Colo320 cells. Minimal cleavage of PARP was also observed when XIAP was suppressed in the absence of Apo2L/TRAIL administration, suggesting that continued expression of XIAP might be important for Colo320 cell survival. To address this more directly, we compared the viability of one Colo320-shXIAP line, Colo320-shXIAP-4, with that of Colo320-shCtrl cells and parental Colo320 cells. Both Colo320 and Colo320-shCtrl cells displayed comparable viability in the presence or absence of doxycycline or Apo2L/TRAIL, indicating that they remained insensitive to Apo2L/TRAIL (Fig. 5B). In contrast, Colo320-shXIAP-4 cells showed a marked reduction in viability when XIAP expression was suppressed in the presence of doxycycline (Fig. 5B). This was further augmented by the addition of Apo2L/TRAIL. These results suggested that Colo320 cells require XIAP for their survival in vitro and also become sensitized to Apo2L/TRAIL in the absence of XIAP. Some cleavage of PARP and DFF45/DFF35 was also observed in the presence of Apo2L/TRAIL when XIAP was not suppressed (in the absence of doxycycline) (Fig. 5A). As all these lines have been generated by infection with lentivirus, we believe this reflects the increased sensitivity of virally infected cells to Apo2L/TRAIL, which has been previously reported [30, 31]. Similar to our observations with an XIAP specific siRNA in Colo320 cells, we saw no decrease in the levels of cIAP1, cIAP2 or survivin in the presence of doxycycline in Colo320-shXIAP-4 cells (Fig. 5C).
Inducible knockdown of XIAP promotes apoptosis in Colo320 cells in vitro. A, Stable Colo320 cell lines with regulatable XIAP (shXIAP-1, shXIAP-3, shXIAP-4) or control (shCtrl) shRNA triggers were generated. Cells were grown in media alone (−Dox) or media containing 500 ng/ml doxycycline (+Dox) for 3 days and then stimulated with 200 ng/ml Apo2L/TRAIL or left untreated. After 17 h, lysates were generated and analyzed by Western blotting using antibodies against the indicated proteins. B, Colo320, Colo320shXIAP-4 and Colo320-shCtrl cells were assayed for cell viability with resazurin. Cells were cultured either in media alone (−Dox) or media containing 500 ng/ml of doxycycline (+Dox) for 5 days and then stimulated with 100 ng/ml of Apo2L/TRAIL (+Apo2L) or left untreated (−Apo2L) for 48 h. Absorbance at 544/590 was read at 2 h after addition of resazurin. C, Colo320, Colo320-shCtrl and Colo320-shXIAP-4 cells were cultured in either media alone (−Dox) or media containing 500 ng/ml doxycycline (+Dox) for 3 days. Lysates were generated and analyzed by Western blotting using antibodies against the indicated proteins
We then wanted to test whether Colo320-shXIAP-4 cells would also be sensitive to Apo2L/TRAIL in vivo. Colo320-shXIAP-4 and control Colo320-shCtrl cells were injected into SCID mice and tumors were allowed to grow until they were approximately 100 mm3. Mice were then switched to doxycycline-containing water to induce knockdown of XIAP or a control scrambled shRNA and treated with vehicle or Apo2L/TRAIL five days later. In both vehicle and Apo2L/TRAIL treated Colo320-shCtrl tumors, there was no significant growth inhibition (Fig. 6A). In contrast, we observed significant tumor growth inhibition in mice with Colo320-shXIAP-4 tumors, even in the absence of Apo2L/TRAIL. Vehicle treated Colo320-shXIAP-4 tumors were 69.7% smaller than vehicle treated Colo320-shCtrl tumors (p-value 0.0004, n = 6). When mice with Colo320-shXIAP-4 tumors were also treated with Apo2L/TRAIL, the majority of tumors completely regressed. The combination of Apo2L/TRAIL treatment when XIAP was suppressed in Colo320-shXIAP-4 tumors resulted in 98% tumor inhibition compared to Apo2L/TRAIL treated Colo320-shCtrl tumors (p < 0.0001, n = 6) and 90% inhibition over vehicle treated Colo320-shXIAP-4 tumors (p-value 0.0350, n = 6). Thus, knockdown of XIAP in Colo320 tumor xenografts dramatically suppressed their tumor growth, and these tumors could be inhibited further by treatment with Apo2L/TRAIL.
