Abstract
From June 2013 to January 2014, blood sera samples and ticks were collected from domestic dogs and wild small mammals, and ticks from the vegetation in a preservation area of the Atlantic Forest biome (Turvo State Park), and the rural area surrounding the Park in Derrubadas municipality, state of Rio Grande do Sul, southern Brazil. Dogs were infested by Amblyomma ovale and Amblyomma aureolatum adult ticks, whereas small mammals were infested by immature stages of A. ovale, Amblyomma yucumense, Amblyomma brasiliense, Ixodes loricatus, and adults of I. loricatus. Ticks collected on vegetation were A. brasiliense, A. ovale, A. yucumense, Amblyomma incisum, and Haemaphysalis juxtakochi. Three Rickettsia species were molecularly detected in ticks: Rickettsia bellii in I. loricatus (also isolated through cell culture inoculation), Rickettsia amblyommatis in A. brasiliense, and Rickettsia rhipicephali in A. yucumense. The latter two are tick-rickettsia associations reported for the first time. Seroreactivity to Rickettsia antigens were detected in 33.5% (55/164) small mammals and 8.3% (3/36) canine sera. The present study reveals a richness of ticks and associated-rickettsiae in the largest Atlantic Forest Reserve of the state of Rio Grande do Sul, which is characterized by a rich fauna of wild mammals, typical of more preserved areas of this biome. Noteworthy, none of the detected Rickettsia species have been associated to human or animal diseases. This result contrasts to other areas of this biome in Brazil, which are endemic for tick-borne spotted fever caused by Rickettsia rickettsii or Rickettsia parkeri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Brazil, the emergence and reemergence of infections caused by rickettsial agents has been evident in some regions during recent decades (Labruna 2009; Krawczak et al. 2014, 2016a; Oliveira et al. 2016). Several factors have been associated to their emergences, including changes in the diversity and geographic distribution of animals and plants, conservation measures favoring certain species and increase of the anthropization process (Ogrzewalska et al. 2011; Barros e Silva et al. 2014; Luz et al. 2019). Rickettsioses are associated with several arthropods, such as lice, fleas, ticks and other mites and, in nature, the maintenance of the rickettsiae cycle is guaranteed by the ability of ticks to act as reservoirs and vertebrates as amplifier hosts (Parola et al. 2013).
Currently, only two tick-borne Rickettsia species of the spotted fever group (SFG) are recognized as human pathogens in Brazil: Rickettsia rickettsii, the agent of Brazilian spotted fever, and Rickettsia parkeri strain ‘Atlantic rainforest’, the agent of R. parkeri spotted fever/rickettsiosis (Parola et al. 2013; Oliveira et al. 2016; Faccini-Martínez et al. 2018). Among the five regions of Brazil, the Southern region occupies the second position in relation to the casuistry of the tick-borne rickettsioses, behind only the Southeastern region. The state of Rio Grande do Sul, located in the Southern region, occupies the seventh place in relation to the number of confirmed cases of tick-borne SFG rickettsiosis, among the 27 federative units of Brazil (Barros e Silva et al. 2014; Brazil 2022). From 2007 to 2021, Rio Grande do Sul reported 14 laboratory confirmed cases of SFG rickettsiosis distributed in seven municipalities (Brazil 2022). Several studies from this state have reported ticks of the genus Amblyomma and Haemaphysalis infected with SFG rickettsiae, namely R. parkeri sensu stricto (s.s.) in Amblyomma tigrinum and Haemaphysalis juxtakochi, R. parkeri strain Atlantic rainforest in Amblyomma ovale, and Ricketsia amblyommatis in Amblyomma longirostre (Krawczak et al. 2016c; Souza et al. 2018; Weck et al. 2020). However, these studies were carried out in areas of the Pampa biome or in transition areas between this biome and the Atlantic Forest biome. Studies aiming at detecting rickettsiae in areas of natural Atlantic Forest do not exist for the state of Rio Grande do Sul, southern Brazil.
