Abstract
We report tick infestations and rickettsial detection in ticks infesting free-living wild mammals (Monodelphis domestica, Tolypeutes tricinctus, Thrichomys inermis and Kerodon rupestris) captured in the Caatinga ecoregion of Bahia state, northeastern Brazil, during September to December 2016. Overall, 117 ticks (61 larvae, 25 nymphs, 25 males, 6 females) belonging to two genera, and at least three species were collected: Amblyomma auricularium, Amblyomma parvum, Amblyomma sp., Ornithodoros rietcorreai and an unidentified Ornithodoros sp. We provide new host records to the rodent T. inermis parasitized by larva and nymphs of A. auricularium and to the marsupial M. domestica infested by larvae of A. auricularium. Furthermore, we describe new tick-host association for larvae of O. rietcorreai on the rodents K. rupestris and T. inermis. Concerning tick-Rickettsia associations, we detected Rickettsia amblyommatis and an uncharacterized species of Rickettsia belonging to the spotted fever group (SFG) in both A. auricularium and A. parvum. Additionally, ‘Candidatus Rickettsia andeanae’ was detected in A. parvum as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria of the genus Rickettsia (Rickettsiales: Rickettsiaceae) are obligatory intracellular microorganisms that infect invertebrate hosts worldwide (Dumler et al. 2001). Currently, the diversity of Rickettsia has been reclassified into different groups, including the spotted fever group (SFG), the typhus group (TG), the transitional group Rickettsia (TRG), the bellii group (BG), the canadensis group (CG), and several other basal groups (Weinert et al. 2009).
In Brazil, various rickettsial agents have been reported, namely the SFG species Rickettsia rickettsii, Rickettsia parkeri, Rickettsia amblyommatis (formerly ‘Candidatus R. amblyommii’), Rickettsia rhipicephali, ‘Candidatus Rickettsia andeanae’; the TRG species Rickettsia felis, Rickettsia asembonensis; the TG species Rickettsia typhi; the BG species Rickettsia bellii; and the CG species Rickettsia monteiroi (Parola et al. 2013; Dall’Agnol et al. 2017).
The most important zoonotic disease transmitted by ticks in Brazil is Brazilian spotted fever (BSF), caused by R. rickettsii, being more frequently reported in the southeastern region of the country, which encompasses the states of Minas Gerais, Rio de Janeiro, São Paulo, and Espírito Santo (Labruna 2009; Szabó et al. 2013). Recently a second and milder tick-borne SFG human rickettsiosis, caused by R. parkeri strain Atlantic rainforest, has also been described in the states of São Paulo (Spolidorio et al. 2010), Bahia (Silva et al. 2011) and Santa Catarina (Krawczak et al. 2016).
Studies on rickettsial infections in ticks parasitizing wild and domestic animals in the state of Bahia (northeastern region of Brazil) have encompassed ticks from wild birds (Ogrzewalska et al. 2011; Lugarini et al. 2015), porcupines (McIntosh et al. 2015), and domestic dogs (Nieri-Bastos et al. 2016). We provide, for the first time, information on rickettsial detection in ticks infesting wild mammals from the orders Cingulata, Didelphimorphia and Rodentia in the state of Bahia, Brazil.
Materials and methods
Capture of mammals and collection of ticks
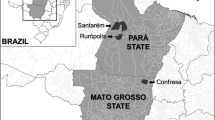
During September to December of 2016, a sampling of wild mammals associated with caves was carried out in a semi-arid region from Santo Inácio, a small village in the municipality of Gentio do Ouro, in the state of Bahia, northeastern Brazil (Fig. 1). Except for the Brazilian three-banded armadillo, Tolypeutes tricinctus (L.), which was captured manually, all other mammals were captured using Tomahawk and Sherman traps installed at the entrance or inside the caves. Fifteen traps were set in each of 19 sampled caves, and remained active for four nights, resulting into a sampling effort of 1200 trap nights. Collected mammals were identified according to Gardner (2008) and Patton et al. (2015), and then deposited at the Zoological Collection of the Federal University of Mato Grosso, Cuiabá Campus, Mato Grosso, Brazil. Ticks were collected from the body of the animals with the aid of brush and forceps, paying special attention to auricular pavilions, a region where ticks use to attach. Collected specimens were preserved in absolute isopropanol and sent to the laboratory.