Inducible knockdown of XIAP promotes apoptosis in Colo320 xenografts. A, SCID mice with either Colo320-shCtrl or Colo320-shXIAP-4 derived s.c. tumors (6/group) were treated with vehicle or Apo2L/TRAIL (60 mg/kg/day, i.p.) on days 0–4. Five days prior to treatment mice were placed on doxycycline-containing water (2 mg/ml in 5% sucrose). To adjust to the initial tumor size in individual mice (day – 3), the fold changes were calculated at each time point and used for the analysis. B, Histological sections from Colo320-shCtrl and Colo320-shXIAP-4 tumors collected from mice receiving no doxycycline were stained for XIAP expression (brown stain). Images are 20× magnification. C, D, Histological sections from Colo320-shCtrl and Colo320-shXIAP-4 tumors from mice receiving doxycycline and either vehicle or Apo2L/TRAIL for 24 h were stained for XIAP (C) and active caspase 3 (D) expression. Images are 20× magnification
To confirm that XIAP expression was reduced in doxycycline treated Colo320-shXIAP-4 tumors, we analyzed histological sections from Colo320-shXIAP-4 and Colo320-shCtrl tumors from mice that did or did not receive doxycycline. In the absence of doxycycline, XIAP protein was detectable at equivalent levels in Colo320-shCtrl tumors and Colo320-shXIAP-4 tumors (Fig. 6B). In the presence of doxycycline, we saw a marked decrease in XIAP expression levels in Colo320-shXIAP-4 tumors but not Colo320-shCtrl tumors (Fig. 6C). This indicated that XIAP expression was specifically suppressed in Colo320-shXIAP-4 tumors in vivo in response to doxycycline. We also observed a concomitant increase in active caspase 3 staining in Colo320-shXIAP-4 tumors over Colo320-shCtrl tumors, and in Colo320-shXIAP-4 tumors exposed to Apo2L/TRAIL (Fig. 6D). Thus, increased caspase 3 activity in vivo corresponded with decreased XIAP expression and Apo2L/TRAIL treatment.
Discussion
Apo2L/TRAIL is a promising anti-cancer therapeutic in early clinical development. Despite its potent anti-tumor activity in xenograft models of colorectal, breast and lung [3, 32–34] cancers as well as others, some tumor cell lines remain resistant to Apo2L/TRAIL when administered as a single agent. This suggests that using Apo2L/TRAIL in combination with agents that target underlying resistance mechanisms will have significantly greater clinical impact than Apo2L/TRAIL alone. Indeed, Apo2L/TRAIL has been shown to cooperate with a wide variety of agents in various models. Tumor cell lines reported to be Apo2L/TRAIL resistant have been rendered Apo2L/TRAIL sensitive by co-treatment with chemotherapeutic agents such as irinotecan (CPT-11), gemcitabine, 5-FU, taxol and carboplatin [32, 34, 35]. Moreover, proteasome inhibitors [36], histone deacetylase inhibitors [37, 38] and cdk inhibitors such as roscovitine [39] can also cooperate with Apo2L/TRAIL to promote apoptosis.
Regulation of death receptor mediated apoptosis occurs at multiple tiers, including the level of receptor expression, assembly of a productive DISC following receptor ligation, recruitment of the Bcl-2-regulated mitochondrial pathway and antagonism of caspase activity by members of the IAP family. Relatively small changes at these multiple regulatory levels can have a significant impact on the apoptotic threshold of the cell and therefore its response to death stimuli. It is becoming apparent that, in addition to resistance being a multi-factorial process, different tumor cell types invoke different mechanisms of death receptor resistance. This suggests that no single agent may be capable of sensitizing all tumor cells to Apo2L/TRAIL and that intervention may be required at multiple points in the pathway.