The Atlantic Forest biome originally occupied 37% of the territory of the state of Rio Grande do Sul. However, data from the Department of Environment and Sustainable Development (https://www.sema.rs.gov.br) show that only 12.9% of natural remnants remain in relation to the original vegetation cover. Recent studies have shown that the pathogen R. parkeri strain Atlantic rainforest has affected the human population residing in preserved areas of this biome in other Brazilian states such as São Paulo, Bahia, Santa Catarina and Espírito Santo (Spolidorio et al. 2010; Silva et al. 2011; Krawczak et al. 2016b; Faccini-Martínez et al. 2020).
Here, we investigated rickettsial infection in ticks, small mammals and dogs in a natural Reserve of the Atlantic Forest biome in Rio Grande do Sul, the largest preserved area of this biome in the southernmost state of Brazil.
Materials and methods
Study area
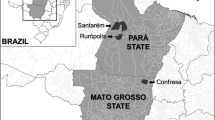
This study was performed in a deciduous forest of the Turvo State Park ‘Parque Estadual do Turvo’ (PET) (27°00’–27°20’S, 53°40’–54°10’W) and in the rural area surrounding the Park, both located within Derrubadas municipality, in the northwestern region of the Rio Grande do Sul state, Brazil. The PET was established in March 11th, 1947, as an Atlantic Forest Reserve (Law no. 2.440, of 2 October 1954), and stands out for being the largest full protection area in Rio Grande do Sul, with 17,491 ha.
The PET is located at the east bank of the Uruguay River (Fig. 1), its mean temperature during the warmest month (January) is above 22 °C, and in the coldest month (July) it ranges from −3 to 18 °C (Melo et al. 2011). In 2021, the municipality of Derrubadas had an estimated population of 2,718 habitants and the preservation area occupied by the park was approximately 50% of the municipality (https://cidades.ibge.gov.br/brasil/rs/derrubadas/panorama). The PET bears a rich fauna of medium-sized to large wild mammals, including tapirs (Tapirus terrestris), jaguar (Panthera onca), puma (Puma concolor), capybaras (Hydrochoerus hydrochaeris), collared peccaries (Dicotyles tajacu), and deer (Mazama spp.) (Kasper et al. 2007).
For the present study, domestic dogs, small mammals and ticks were collected during three field campaigns (June and October 2013, and January 2014). This study was previously approved by the Chico Mendes Institute for Biodiversity (ICMBio Permit No. 38502-1) and the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Medicine of the University of São Paulo (protocol 2908/2013) and State Secretary for the Environment of the Rio Grande do Sul (protocol 05/2013 registration number 428).
Domestic dogs
The primary reason to sample dogs was to test them by serology for the presence of reactive antibodies to SFG rickettsiae. Thus, to determine the number of dogs to be tested, a 3.16:1 ratio (number of humans/number of dogs) for rural area was used as previously described (Soto et al. 2006), and to calculate the sample size, we considered an expected prevalence of 10% of canine seropositivity to SFG rickettsiae (Labruna et al. 2007a), and 95% accuracy according to the formula n = [(z2)P(1 – P)]/d2 of Arya et al. (2012), where n = sample size, z = z statistic for the level of confidence, P = expected prevalence, and d = allowable error. This procedure indicated a minimum sample size of 29 dogs.
During the first field campaign in the rural area of Derrubadas, in the surroundings of the PET, blood serum samples were obtained from 36 dogs, which were also examined for tick infestations. All dogs were reported to have free access to forests. In the second and third field campaigns, 18 and 15 of these dogs were re-examined for tick infestations, respectively, resulting in a total of 69 canine examinations during the three field campaigns. Each dog had its entire body examined by two observers for a period of 3–5 min for the presence of ticks, which were collected in plastic tubes and transported to the laboratory for identification. The adult ticks and engorged nymphs were held alive, whereas the remaining immature ticks were stored in absolute ethanol.