The capture and collection of mammals was authorized by the “Instituto do Meio Ambiente e Recursos Hídricos do Estado da Bahia (INEMA-BA)”, through the Portarias INEMA no. 11.044, 11.046, 11.086, 11.097, 11.098 and 11.104. Tick collection was previously approved by the “Instituto Chico Mendes de Conservação da Biodiversidade” (ICMBio collection permit no. 40617).
Morphological identification of ticks
Post larval stages of hard ticks (Ixodidae) were identified following Barros-Battesti et al. (2006) and Martins et al. (2010). Part of soft tick larvae (Argasidae) was clarified in KOH 25%, hydrated in distilled water, and mounted in slides using Hoyer’s solution. After 2 days at 25 °C, slide-mounted ticks were observed through optical microscopy (Olympus BX40 optical microscope), and morphological traits were compared with other Neotropical species (Cooley and Kohls 1944; Kohls et al. 1965, 1969; Jones and Clifford 1972; Endris et al. 1989; Venzal et al. 2008, 2013; Labruna et al. 2016; Muñoz-Leal et al. 2016). One slide-mounted larva was photographed with software Image-Pro Plus 5.1 to illustrate morphological characters.
DNA extraction, Rickettsia detection and molecular identification of ticks
Four larvae of Ornithodoros, 5 individual and 8 pools of 3 larvae, 25 individual nymphs and 31 individual adults of Amblyomma ticks were submitted to DNA extraction by the guanidine isothiocyanate protocol, as described by Sangioni et al. (2005). Only DNA extracted from hard ticks were submitted to a screening for rickettsial agents. For this purpose, a first conventional PCR was performed using CS-78 and CS-323 primers, which target a 401 base pairs (bp) fragment of the citrate synthase gene (gltA) common to all Rickettsia species (Labruna et al. 2004). Positive samples were further tested by another PCR protocol using primers Rr190.70p and Rr190.602n, which amplify a ~ 530 bp fragment of the 190-kDa outer membrane protein gene (ompA) present only in Rickettsia of the SFG (Regnery et al. 1991). For molecular identification of Rickettsia-positive Amblyomma larvae, and larvae of Ornithodoros genus, a third PCR targeting a ~ 460 bp fragment of the tick mitochondrial 16S rRNA gene was performed as previously described (Mangold et al. 1998). Expected size amplicons of Rickettsia and tick mitochondrial 16S rRNA gene PCRs were purified and sequenced in an automated sequencer (ABI-PRISM 3500 Genetic Analyzer, Foster City, CA, USA). Obtained sequences were then submitted to a Basic Local Alignment Search Tool analysis (BLAST; Altschul et al. 1990) to determine closest identities with congeneric organisms available in GenBank.
Phylogenetic analysis
In the current study, a phylogenetic analysis was performed for Ornithodoros haplotypes from obtained consensus sequences of the mitochondrial 16S rRNA gene. The phylogenetic tree was constructed using Maximum Parsimony (MP) and Bayesian (B) methods. Sixty-four sequences of soft ticks available in GenBank, and the herein obtained haplotypes were aligned using Clustal X (Thompson et al. 1997), and were adjusted manually using GeneDoc (Nicholas et al. 1997). MP analysis was performed in PAUP version 4.0b10 (Swofford 2002) with 500 bootstrap replicates, random stepwise addition starting trees and TBR branch swapping. The Bayesian analysis was performed using MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001) with four independent Markov chain runs for 1,500,000 metropolis-coupled MCMC generations, sampling a tree every 100th generation. The first 25% of the trees represented burn-in, and the remaining trees were used to calculate Bayesian posterior probability. Sequences from Ixodes holocyclus Neumann and Ixodes uriae White were used as outgroup. GenBank accession numbers for all the sequences used in the analysis are indicated in the phylogenetic tree.