In this study we used the colon adenocarcinoma lines Colo320 and Colo205 as a model system to delineate the contribution of these mechanisms to Apo2L/TRAIL resistance. Compared to Colo205, Colo320 cells exhibit significantly less sensitivity to Apo2L/TRAIL mediated apoptosis. Both Colo320 and Colo205 cells express similar levels of surface death and decoy Apo2L/TRAIL receptors, suggesting that differences in receptor expression levels are unlikely to be a major determinant of Apo2L/TRAIL sensitivity. However, significant differences between Colo205 and Colo320 were apparent when the expression level of DISC components was compared. Whereas FLIP levels were comparable between the two cells types, levels of FADD, caspase 8, and caspase 10 were lower in Colo320 than in Colo205. In addition, significantly less processing of caspase 8 and caspase 3 was observed following stimulation with Apo2L/TRAIL in Colo320 than in Colo205. Although some initial cleavage of caspase-3 was detected in Colo320, further maturation of active caspase-3 and processing of downstream substrates was not observed. Both the low FADD levels and high ratio of FLIP to caspase-8 might contribute to the relative inefficiency with which the DISC in Colo320 propagates an apoptotic signal.
The possibility of differential involvement of the intrinsic pathway is suggested by the fact that Colo320 cells expressed higher levels of Bcl-2 than Colo205. Bcl-2 overexpression has been reported to be a mechanism for preventing Apo2L/TRAIL induced apoptosis in several cell lines and siRNA mediated Bcl-2 downregulation has been shown to sensitize resistant melanoma cells to Apo2L/TRAIL induced apoptosis [40, 41]. Another potential explanation for the low level of caspase 3 activity upon Apo2L/TRAIL stimulation of Colo320 cells might be provided by high XIAP expression. However, as similar levels of XIAP were detected in Colo320 and Colo205 cells, it appears unlikely that differential expression of XIAP underlies the mechanism of resistance in Colo320 cells. While these results do not rule out a contribution by downstream mechanisms, they are consistent with a model in which the primary defect in Apo2L/TRAIL induced signaling occurs at the level of the DISC in Colo320 cells.
Exposure to the protein synthesis inhibitor cycloheximide or the proteasome inhibitor MG132 sensitized Colo320 cells to Apo2L/TRAIL mediated apoptosis. These findings underscore the fact that regulatory proteins with short half-lives acutely regulate apoptosis pathways. Cycloheximide has previously been shown to sensitize cells to death receptor mediated apoptosis and FLIP is at least one protein described to have a high turnover rate and be influenced by cycloheximide treatment [27, 42]. Proteasome inhibitors have also been shown to either induce apoptosis by themselves or to sensitize cells to apoptotic stimuli, including Apo2L/TRAIL [36, 43]. Candidate mechanisms include stabilization of pro-apoptotic proteins such as the Bcl-2 family members Bid and Bik [44, 45]. Interestingly, pro-survival regulators of the Bcl-2 and IAP families are also direct proteasomal targets [46]. In addition, proteasome inhibitors can block NF-κB activity by stabilizing IκB. Since NF-κB transcriptionally regulates many anti-apoptotic genes, including IAP family members, FLIP, Bcl-2, and Bcl-XL, blocking NF-κB can have a profound effect upon cell survival [47]. Proteasome inhibitors can also sensitize cells to Apo2L/TRAIL by transcriptional upregulation of DR5 resulting from stabilization of p53 and the CCAAT/enhancer-binding protein homologous protein (CHOP) [48, 49]. This highlights the complex effects that pharmacological inhibitors are likely to have on apoptotic pathways.
Upon exposure to cycloheximide, we observed reduced FLIP expression in both Colo205 and Colo320 cells, and this resulted in sensitizing Colo320 cells to Apo2L/TRAIL. Interestingly, FLIPL levels were considerably more sensitive to cycloheximide than FLIPS in both cell lines tested, suggesting that turnover of these FLIP variants may be differentially regulated in the cell. Selective knockdown of FLIPL has been shown to enhance effector-caspase stimulation and apoptosis, whereas FLIPS reduction does not have this effect [26]. Reduction of steady state levels of FLIPL in Colo320 cells also resulted in reduced FLIPL levels in the Colo320 DISC, but had no effect on FLIPS.
