Abstract
Varroa destructor is the most common ectoparasite of the Western honey bee (Apis mellifera L.) worldwide and poses a serious threat to bee health. Synthetic acaricides, particularly pyrethroids, are frequently used to control Varroa mites. However, long-term and repeated use of synthetic pyrethroids has led to the development of resistance. In this study, we report on the presence of resistance mutations in the voltage-gated sodium channel in V. destructor populations from Turkish beekeeping areas. Two resistance mutations, L925V and L925I, that were previously associated with pyrethroid resistance, were found in more than 75% of the populations. A general correlation between the presence of mutations and the history of acaricide usage was observed for the sampled hives. In addition, we show there is only a low genetic distance among the sampled V. destructor populations, based on the analysis of three mitochondrial genes: cytochrome b (cytb), ATP synthase subunit 6 (atp6), and cytochrome c oxidase subunit III (cox3). Revealing the presence and geographical distribution of pyrethroid resistance mutations in V. destructor populations from Turkish apiaries will contribute to create more effective mite management programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turkey is the second largest honey-producing country across the globe, with 8 million hives and a honey production of 110 thousand tons annually (Özkırım 2018; FAOSTAT 2021). However, the average yield per hive in Turkey is far below the world average (15 vs. 40 kg, respectively) (Terin et al. 2018). One of the main reasons for the decreased yield is the inadequate control of the pests of honey bees. Varroa spp. are recognised among the most important pests and drivers of honey bee colony loss throughout the world (Potts et al. 2010; Steinhauer et al. 2018; Noël et al. 2020).
Varroa destructor Anderson & Trueman (Acari: Varroidae) is the most important obligate ectoparasite of the Western honey bee, Apis mellifera L. (Hymenoptera: Apidae), worldwide (Thoms et al. 2019; Traynor et al. 2020). Varroa spp. can cause significant damage to bee colonies by feeding on hemolymph and fat body tissues, leading to a decreased body weight and a shortened life span in honey bees (Rosenkranz et al. 2010; Ramsey et al. 2019). Besides, Varroa mites are known as virus vectors, transmitting various lethal pathogens to honey bees (McMenamin and Genersch, 2015). The honey bee colonies can collapse within a couple of years when the mites are not adequately controlled, resulting in dramatic decline of bee populations (Rosenkranz et al. 2010). Therefore, the management of V. destructor in apiaries is of great importance to maintain colony health.
Application of genetic markers to determine the genetic variation and haplotype of Varroa mites has been considered very important for several reasons, e.g., for species discrimination (Anderson and Trueman 2000), yet also for haplotype discrimination, as Korean haplotypes are considered more harmful than Japanese ones (Mendoza et al. 2020). As the usage of single genes is often insufficient, multiple genes should be employed to reveal genetic variation (Navajas et al. 2010; Muntaabski et al. 2020). Here, we use three mitochondrial genes, cytochrome b (cytb), ATP synthase subunit 6 (atp6), and cytochrome c oxidase subunit III (cox3), to assess genetic distance between various V. destructor populations.
Synthetic acaricides, including pyrethroids, formamidines, and organophosphates, have been the major effective tools used in the control of Varroa mites for years (Rosenkranz et al. 2010). Among them, the pyrethroids tau-fluvalinate and flumethrin are commonly preferred due to their in-hive selectivity, when used appropriately (Johnson et al. 2010; Blacquière et al. 2017). However, as a result of the long-term and repeated use of these pyrethroids in Varroa control, the beekeeping industry is facing the development of resistance and also the presence of chemical residues in bee products such as beeswax and honey (Bogdanov 2006; Rosenkranz et al. 2010; Smodiš Škerl et al. 2011).
Similar to other arthropods, mites can develop resistance to pesticides via pharmacokinetic and pharmacodynamic mechanisms (Feyereisen et al. 2015; Van Leeuwen and Dermauw 2016). The former often occurs by increased activity of major detoxification enzymes such as cytochrome P450-monooxygenases, glutathione S-transferases and carboxyl/cholinesterases (Surlis et al. 2016; Panini et al. 2019), whereas mutations that alter the target-site structure or expression are most frequent in the latter (Feyereisen et al. 2015). Recently, decreased activation of coumaphos to its toxic oxon form, mediated by decreased expression of CYP4EP4, has been uncovered as a rare but evolutionarily powerful solution to achieve resistance in V. destructor (Vlogiannitis et al. 2021).