Results
Capture of mammals and morphological identification of ticks
Overall, 25 wild mammals represented by the following 4 species were captured: T. tricinctus, Monodelphis domestica (Wagner), Thrichomys inermis (Pictet) and Kerodon rupestris (Wied-Neuwied). Upon these vertebrates, 117 ticks (61 larvae, 25 nymphs, 25 males, 6 females) were collected (Table 1). Nymphs and adults were recognized by morphological characters into 2 Amblyomma species, Amblyomma auricularium (Conil) and Amblyomma parvum (Aragão), while 25 larvae were identified as A. auricularium by sequencing as described below. Four larvae were retained as Amblyomma sp.
On the other hand, 32 larvae of Ornithodoros genus were identified as 2 taxa by the following morphological characters: (1) Ornithodoros rietcorreai Labruna, Nava and Venzal 2016: dorsal surface provided with four pairs of posterolateral, and three pairs of central setae; dorsal plate pyriform with posterior margin slightly concave; hypostome pointed apically, provided with three files of denticles in the anterior half, and two posteriorly almost to base, anal valves long with leaf-shaped ends (Labruna et al. 2016); and (2) Ornithodoros sp.: dorsal surface with seven pairs of anterolateral, ten pairs of posterolateral, and five pairs of central setae; dorsal plate pyriform; hypostome pointed apically, with three files of denticles in the anterior third, then two files almost towards the base; file one provided with 23, file two with 21 and file three with eight denticles (Fig. 2). Ethanol-preserved and slide-mounted larvae of O. rietcorreai, and two slides with the unidentified Ornithodoros sp. were deposited at the tick collection “Colecão Nacional de Carrapatos Danilo Gonçalves Saraiva” (CNC) at the University of São Paulo, São Paulo, Brazil (accession numbers: CNC 3708–3709, and CNC 3711–3712).
Rickettsia detection
A total of 61 individual specimens and eight larval pools of Amblyomma spp. were screened by PCR for rickettsial infection (Table 2). R. amblyommatis was detected in A. auricularium larvae, nymphs, and adults. Partial sequences of the ompA gene obtained from A. auricularium ticks were identical to each other and matched 100% (488/488 bp) to sequences of R. amblyommatis available in GenBank (KX434739, JX867426). Moreover, a sequence of ompA gene obtained from a female of A. parvum showed an identity of 98% (478/488 bp) with sequences of Rickettsia sp. Gu263 (F523328) from Guatemala, here designated as Rickettsia sp. haplotype ApBA1. Because of the quality of amplified DNA, it was not possible to sequence ompA amplicons obtained upon a pool of larvae, from two nymphs and from one male of A. auricularium. However, for all these samples we obtained gltA sequences identical to each other and to several sequences of R. amblyommatis (MG674587, CP015012, KY674356, KY273595, KY628365). Furthermore, partial sequences of the gltA gene from two nymphs of A. parvum yielded perfect matches (350/3350 bp) to ‘Ca. R. andeanae’ (KY628369). Finally, the gltA sequence from two ticks (a nymph of A. auricularium and a female of A. parvum) matched 99% (347/350 bp) with several SFG rickettsiae (KY474576, KX591657, MF002509), here referred as Rickettsia sp. haplotype ApBA2. All sequences of Rickettsia spp. obtained in this study were deposited in GenBank and the accession numbers are listed in Table 2.
Molecular identification of Ornithodoros spp. and Amblyomma larvae
Molecular tools confirmed morphological identification of soft ticks since two larvae of O. rietcorreai submitted to DNA extraction yielded a sole haplotype of 427 bp 99% (423/427 bp) and 96% (411/428 bp) identical to conspecific sequences from Paraiba (KX130781) and Piauí states (KX130782), respectively. On the other hand, two larvae of the unidentified Ornithodoros morphotype yielded a unique haplotype of 422 bp, that matched Ornithodoros atacamensis Muñoz-Leal, González-Acuña and Venzal 2016 from Chile (KT894587) as closest relative with 90% (387/428 bp, eight gaps) of identity. Morphological diagnoses of A. auricularium ticks positive to Rickettsia detection were confirmed as well, since 16S rDNA haplotypes obtained for four individual larvae and seven larval pools were 99% (400/404 pb) and 100% (404/404 pb) identical to the sequence of A. auricularium clone Aa2 from the state of Pernambuco, Brazil (KR869155), respectively.