In Colo320 cells, both cycloheximide and MG132 increased processing of caspase 8 and its substrate Bid, indicating that either agent was capable of enhancing caspase 8 activation in response to Apo2L/TRAIL. Whereas cycloheximide resulted in decreased FLIP L in the Colo320 DISC, MG132 treatment increased levels of caspase 8 in the DISC. This indicates that caspase 8 may be stabilized in the DISC by proteasome inhibitors. The ratio of FLIPL to caspase 8 in the DISC has been shown to be a critical determinant of signaling downstream of Apo2L/TRAIL [26]. Collectively, our data suggest that changes in either FLIPL or caspase 8 levels could explain the increased ability of the DISC to activate downstream effectors. In this context, it is possible that the primary mechanism by which cycloheximide promotes formation of a productive DISC is by reducing the level of FLIPL available for recruitment while proteasome inhibitors may increase caspase-8 levels by stabilizing its turnover.
Downstream of the DISC, several additional pathways are likely to contribute to the sensitization of Colo320 cells by cycloheximide and MG132. Increased processing of Bid implies that the intrinsic pathway is differentially recruited in sensitized Colo320 cells. In conjunction with the higher levels of Bcl-2 observed in Colo320 relative to Colo205, this suggests that the intrinsic pathway may be a target for sensitizing agents in these cells. XIAP-mediated ubiquitination and degradation of caspases is likely to be another important target of proteasome inhibitors in the sensitization of Colo320 cells. It is worth noting that proteasome inhibitors can have opposing effects on XIAP. Proteasome inhibitors can prevent NFkB dependent expression of XIAP and XIAP mediated caspase degradation, yet also block XIAP auto-ubiquitination and self-degradation, thereby stabilizing its turnover [50, 51]. In response to Apo2L/TRAIL alone, some initial cleavage of caspase 3 was observed. However, in combination with either sensitizing agent, Apo2L/TRAIL enhanced processing and maturation of caspase 3. Both the enhanced output of active caspase 8 from the DISC and inhibition of caspase 3 turnover could contribute to this result.
Since pharmacological agents are likely to influence Apo2L/TRAIL sensitivity at multiple levels, we sought to elucidate the contribution of specific regulatory components in Colo320 cells by siRNA-mediated knockdown. Based upon our previous data, we focused on Bcl-2, FLIP, and XIAP. Despite the fact that they express higher levels of Bcl-2 than Colo205, Colo320 cells were not sensitized to Apo2L/TRAIL by siRNA-mediated knockdown of Bcl-2. Although Bcl-2 can mediate Apo2L/TRAIL resistance in some cells, this result argues against Bcl-2 playing an important role in the Apo2L/TRAIL resistance of Colo320 cells [52]. In contrast, specific knockdown of either XIAP or FLIP in Colo320 cells sensitized them to Apo2L/TRAIL. Other reports have also demonstrated that specific targeting of XIAP or FLIP can restore Apo2L/TRAIL sensitivity [26, 41, 53, 54]. The fact that both XIAP and FLIP inhibition could induce Apo2L/TRAIL sensitivity yet function by blocking the activity of different caspases indicates that Apo2L/TRAIL resistance in Colo320 cells can be overcome via multiple mechanisms.