Resistance to pyrethroids is well documented for many arthropods (Dong et al. 2014; Feyereisen et al. 2015) and has been firstly reported in the early 1990s in European V. destructor populations (Martin 2004). Although pyrethroid resistance has been associated with increased P450 monooxygenase and esterase activity (Hillesheim et al. 1996; Mozes-Koch et al. 2000), the L925V/I/M and M918L mutations in the voltage-gated sodium channel have been reported as major resistance mechanism in V. destructor populations from different continents (González-Cabrera et al. 2013, 2016, 2018; Millán-Leiva et al. 2021a, b).
The presence of Varroa has been documented in Turkey since the 1980s (Çakmak et al. 2003) and increasing bee health problems caused by these mites have been reported (Warrit et al. 2004; Çakmak and Sevençakmak 2016). However, the resistance status and genetic population structure have been rarely investigated in beekeeping areas of Turkey. In this study, we determined the genetic variation among V. destructor populations from Turkey and the presence of pyrethroid resistance mutations.
Materials and methods
Sampling of Varroa destructor populations
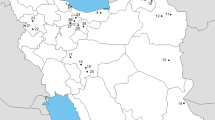
Using the powdered sugar method (Gregorc et al. 2017), a total of 22 Varroa populations were collected from 17 locations in Turkey during 2020 (Fig. 1). Mite samples were transferred to the laboratory in 90% ethanol for further processing. Detailed information about pesticide usage history, beekeeping practice and the location of the apiaries is presented in Table 1 and Fig. 1.
Total DNA isolation
Total DNA was extracted from pools of ten adult female mites per population using the Qiagen DNeasy Blood & Tissue Kit following the manufacturer’s instructions. At the final step, DNA was dissolved in 100 μl elution buffer. The purity and quantity of genomic DNA were checked using agarose gel electrophoresis (1.5%) and a NanoDrop 2000 (Thermo Scientific) spectrophotometer. DNA extracts were stored at − 20 °C until used.
PCR amplification conditions
The mtDNA sequences of the cytb, atp6 and cox3 genes of Varroa mites were used to determine the genetic distance among populations from different geographical origins. PCR conditions for above-mentioned genes were as follows: 4 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 51 °C (55 °C for cytb) and 75 s at 72 °C and a final extension of 8 min at 72 °C. Screening of the resistance mutations located at the IIS4–IIS5 region of the voltage-gated sodium channel (VGSC) gene was performed as previously described (Alissandrakis et al. 2017).
All primers used in this study are given in Table S1. Each PCR reaction was performed in a total volume of 30 μl containing 3 μl of mite DNA (30–50 ng/µL), 0.5 μl each of forward and reverse primer (10 µM stock), 20 μl of PCR-grade water and 6 μl of FIREPol Master Mix (Solis Biodyne). The presence of PCR products was confirmed by gel electrophoresis on a 1.5% agarose gel in 0.5× TAE buffer at 100 V for 40 min, stained with SAFE-T-STAIN (BioShop, Canada), and visualised with a UV transilluminator.
The PCR products were purified using the HighPrep PCR clean-up system (MagBio Genomics) and subsequent sequencing of PCR products was performed by Macrogen (Seoul, Korea).
Data analysis and screening for resistance mutations
Multiple sequence alignment was performed using MAFFT v.7 with ‘Auto’ strategy (Katoh et al. 2019) and edited using Bioedit v.7.0.5 software (Hall 1999). The incidence of mutations was determined by inspecting the sequencing chromatographs as previously described (İnak et al. 2019).
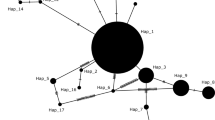
To investigate the genetic variation in different Varroa species, sequences obtained in this study were analysed together with sequences retrieved from the public GenBank database (accession numbers are provided in Table 2). A maximum likelihood phylogenetic tree was constructed with Molecular Evolutionary Genetics Analysis (MEGA X) using the T92 model (identified to be the best-fit model by MEGA X) and with 1000 bootstraps (Kumar et al. 2018) based on atp6 sequences that showed the highest mean genetic variation among Varroa species.