GenBank accession numbers for partial sequences of mitochondrial 16S rDNA gene generated in the present study are as follows: O. rietcorreai, MH061498; Ornithodoros sp., MH061499; and A. auricularium, MG887827, MG887829.
Phylogenetic analysis
The phylogeny inferred by Parsimony and Bayesian methods for mitochondrial 16S rDNA sequences of Neotropical Argasidae is presented in Fig. 3. As it would be expected, the obtained haplotype of O. rietcorreai clustered into a highly supported monophyletic group with other conspecific sequences from Paraiba and Piauí states. On the other hand, the haplotype from two larvae of the unidentified Ornithodoros sp. resulted into a sister taxon of O. atacamensis, and together with Ornithodoros puertoricensis (Fox), all three species formed a well-supported clade between other representatives of the Ornithodoros talaje (Guérin-Méneville) species group.
Discussion
The present study reports field data on rickettsial infection in ticks infesting free-living wild animals in the Caatinga ecoregion of northeastern Brazil, in the Bahia state. Herein, we provide the first records A. auricularium and O. rietcorreai from T. inermis.
Amblyomma auricularium was the most abundant hard tick. This tick species is widely distributed from southern USA to Argentina (Mertins et al. 2017; Lord and Day 2000; Guglielmone et al. 2003), and all parasitic stages of A. auricularium have already be found feeding mainly on armadillos (Dasypodidae), including records of adults on the Brazilian three-banded armadillo T. tricinctus (Nava et al. 2017), as observed in the present study. However, immature stages of this tick have also been described feeding on other hosts, such as small rodents and marsupials (Guglielmone et al. 2003; Horta et al. 2011). In line with these reports, in the present study we record the rodent T. inermis as a new host for the nymph and larva of A. auricularium and the marsupial M. domestica for the larva of A. auricularium.
Amblyomma parvum is distributed from Mexico to Argentina (Nava et al. 2008), with a single specimen reported from Florida (Corn et al. 2012). Despite its wide geographical distribution, most records on this tick species were from dry areas (Nava et al. 2008), including Caatinga ecoregion in Brazil (Horta et al. 2011; Lugarini et al. 2015; Pereira et al. 2017). Hosts of A. parvum comprise medium- to large-sized mammals for adult stages, while larvae and nymphs prefer parasitizing small mammals (Nava et al. 2008, 2017). Although A. parvum has already been recorded in the Caatinga ecoregion on Galea spixii and Thrichomys apereoides (Horta et al. 2011), to authors’ knowledge, we provide the first records for immature stages on M. domestica and T. inermis.
The argasid tick O. rietcorreai was recently described from K. rupestris resting places in three different areas of the Caatinga ecoregion, two in Piauí State, and one in Paraíba State (Labruna et al. 2016). Confirmed by a morphological and molecular approach, the present study expands the distributional range of this species to Bahia state. According to Labruna et al. (2016), it is possible that at least part of the earlier records of O. talaje from the Caatinga ecoregion relied primarily on a morphological examination of external characters of post-larval stages and could refer to O. rietcorreai. Furthermore, we record, for the first time infestation by larvae of O. rietcorreai on its probable type host K. rupestris, and also on T. inermis, two small mammals species inhabiting rocky environments.