While siRNA allowed us to dissect the mechanisms of Apo2L/TRAIL resistance in vitro, we wanted to specifically address the role of XIAP in vivo. We utilized a doxycycline-regulated promoter to express XIAP specific shRNAs in conjunction with a lentiviral vector delivery system. This method has the advantage of being able to stably knockdown expression of a target gene in > 90% of cells at various stages of tumor development [55]. Analysis of Colo320-shXIAP cells in vitro revealed that knockdown of XIAP was sufficient to sensitize Colo320 cells to Apo2L/TRAIL induced apoptosis. Moreover, loss of XIAP in Colo320 cells in the absence of Apo2L/TRAIL resulted in reduced cell viability, suggesting that Colo320 cells were dependent on XIAP for survival. This may be a cell-specific effect since down-regulation of XIAP fails to induce apoptosis in the absence of an externally applied apoptotic trigger in other cells such as MDA-MB-231 [54]. Importantly, reduction of XIAP in Colo320 cells by either siRNA or shRNA did not result in compensatory changes in the expression of other IAP family members.
Significant reduction of XIAP expression was observed in Colo320shXIAP tumors from mice that received doxycycline. Reduced XIAP expression in tumors also corresponded with increased active caspase 3 expression as measured by inmmunohistochemistry and this was enhanced when mice received Apo2L/TRAIL. Consistent with this, we observed impaired tumor growth in Colo320shXIAP derived tumors following down-regulation of XIAP. These findings indicate that reducing XIAP expression in Colo320 tumors compromises their ability to grow and increases their susceptibility to Apo2L/TRAIL. It is worth noting that although Colo320 cells are sensitized to Apo2L/TRAIL induced apoptosis by modulating Flip or XIAP levels, it is likely that other resistant colon cancer lines may depend on different anti-apoptotic factors or mechanisms for mediating those effects. To date, no consistent correlation has been described that identifies a single determinant of Apo2L/TRAIL sensitivity.
Conclusion
Our studies show that Apo2L/TRAIL resistance can be modulated at multiple points of intervention and provide insights into the mechanisms that regulate susceptibility to death receptor signals. These findings support the hypothesis that apoptosis promoting therapies may function optimally when combined with agents that modulate expression of anti-apoptotic factors. Current attempts to improve survival in cancer patients depend upon approaches to target tumor cell resistance and on the development of new combination therapeutic strategies. Thus, combination of Apo2L/TRAIL with agents that block intracellular inhibitory factors either directly or indirectly should prove to be a useful combination strategy in the treatment of cancer.
References
Wiley SR, Schooley K, Smolak PJ et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682
Pitti RM, Donahue CJ, Ruppert S, Bauer KD, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. Curr Biol 6:750–752
Ashkenazi A, Pai RC, Fong S et al (1999) Safety and anti-tumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104:155–162
Walczak H (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. [see comment]. Nat Med 5:157–163
Pan G, O’Rourke K, Chinnaiyan AM et al (1997) The receptor for the cytotoxic ligand TRAIL. Science 276:111–113
Walczak H, Degli-Esposti MA, Johnson RS et al (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 16:5386–5397
Sheridan JP, Marsters SA, Pitti RM et al (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. [see comment]. Science 277:818–821
Screaton GR, Mongkolsapaya J, Xiao-Ning X, Cowper A, McMichael AJ, Bell J (1997) TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol 7:693–696
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. [see comment]. Science 277:815–818
Degli-Esposti MA, Smolak PJ, Walczak H et al (1997) Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186:1165–1170
Emery JG, McDonnell P, Burke MB et al (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 273:14363–14367
Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A (2000) Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611–620
Irmler M, Thome M, Hahne M et al (1997) Inhibition of death receptor signals by cellular FLIP. [see comment]. Nature 388:190–195
Sprick MR, Weigand MA, Rieser E et al (2000) FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599–609
Bodmer JL, Holler N, Reynard S et al (2000) TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2:241–243
LeBlanc H, Lawrence D, Varfolomeev E et al (2002) Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. [see comment]. Nat Med 8:274–281
Deng Y, Lin Y, Wu X (2002) TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev 16:33–45
Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 3:401–410
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245