Results and discussion
Varroa spp. are among the most destructive pests in apiculture and are considered to be among the main drivers of colony collapse disorder (Le Conte et al. 2010; Gajic et al. 2013; Steinhauer et al. 2018; Noël et al. 2020). The occurrence of Varroa mites in Turkey has been first documented in 1977 (Çakmak and Sevençakmak 2016) and only 40% Varroa infestation rate has been reported in Turkish apiaries in 2003 (Çakmak et al. 2003). However, although we did not systematically record the exact number of mites, all sampled apiaries in the present study were infested with V. destructor, similar to the findings of Yalçınkaya and Keskin (2010) that reported 100% Varroa infestation in apiaries from southern Turkey. Rapid spread of Varroa mites in Turkey may be associated with the geographical location of the country, honey bee importation and migratory beekeeping practices (Warrit et al. 2004). Here, we investigated the genetic variation among V. destructor populations from Turkey and compared them with other populations as well as with other Varroa species.
Application of mitochondrial gene sequences in Varroa species allows to identify different haplotypes and to discriminate between species (Navajas et al. 2010; Muntaabski et al. 2020). In the present study, three mitochondrial markers (atp6, cox3, cytb) were used to determine the haplotypes and genetic variation among V. destructor populations. Similar to other reports from Turkey (Warrit et al. 2004; Ayan et al. 2017; Ayan and Aldemir 2018) and other closely located countries (Solignac et al. 2005; Farjamfar et al. 2018; Traynor et al. 2020), all sampled populations belonged to the Korean haplotype according to the discrimination method suggested by Navajas et al. (2010). The average genetic distance among Turkish V. destructor populations was 0.06 and 0.55% for cytb and cox3, respectively. Genetic distance based on mt-markers between Turkish V. destructor populations was very low (even no distance for atp6) (Table 2), which is in line with previous studies (Solignac et al. 2005; Navajas et al. 2010; Gajic et al. 2013; Farjamfar et al. 2018). The low genetic distance among V. destructor populations is probably caused by the shift from its original host, Apis cerana, to A. mellifera and subsequent spread over wide areas resulting in a ‘genetic bottleneck’ (Solignac et al. 2005; Navajas et al. 2010). As a result, low genetic structuring between V. destructor and V. jacobsoni colonies from similar locations has been reported (Dietemann et al. 2019).
The highest genetic distance between V. destructor and other Varroa species was determined in the sequences of atp6. Similarly, atp6 sequences had the highest average genetic distance within the genus Varroa (Table 2). In addition, the phylogenetic tree based on atp6 has successfully discriminated all Varroa species herein considered (Fig. 2). Recently, a mitochondrial gene, ND4, has been reported as a sensitive marker to assess genetic variability since the variation significantly correlates with geographic distance among various V. destructor populations (Muntaabski et al. 2020). The sequences of ND4 could be analyzed in future studies to get a better understanding of the population genetics of Varroa mites.
Together with organic acids and essential oils, the control of Varroa mites is mainly based on acaricides, in particular synthetic pyrethroids. However, development of resistance, causing failure in chemical control of Varroa mites, has been reported from many countries (Trouiller 1998; Martin 2004; Tutun et al. 2018; Higes et al. 2020; Millán-Leiva et al. 2021a; Hernández-Rodríguez et al. 2021). Synthetic pyrethroids are able to bind to voltage-gated sodium channels and cause prolonged channel opening (Soderlund 2012; Rinkevich et al. 2013) and are recognized as the second most widely used insecticide/acaricide group, corresponding to 15% of the total insecticide/acaricide market share (Sparks et al. 2020). Due to the low mammalian toxicity, relatively rapid degradation (Tang et al. 2018) and arthropod selectivity of pyrethroids (Khambay and Jewess 2005), this pesticide group is commonly used to control Varroa mites in apiaries. However, the limited number of active ingredients has resulted in repeated selection pressure and resistance development (Van Leeuwen and Dermauw 2016).