By the morphological study of larvae, two identical specimens of Ornithodoros were not classified to species level by means of available data on Ornithodoros descriptions from the Neotropical region. Although morphological traits were somehow related to other representatives of the O. talaje group (i.e. dorsal setae rather short, pointed hypostome, pyriform dorsal plate, long palpi), the phenotype of this unidentified immature form of Ornithodoros collected on M. domestica did not match any published description from South America. Interestingly, a recent report of an unidentified Ornithodoros collected from the same mammal host (Pereira et al. 2017) coincides at least by sharing a pyriform dorsal plate and a pointed hypostome. Unfortunately, the report of this specimen lacks a molecular characterization, therefore comparisons of mitochondrial sequences were impossible to perform. Phylogenetic analyses of the current study pointed that our unidentified Ornithodoros sp. is closely related to O. atacamensis, a lizard-associated soft tick that occurs in the Atacama Desert in Chile (Muñoz-Leal et al. 2016). Although phylogenetically related, O. atacamensis differs from the phenotype of herein characterized Ornithodoros larva by having five rather than ten posterolateral pairs of setae. The presence of 22 dorsal setae constitutes an exclusive combination of discrete characters in both slide-mounted larvae (Fig. 2), and separate herein reported Ornithodoros morphotype from all the rest of O. talaje group as well (i.e. Ornithodoros guaporensis, Ornithodoros hasei, O. kohlsi, O. puertoricensis, and Ornithodoros rioplatensis). So far, it is highly possible that the Ornithodoros sp. collected from M. domestica could indeed represent a new taxon within the Argasidae. The obtention of more specimens, including adult forms, would be now needed in order to perform a formal description.
Rickettsia amblyommatis has already been reported infecting A. auricularium and A. parvum in the northeastern region of Brazil (Saraiva et al. 2013; Lugarini et al. 2015), and other Amblyomma species including Amblyomma longirostre (Ogrzewalska et al. 2011; Lugarini et al. 2015; McIntosh et al. 2015), Amblyomma cajennense sensu stricto (s.s.) (Costa et al. 2017), Amblyomma pseudoconcolor (Silva et al. 2018), and Amblyomma varium (Lugarini et al. 2015). Despite R. amblyommatis has not yet been confirmed as a human pathogen (Karpathy Sandor et al. 2016), there is evidence supporting the natural infection of dogs by tick transmission in northeastern Brazil (Costa et al. 2017), and in the United States (Barrett et al. 2014). Furthermore, there has been laboratory evidence has pointed that naturally infected A. auricularium are competent vectors of R. amblyommatis to rabbits (Saraiva et al. 2013).
In Brazil, ‘Candidatus R. andeanae’ was originally detected in A. parvum from ticks of the Pantanal and Cerrado ecoregions (Nieri-Bastos et al. 2014). Subsequently, reports included infection of A. parvum ticks in Caatinga (Lugarini et al. 2015) and Cerrado ecosystem (Costa et al. 2017). Further, two other tick species have been reported to harbour ‘Ca. R. andeanae’ in Brazil namely A. sculptum (Witter et al. 2016) and A. auricularium (Lugarini et al. 2015). However, the capacity of ‘Ca. R. andeanae’ to infect humans or other vertebrate hosts as well as causing disease is unknown.