LeBlanc HN, Ashkenazi A (2003) Apo2L/TRAIL and its death and decoy receptors Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Cell Death Differ 10:66–75
Griffith TS, Rauch CT, Smolak PJ et al (1999) Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol 162:2597–2605
Barry SC, Harder B, Brzezinski M, Flint LY, Seppen J, Osborne WR (2001) Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum Gene Ther 12:1103–1108
Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O (2006) Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol 26:7046–7055
Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S (2001) Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem 276:20633–20640
Thome M, Tschopp J (2001) Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol 1:50–58
Sharp DA, Lawrence DA, Ashkenazi A (2005) Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem 280:19401–19409
Fulda S, Meyer E, Debatin KM (2000) Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res 60:3947–3956
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18:5242–5251
Cheng EH, Kirsch DG, Clem RJ et al (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278:1966–1968
Sedger LM, Shows DM, Blanton RA et al (1999) IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol 163:920–926
Strater J, Moller P (2004) TRAIL and viral infection. Vitam Horm 67:257–274
Gliniak B, Le T (1999) Tumor necrosis factor-related apoptosis-inducing ligand’s antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res 59:6153–6158
Walczak H, Miller RE, Ariail K et al (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. [see comment]. Nat Med 5:157–163
Jin H, Yang R, Fong S et al (2004) Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res 64:4900–4905
Zisman A, Ng CP, Pantuck AJ, Bonavida B, Belldegrun AS (2001) Actinomycin D and gemcitabine synergistically sensitize androgen-independent prostate cancer cells to Apo2L/TRAIL-mediated apoptosis. J Immunother 24:459–471
Mitsiades CS, Treon SP, Mitsiades N et al (2001) TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 98:795–804
Insinga A, Monestiroli S, Ronzoni S et al (2005) Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med 11:71–76 [erratum appears in Nat Med 2005 11(2):233]
Earel JK Jr, Van Oosten RL, Griffith TS (2006) Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res 66:499–507
Kim EH, Kim SU, Shin DY, Choi KS (2004) Roscovitine sensitizes glioma cells to TRAIL-mediated apoptosis by downregulation of survivin and XIAP. Oncogene 23:446–456
Fulda S, Meyer E, Debatin KM (2002) Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 21:2283–2294
Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC (2004) Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ 11:915–923
Van Geelen CM, de Vries EG, Ltk P, van Weeghel RP, de Jong S (2003) Differential modulation of the TRAIL receptors and the CD95 receptor in colon carcinoma cell lines. Br J Cancer 89:363–373
Brooks AD, Ramirez T, Toh U, Onksen J, Elliott PJ, Murphy WJ, Sayers TJ (2005) The proteasome inhibitor bortezomib (Velcade) sensitizes some human tumor cells to Apo2L/TRAIL-mediated apoptosis. Ann NY Acad Sci 1059:160–167
Breitschopf K, Zeiher AM, Dimmeler S (2000) Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem 275:21648–21652
Marshansky V, Wang X, Bertrand R et al (2001) Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells. J Immunol 166:3130–3142
Jesenberger V, Jentsch S (2002) Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol 3:112–121
Shishodia S, Aggarwal BB (2002) Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol 35:28–40
Wu GS, Burns TF, McDonald ER 3rd et al (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17:141–143
Yoshida T, Shiraishi T, Nakata S et al (2005) Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res 65:5662–5667
Suzuki Y, Nakabayashi Y, Takahashi R (2001) Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Nat Acad Sci USA 98:8662–8667
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288:874–877
Kuwana T, Smith JJ, Muzio M, Dixit V, Newmeyer DD, Kornbluth S (1998) Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem 273:16589–16594
Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F (2004) X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res 64:3006–3008
McManus DC, Lefebvre CA, Cherton-Horvat G et al (2004) Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene 23:8105–8117
Czauderna F, Santel A, Hinz M et al (2003) Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res 31:e127
Acknowledgements
We thank J. Graves and A. R. v.d. Vuurst de Vries for reading the manuscript, G. Chen for help with statistical analysis and the Amgen Washington pathology, flow cytometry and animal care groups for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lippa, M.S., Strockbine, L.D., Le, T.T. et al. Expression of anti-apoptotic factors modulates Apo2L/TRAIL resistance in colon carcinoma cells. Apoptosis 12, 1465–1478 (2007). https://doi.org/10.1007/s10495-007-0076-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0076-6