The development of pyrethroid resistance in Varroa mites has been observed since the early 1990s in Europe (Martin 2004). Thus far, different resistance mechanisms such as detoxification and point mutations have been associated with pyrethroid resistance in V. destructor (Wang et al. 2002; Liu et al. 2006). A leucine to valine substitution at position 925 (Musca domestica numbering) in the transmembrane segment 5 of domain II of the VGSC has been reported to confer pyrethroid resistance in V. destructor from UK and Czech Republic (González-Cabrera et al. 2013; Hubert et al. 2014). More recently, the presence of the L925V (CTG leucine to GTG valine) mutation in V. destructor populations has been reported throughout Europe and is considered to be a driving factor of pyrethroid resistance (González-Cabrera et al. 2018; Panini et al. 2019; Stara et al. 2019a; Hernández-Rodríguez et al. 2021). Other mutations in the same position, L925M and L925I, have been found in V. destructor populations from USA and Greece (González-Cabrera et al. 2016; Alissandrakis et al. 2017; Millán-Leiva et al. 2021a). On the contrary, no mutation has been found in V. destructor populations collected from 28 different locations in Iran (Farjamfar et al. 2018). Just recently, the kdr-like mutation M918L in combination with L925V, associated with pyrethroid resistance in other arthropod species, has been detected in V. destructor for the first time (Millán-Leiva et al. 2021b). All Varroa populations from Turkey, except the PLT population, harboured either one or a combination of L925V and L925I mutations (Fig. 3), whereas the L925M substitution was not detected. More than 75% of sampled Varroa populations contained the L925V mutation (yet never in a fixed state, see Figure S1) that reflects common usage of pyrethroids in beekeeping areas of Turkey (18 out of 22 sampled apiaries of sampled apiaries, see Table 1). In the KZ1 population, based on the mites analysed, it was concluded that the L925I mutation was fixed, probably caused by usage of flumethrin without any rotation in last treatments. The only population that did not contain any known resistance mutations in the target-site, was PLT, even though treated with flumethrin. Last, none of the populations harboured the M918L substitution.
Venn diagram of the amino-acid substitutions at position 925 of the voltage-gated sodium channel in Varroa destructor populations (VENNY, Oliveros 2007)
As a putative fitness cost associated with the L925V mutation has been suggested (González-Cabrera et al. 2018), the use of pyrethroids in rotation with other chemical control options such as organic acids and amitraz in Varroa control will result in decreasing the frequency of the resistance mutations and allow for more sustainable chemical control. The populations that were not exposed to flumethrin (KL2, BAL, CBK, GDL) also contained mutant individuals; however, susceptible alleles were determined as more frequent (L/V or L/I) based on visual inspection of sequencing chromatographs (Figure S1). This may reflect the decreasing resistance allel frequency in the absence of selection pressure. In addition, a vial test and a PCR–RFLP method have been reported to be fast and cost-effective alternatives to detect resistance and L925V/M/I mutations, respectively, allowing to monitor resistance widely and to design effective Varroa control management (Millán-Leiva et al. 2018; Stara et al. 2019b).
Considering the economic importance of beekeeping, the knowledge about genetic variation and pyrethroid resistance mutations in V. destructor populations in Turkey was very limited. In the current study, we revealed the presence and geographical distribution of pyrethroid resistance mutations (L925V and L925I) in Turkey. The results indicated that pyrethroids should include only a small fraction of the chemical treatments used within a rotation sheme, in order to decrease the frequency of pyrethroid resistance in apiaries of Turkey.
Data availability
All relevant data are within the manuscript.