Finally, an uncharacterized Rickettsia agent related to SFG was detected in both A. auricularium and A. parvum. However, collection of more ticks and isolation for further characterization using others sets of primers of this undescribed Rickettsia sp. are required to determine its taxonomic status, beyond possible pathogenic potential.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Barrett A, Little SE, Shaw E (2014) Rickettsia amblyommii and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis 14:20–25. https://doi.org/10.1089/vbz.2013.1325
Barros-Battesti DM, Arzua M, Bechara GH (2006) Carrapatos de importância médico-veterinária da região neotropical: um guia ilustrado para identificação de espécies. Vox/ICTTD-3/Butantan, São Paulo, p 223
Cooley RA, Kohls GM (1944) The Argasidae of North America, Central America and Cuba. In: The American midland naturalist. Monograph n.1. The University Press, Notre Dame, p 152
Corn JL, Hanson BA, Okraska CR, Muizneiks B, Morgan V, Mertins JW (2012) First at-large record of Amblyomma parvum (Acari: Ixodidae) in the United States. Syst Appl Acarol 17:3–6. http://www.bioone.org/doi/full/ https://doi.org/10.11158/saa.17.1.2
Costa FB, Costa AP, Moraes-Filho J, Martins TF, Soares HS, Ramirez DG, Dias RA, Labruna MB (2017) Rickettsia amblyommatis infecting ticks and exposure of domestic dogs to Rickettsia spp. in an Amazon-Cerrado transition region of northeastern Brazil. PLoS ONE. https://doi.org/10.1371/journal.pone.0179163
Dall’Agnol B, Souza U, Webster A, Weck B, Stenzel B, Labruna M, Klafke G, Martins JR, Ferreira CAS, Reck J (2017) “Candidatus Rickettsia asemboensis” in Rhipicephalus sanguineus ticks, Brazil. Acta Trop 167:18–20. https://doi.org/10.1016/j.actatropica.2016.12.008
Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145–2165. https://doi.org/10.1099/00207713-51-6-2145
Endris RG, Keirans JE, Robbins RG, Hess WR (1989) Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae): redescription by scanning electron microscopy. J Med Entomol 26:146–154
Gardner AL (2008) Mammals of South America: marsupials, xenarthrans, shrews and bats, vol 1. University of Chicago Press, Chicago, p 669
Guglielmone AA, Estrada-Peña A, Luciani CA, Mangold AJ, Keiran JE (2003) Hosts and distribution of Amblyomma auricularium (Conil 1878) and Amblyomma pseudoconcolor Aragão, 1908 (Acari: Ixodida). Exp Appl Acarol 29:131–139. https://doi.org/10.1023/A:1024251020035
Horta MC, Nascimento GF, Martins TF, Labruna MB, Machado LCP, Nicola PA (2011) Ticks (Acari: Ixodida) parasitizing free-living wild animals in the Caatinga biome in the State of Pernambuco, northeastern Brazil. Syst Appl Acarol 16:207–211. https://doi.org/10.11158/saa.16.3.3
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jones EK, Clifford CM (1972) The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). V. A revised key to larval Argasidae of the Western Hemisphere and description of seven new species of Ornithodoros. Ann Entomol Soc Am 65:730–740
Karpathy Sandor E, Slater Kimetha S, Goldsmith CS, Nicholson WL, Paddock CD (2016) Rickettsia amblyommatis sp. nov., a spotted fever group rickettsia associated with multiple species of Amblyomma ticks in North and South America. Int J Syst Evol Microbiol 66:5236–5243. https://doi.org/10.1099/ijsem.0.001502
Kohls GM, Sonenshine DE, Clifford CM (1965) The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions of three new species. Ann Entomol Soc Am 58:331–364
Kohls GM, Clifford CM, Jones EK (1969) The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from the Western Hemisphere. Ann Entomol Soc Am 62:1035–1043
Krawczak FS, Muñoz-Leal S, Guztzazky AC, Oliveira SV, Santos FC, Angerami RN, Moraes-Filho J, de Souza xJC Jr, Labruna MB (2016) Rickettsia sp. strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am J Trop Med Hyg 95:551–553. https://doi.org/10.1016/j.ttbdis.2014.07.010