References
Anderson DL, Trueman JWH (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol 24(3):165–189
Alissandrakis E, Ilias A, Tsagkarakou A (2017) Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J Apic Res 56(5):625–630
Ayan A, Aldemir OS (2018) Genetic characterization of Varroa destructor (Family: Varroidae) prevalent in honeybees (Apis mellifera) in the province of Aydin in Turkey. MAKÜ Sag Bil Enst Derg 6(1):26–32
Ayan A, Aldemir OS, Selamoğlu Z (2017) Analysis of COI gene region of Varroa destructor in honey bees (Apis mellifera) in province of Siirt. TJVR 1(1):20–23
Blacquière T, Altreuther G, Krieger KJ (2017) Evaluation of the efficacy and safety of flumethrin 275 mg bee-hive strips (PolyVar Yellow®) against Varroa destructor in naturally infested honey bee colonies in a controlled study. Parasitol Res 116(1):109–122
Bogdanov S (2006) Contaminants of bee products. Apidologie 37(1):1–18
Çakmak I, Sevençakmak S (2016) Beekeeping and recent colony losses in Turkey. Uludag Bee J 16(1):31–48
Çakmak I, Aydin L, Gulegen E, Wells H (2003) Varroa (Varroa destructor) and tracheal mite (Acarapis woodi) incidence in the Republic of Turkey. J Apic Res 42(4):57–60
Dietemann V, Beaurepaire A, Page P, Yañez O, Buawangpong N, Chantawannakul P, Neumann P (2019) Population genetics of ectoparasitic mites Varroa spp. in Eastern and Western honey bees. Parasitology 146(11):1429–1439
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS (2014) Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol 50:1–17
FAOSTAT (2021) Livestock primary production. http://www.fao.org/faostat/en/#data/QL. Accessed 15 Jan 2021
Farjamfar M, Saboori A, González-Cabrera J, Rodríguez CSH (2018) Genetic variability and pyrethroid susceptibility of the parasitic honey bee mite Varroa destructor (Acari: Varroidae) in Iran. Exp Appl Acarol 76(1):139–148
Feyereisen R, Dermauw W, Van Leeuwen T (2015) Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol 121:61–77
Gajic B, Radulovic Z, Stevanovic J, Kulisic Z, Vucicevic M, Simeunovic P, Stanimirovic Z (2013) Variability of the honey bee mite Varroa destructor in Serbia, based on mtDNA analysis. Exp Appl Acarol 61(1):97–105
González-Cabrera J, Davies TE, Field LM, Kennedy PJ, Williamson MS (2013) An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS One 8(12):e82941
González-Cabrera J, Rodriguez-Vargas S, Davies TE, Field LM, Schmehl D, Ellis JD, Krieger K, Williamson MS (2016) Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS One 11(5):e0155332
González-Cabrera J, Bumann H, Rodríguez-Vargas S, Kennedy PJ, Krieger K, Altreuther G, Hertel A, Hertlein G, Nauen R, Williamson MS (2018) A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J Pest Sci 91(3):1137–1144
Gregorc A, Knight PR, Adamczyk J (2017) Powdered sugar shake to monitor and oxalic acid treatments to control varroa mites (Varroa destructor Anderson and Trueman) in honey bee (Apis mellifera) colonies. J Apic Res 56(1):71–75
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hernández-Rodríguez CS, Marín Ó, Calatayud F, Mahiques MJ, Mompó A, Segura I, Simó E, González-Cabrera J (2021) Large-scale monitoring of resistance to coumaphos, amitraz, and pyrethroids in Varroa destructor. Insects 12(1):27
Higes M, Martín-Hernández R, Hernández-Rodríguez CS, González-Cabrera J (2020) Assessing the resistance to acaricides in Varroa destructor from several Spanish locations. Parasitol Res 119(11):3595–3601
Hillesheim E, Ritter W, Bassand D (1996) First data on resistance mechanisms of Varroa jacobsoni (Oud.) against tau-fluvalinate. Exp Appl Acarol 20(5):283–296
Hubert J, Nesvorna M, Kamler M, Kopecky J, Tyl J, Titera D, Stara J (2014) Point mutations in the sodium channel gene conferring tau-fluvalinate resistance in Varroa destructor. Pest Manag Sci 70(6):889–894
İnak E, Alpkent YN, Çobanoğlu S, Dermauw W, Van Leeuwen T (2019) Resistance incidence and presence of resistance mutations in populations of Tetranychus urticae from vegetable crops in Turkey. Exp Appl Acarol 78(3):343–360
Johnson RM, Huang ZY, Berenbaum MR (2010) Role of detoxification in Varroa destructor (Acari: Varroidae) tolerance of the miticide tau-fluvalinate. Int J Acarol 36(1):1–6
Khambay B, Jewess P (2005) Pyrethroids. In: Iatrou K, Gilbert LI, Gill SS (eds) Comprehensive molecular insect science, vol 6. Elsevier, Oxford, pp 1–29
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41(3):353–363
Liu Z, Tan J, Huang ZY, Dong K (2006) Effect of a fluvalinate-resistance-associated sodium channel mutation from varroa mites on cockroach sodium channel sensitivity to fluvalinate, a pyrethroid insecticide. Insect Biochem Mol Biol 36(11):885–889
Martin SJ (2004) Acaricide (pyrethroid) resistance in Varroa destructor. Bee World 85(4):67–69
McMenamin AJ, Genersch E (2015) Honey bee colony losses and associated viruses. Curr Opin Insect Sci 8:121–129
Mendoza Y, Gramajo E, Invernizzi C, Tomasco IH (2020) Mitochondrial haplotype analyses of the mite Varroa destructor (Acari: Varroidae) collected from honeybees Apis mellifera (Hymenoptera, Apidae) in Uruguay. Syst Appl Acarol 25(8):1526–1529
Millán-Leiva A, Hernández-Rodríguez CS, González-Cabrera J (2018) New PCR–RFLP diagnostics methodology for detecting Varroa destructor resistant to synthetic pyrethroids. J Pest Sci 91(3):937–941
Millán-Leiva A, Marín Ó, Christmon K, VanEngelsdorp D, González-Cabrera J (2021a) Mutations associated with pyrethroid resistance in Varroa mites, a parasite of honey bees, are widespread across the USA. Pest Manag Sci. https://doi.org/10.1002/ps.6366
Millán-Leiva A, Marín Ó, De la Rúa P, Muñoz I, Tsagkarakou A, Eversol H, Christmon K, Van Engelsdorp D, González-Cabrera J (2021b) Mutations associated with pyrethroid resistance in the honey bee parasite Varroa destructor evolved as a series of parallel and sequential events. J Pest Sci. https://doi.org/10.1007/s10340-020-01321-8
Mozes-Koch R, Slabezki Y, Efrat H, Kalev H, Kamer Y, Yakobson BA, Dag A (2000) First detection in Israel of fluvalinate resistance in the varroa mite using bioassay and biochemical methods. Exp Appl Acarol 24(1):35–43
Muntaabski I, Russo RM, Liendo MC, Palacio MA, Cladera JL, Lanzavecchia SB, Scannapieco AC (2020) Genetic variation and heteroplasmy of Varroa destructor inferred from ND4 mtDNA sequences. Parasitol Res 119(2):411–421
Navajas M, Anderson DL, De Guzman LI, Huang ZY, Clement J, Zhou T, Le Conte Y (2010) New Asian types of Varroa destructor: a potential new threat for world apiculture. Apidologie 41(2):181–193
Noël A, Le Conte Y, Mondet F (2020) Varroa destructor: how does it harm Apis mellifera honey bees and what can be done about it? Emerg Top Life Sci 4(1):45–57
Oliveros JC (2007) VENNY. An interactive tool for comparing lists with Venn's diagrams. https://bioinfogp.cnb.csic.es/tools/venny/index.html. Accessed 01 Febr 2021
Özkırım A (2018) Beekeeping in Turkey: bridging Asia and Europe. In: Chantawannakul P, Williams G, Neumann P (eds) Asian beekeeping in the 21st century. Springer, Singapore, pp 41–69
Panini M, Reguzzi MC, Chiesa O, Cominelli F, Lupi D, Moores G, Mazzoni E (2019) Pyrethroid resistance in Italian populations of the mite Varroa destructor: a focus on the Lombardy region. Bull Insectol 72(2):227–232
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353
Ramsey SD, Ochoa R, Bauchan G, Gulbronson C, Mowery JD, Cohen A, Lim D, Joklik J, Cicero JM, Ellis JD, Hawthorne D, vanEngelsdorp D (2019) Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc Natl Acad Sci USA 116(5):1792–1801
Rinkevich FD, Du Y, Dong K (2013) Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106(3):93–100
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96-S119
Soderlund DM (2012) Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol 86(2):165–181