Labruna MB (2009) Ecology of rickettsia in South America. Ann N Y Acad Sci 1166:156–166. https://doi.org/10.1111/j.1749-6632.2009.04516.x
Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, Popov V, Walker DH (2004) Rickettsia belli and Rickettsia amblyommii in Amblyomma. ticks from the State of Rondônia, Western Amazon, Brazil. J Med Entomol 41:1073–1081. https://doi.org/10.1603/0022-2585-41.6.1073
Labruna MB, Nava S, Marcili A, Barbieri ARM, Nunes PH, Horta MC, Venzal JM (2016) A new argasid tick species (Acari: Argasidae) associated with the rock cavy, Kerodon rupestris Wied-Neuwied (Rodentia: Caviidae), in a semiarid region of Brazil. Parasites Vectors 9:511. https://doi.org/10.1186/s13071-016-1796-7
Lord CC, Day JF (2000) First record of Amblyomma auricularium (Acari: Ixodidae) in the United States. J Med Entomol 37:977–978. https://doi.org/10.1603/0022-2585-37.6.977
Lugarini C, Martins TF, Ogrzewalska M, De Vasconcelos NC, Ellis VA, De Oliveira JB, Pinter A, Labruna MB, Silva JC (2015) Rickettsial agents in avian ixodid ticks in northeast Brazil. Ticks Tick Borne Dis 6:364–375. https://doi.org/10.1016/j.ttbdis.2015.02.011
Mangold AJ, Bargues MD, Mas-Coma S (1998) Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res 84:478–484. https://doi.org/10.1007/s004360050433
Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB (2010) Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 1:75–99. https://doi.org/10.1016/j.ttbdis.2010.03.002
McIntosh D, Bezerra RA, Luz HR, Faccini JL, Gaiotto FA, Giné GA, Albuquerque GR (2015) Detection of Rickettsia belli and Rickettsia amblyommii in Amblyomma longirostre (Acari: Ixodidae) from Bahia state, Northeast Brazil. Braz J Microbiol 46:879–883. https://doi.org/10.1590/S1517-838246320140623
Mertins JW, Vigil SL, Corn JL (2017) Amblyomma auricularium (Ixodida: Ixodidae) in Florida: new hosts and distribution records. J Med Entomol 54:132–141. https://doi.org/10.1093/jme/tjw159
Muñoz-Leal S, Venzal JM, González-Acuña D, Nava S, Lopes MG, Martins TF, Figueroa C, Fernández N, Labruna MB (2016) A new species of Ornithodoros (Acari: Argasidae) from desert areas of northern Chile. Ticks Tick Borne Dis 7:901–910. https://doi.org/10.1016/j.ttbdis.2016.04.008
Nava S, Mangoldi AJ, Guglielmone AA (2008) Aspects of the life cycle of Amblyomma parvum (Acari: Ixodidae) under natural conditions. Vet Parasitol 156:270–276. https://doi.org/10.1016/j.vetpar.2008.05.029
Nava S, Venzal JM, González-Acuña D, Martins TF, Guglielmone AA (2017) Ticks of the Southern Cone of America: diagnosis, distribution and hosts with taxonomy, ecology and sanitary importance. Elsevier, London
Nicholas KB, Nicholas HB Jr, Deerfield DW (1997) GeneDoc: analysis and visualization of genetic variation. Embnew News 4:14
Nieri-Bastos FA, Lopes MG, CancËado PHD, Rossa GAR, Faccini JLH, Gennari SM, Labruna MB (2014) Candidatus Rickettsia andeanae, a spotted fever group agent infecting Amblyomma parvum ticks in two Brazilian biomes. Mem Inst Oswaldo Cruz 109:259–261. https://doi.org/10.1590/0074-0276140283
Nieri-Bastos FA, Horta MC, Barros-Battesti DM, Moraes-Filho J, Ramirez DG, Martins TF, Labruna MB (2016) Isolation of the Pathogen Rickettsia sp. strain atlantic rainforest from its presumed tick vector, Amblyomma ovale (Acari: Ixodidae), from two areas of Brazil. J Med Entomol 53:977–981. https://doi.org/10.1093/jme/tjw062
Ogrzewalska M, Uezu A, Labruna MB (2011) Ticks (Acari: Ixodidae) infesting wild birds in the Atlantic Forest in northeastern Brazil, with notes on rickettsial infection in ticks. Parasitol Res 108:665–670. https://doi.org/10.1007/s00436-010-2111-8
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdal MY, Stenos J, Bitam I, Fournier PE, Raoult D (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702. http://cmr.asm.org/content/26/4/657
Patton JL, Pardiñas UF, D’elía G (2015) Mammals of South America: rodents, vol 2. University of Chicago Press, Chicago, p 1384
Pereira JS, Martins TF, Muñoz-Leal S, Lopes MG, Labruna MB, Paiva KAR, Oliveira MF, Ahid SMM (2017) Infestação por carrapatos Argasidae e Ixodidae em pequenos mamíferos silvestres da Estação Experimental Rafael Fernandes, Mossoró/RN. Pesqu Vet Bras 37:741–748. https://doi.org/10.1590/s0100-736x2017000700015
Regnery RL, Spruill CL, Plikaytis BD (1991) Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. Int J Bacteriol 173:1576–1589
Sangioni LA, Horta MC, Vianna MCB, Gennari SM, Soares RS, Galvão MAM, Schumaker TTS, Ferreira F, Vidotto O, Labruna MB (2005) Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 11:265–270. https://doi.org/10.3201/eid1102.040656
Saraiva DG, Nieri-Bastos FA, Horta MC, Soares HS, Nicola PA, Pereira LCM, Labruna MB (2013) Rickettsia amblyommii infecting Amblyomma auricularium ticks in Pernambuco, northeastern Brazil: isolation, transovarial transmission, and transstadial perpetuation. Vector Borne Zoonotic Dis 13:1–4. https://doi.org/10.1089/vbz.2012.1223
Silva N, Eremeeva ME, Rozental T, Ribeiro GS, Paddock CD, Ramos EAG, Favacho ARM, Reis MG, Dasch GA, de Lemos ERS, Ko AI (2011) Eschar-associated spotted fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 17:275–278. https://doi.org/10.3201/eid1702.100859
Silva AB, Cardoso KM, de Oliveira SV, Costa RMF, Oliveira G, Amorim M, Alves LC, Monteiro MFM, Gazeta GS (2018) Rickettsia amblyommatis infecting Amblyomma pseudoconcolor in area of new focus of spotted fever in northeast Brazil. Acta Trop 182:305–308. https://doi.org/10.1016/j.actatropica.2018.03.005
Spolidorio MG, Labruna MB, Mantovani E, Brandão PE, Richtzenhain LJ, Yoshinari NH (2010) Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 16:521–523. https://doi.org/10.3201/eid1603.091338
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony. Beta Version 4.0b10. Sinauer and Associates, Sunderland
Szabó MPJ, Nieri-Bastos FA, Spolidorio MG, Martins TF, Barbier AM, Labruna MB (2013) In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology 140:719–728. https://doi.org/10.1017/S0031182012002065
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Venzal JM, Estrada-Peña A, Mangold AJ, González-Acuña D, Guglielmone AA (2008) The Ornithodoros (Alectorobius) talaje species group (Acari: Ixodida: Argasidae): description of Ornithodoros (Alectorobius) rioplatensis n. sp. From southern South America. J Med Entomol 45:832–840. https://doi.org/10.1603/0022-2585(2008)45%5B832:TOATSG%5D2.0.CO;2
Venzal JM, Nava S, González-Acuña D, Mangold AJ, Muñoz-Leal S, Lado P, Guglielmone AA (2013) A new species of Ornithodoros (Acari: Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern Chile. Ticks Tick Borne Dis 4:128–132. https://doi.org/10.1016/j.ttbdis.2012.10.038
Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7:6. https://doi.org/10.1186/1741-7007-7-6
Witter R, Martins TF, Campos AK, Melo ALT, Corrêa SHR, Morgado TO, Wolf RW, May-Júnior JA, Sinkoc AL, Strüssmann C, Aguiar DM, Rossi RV, Semedo TBF, Campos Z, Desbiez ALJ, Labruna MB, Pacheco RC (2016) Rickettsial infection in ticks (Acari: Ixodidae) of wild animals in midwestern Brazil. Ticks Tick Borne Dis 7:415–423. https://doi.org/10.1016/j.ttbdis.2015.12.019
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarships of M. O. Maia, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the awarding a research productivity grant to M. B. Labruna and R. C. Pacheco, and to Geo & Bio Ambiental Ltda for financial and logistical support during fieldwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maia, M.O., Koppe, V.C., Muñoz-Leal, S. et al. Detection of Rickettsia spp. in ticks associated to wild mammals in Northeastern Brazil, with notes on an undetermined Ornithodoros sp. collected from marsupials. Exp Appl Acarol 76, 523–535 (2018). https://doi.org/10.1007/s10493-018-0323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0323-2