Solignac M, Cornuet JM, Vautrin D, Le Conte Y, Anderson D, Evans J, Cros-Arteil S, Navajas M (2005) The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc R Soc B 272(1561):411–419
Sparks TC, Crossthwaite AJ, Nauen R, Banba S, Cordova D, Earley F, Ebbinghaus-Kintscher U, Fujioka S, Hirao A, Karmon D, Kennedy R, Nakao T, Popham HJR, Salgado V, Watsona GB, Wedel BJ, Kennedy R (2020) Insecticides, biologics and nematicides: updates to IRAC’s mode of action classification—a tool for resistance management. Pestic Biochem Physiol 167:104587
Smodiš Škerl MI, Nakrst M, Žvokelj L, Gregorc A (2011) The acaricidal effect of flumethrin, oxalic acid and amitraz against Varroa destructor in honey bee (Apis mellifera carnica) colonies. Acta Vet Brno 80(1):51–56
Stara J, Pekar S, Nesvorna M, Kamler M, Doskocil I, Hubert J (2019a) Spatio-temporal dynamics of Varroa destructor resistance to tau-fluvalinate in Czechia, associated with L925V sodium channel point mutation. Pest Manag Sci 75(5):1287–1294
Stara J, Pekar S, Nesvorna M, Erban T, Vinsova H, Kopecky J, Doskocil I, Kamler M, Hubert J (2019b) Detection of tau-fluvalinate resistance in the mite Varroa destructor based on the comparison of vial test and PCR–RFLP of kdr mutation in sodium channel gene. Exp Appl Acarol 77(2):161–171
Steinhauer N, Kulhanek K, Antúnez K, Human H, Chantawannakul P, Chauzat MP (2018) Drivers of colony losses. Curr Opin Insect Sci 26:142–148
Surlis C, Carolan JC, Coffey MF, Kavanagh K (2016) Proteomic analysis of Bayvarol® resistance mechanisms in the honey bee parasite Varroa destructor. J Apic Res 55(1):49–64
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007
Terin M, Yıldırım İ, Aksoy ADEM, Sarı MM (2018) Competition power of Turkey’s honey export and comparison with Balkan Countries. Bulg J Agric Sci 24(1):17–22
Thoms CA, Nelson KC, Kubas A, Steinhauer N, Wilson ME (2019) Beekeeper stewardship, colony loss, and Varroa destructor management. Ambio 48(10):1209–1218
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MA, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36(6):592–602
Trouiller J (1998) Monitoring Varroa jacobsoni resistance to pyrethroids in western Europe. Apidologie 29(6):537–546
Tutun H, Koç N, Kart A (2018) Plant essential oils used against some bee diseases. TURJAF 6(1):34–45
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Entomol 61:475–498
Vlogiannitis S, Mavridis K, Dermauw W, Snoeck S, Katsavou E, Morou E, Harizanis P, Swevers L, Hemingway J, Feyereisen R, Van Leeuwen T, Vontas J (2021) Reduced proinsecticide activation by cytochrome P450 confers coumaphos resistance in the major bee parasite Varroa destructor. PNAS 118(6):e2020380118
Wang R, Liu Z, Dong K, Elzen PJ, Pettis J, Huang Z (2002) Association of novel mutations in a sodium channel gene with fluvalinate resistance in the mite, Varroa destructor. J Apic Res 41(1–2):17–25
Warrit N, Hagen TA, Smith DR, Çakmak I (2004) A survey of Varroa destructor strains on Apis mellifera in Turkey. J Apic Res 43(4):190–191
Yalçınkaya A, Keskin N (2010) The investigation of honey bee diseases after colony losses in Hatay and Adana provinces of Turkey. Mellifera 10(20):24–31
Acknowledgements
We would like to thank the beekeepers of the research area for their kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10493_2021_626_MOESM1_ESM.docx
Figure S1 Partial sequencing chromatographs of PCR amplicons revealing position 925 in the voltage-gated sodium channel (DOCX 4084 kb).
Rights and permissions
About this article
Cite this article
Koç, N., İnak, E., Jonckheere, W. et al. Genetic analysis and screening of pyrethroid resistance mutations in Varroa destructor populations from Turkey. Exp Appl Acarol 84, 433–444 (2021). https://doi.org/10.1007/s10493-021-00626-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00626-2