Small mammals
Attempts to capture wild small mammals were performed along three trails within the deciduous forest of the PET (Fig. 1). For this purpose, a total of 80 live-traps [75 Sherman, (n = 25 in each trail) and five Tomahawk (2 in trail A, 2 in trail B and 1 in trail C)] baited with bacon, banana, apple, peanut butter and ham were installed for four consecutive nights during each field campaign. Additionally, three pitfall station traps with five bucket of 42.5 cm diameter and 60 cm height in each station connected by a plastic fence (of at least 30 m long and 50 cm high) (Umetsu et al. 2006) were installed for the same period. The total sampling effort was 960 trap-nights for live-traps and 180 trap-nights for pitfall-traps. Trapped animals were identified to species following current literature (Bonvicino et al. 2008; Melo et al. 2011), anaesthetized with ketamine and xylazine, and carefully examined for ticks, which were collected in plastic tubes and transported to the laboratory. Blood samples for serological analysis were collected by intracardiac or tail vein venipuncture from all trapped animals. Each animal was marked with a numbered earring (fish and small animal tag size 1; National Band and Tag, Newport, KY, USA), and released at the same capture site after recovery from anesthesia.
Host-seeking ticks
Host-seeking ticks were collected from vegetation in each field campaign using the cloth dragging technique and the visual search method according to Oliveira et al. (2000) and Terassini et al. (2010). Dragging was conducted by passing a cotton flannel (75 × 100 cm) over the ground level vegetation by one person through a 50–100 m trail in each campaign. The same trail was used for the visual search method, in parallel to dragging. Collected ticks were put in plastic tubes and transported to the laboratory.
Tick identification
Collected ticks were morphologically identified to species based on Marques et al. (2004), Barros-Battesti et al. (2006) and Martins et al. (2010). The morphological identification of some Amblyomma larvae was confirmed through molecular analysis. In this case, individual larval DNA was extracted by boiling (Horta et al. 2007) and tested by polymerase chain reaction (PCR) with primers targeting an approximately 460 bp fragment of the tick 16 S rDNA mitochondrial gene (Mangold et al. 1998). PCR products were DNA sequenced in an automatic sequencer (Model ABI 3500 Genetic Analyzer; Applied Biosystems/Thermo Fisher Scientific, Foster City, CA, USA) according to manufacturer’s instructions. Generated sequences were submitted to BLAST analysis to determine the closest identities available in GenBank.
Rickettsial infection in ticks
Adult ticks that arrived alive at the laboratory were stored in a −80 °C freezer until tested for isolation of rickettsiae in Vero cell culture. For this purpose, ticks were thawed at room temperature and processed by the shell vial technique, as previously described (Labruna et al. 2004). A rickettsial isolate was considered to be established in Vero cells after at least three passages at 28 °C with the prevalence of infected cells exceeding 95%. A sample of 4th passage-infected cells was submitted to DNA extraction by the DNeasy Tissue Kit (Qiagen, Chatsworth, CA, USA), and tested by a PCR protocol with primers CS-78 and CS-323, which amplify a 401-bp fragment of the rickettsial citrate synthase gene (gltA) (Labruna et al. 2004). PCR products were DNA sequenced; generated sequences were submitted to BLAST analyses, as cited above.
DNA extraction using the guanidine isothiocyanate and phenol/chloroform technique (Sangioni et al. 2005) was applied to the remnants of ticks processed by the shell vial technique, as well as other frozen or alcohol-preserved adults. Larval and nymphal ticks were processed individually by boiling (Horta et al. 2007). Tick DNA samples were tested by a TaqMan real-time PCR assay targeting a 147-bp fragment of the rickettsial gltA gene (Labruna et al. 2004; Guedes et al. 2005). Once a tick was demonstrated by real-time PCR to contain rickettsial DNA, amplification of a larger fragment of the gltA gene was attempted by two conventional PCR protocols. One used primers CS-78 and CS-323 for the gltA gene (Labruna et al. 2004), and the second protocol used primers Rr190.70 F and Rr190.701R, which amplify an approximately 632 bp fragment of the 190 kDa outer membrane protein gene (ompA) of SFG rickettsiae (Roux et al. 1996). The PCR products were sequenced and submitted to BLAST analysis to determine their closest similarities to Rickettsia sequences available in GenBank.
All real-time PCR negative tick samples were evaluated by conventional PCR, aiming to amplify an approximately 460-bp fragment of the mitochondrial 16 S rDNA gene from ticks (Mangold et al. 1998), in order to validate the presence of viable DNA in the extractions protocol. If the tick sample yielded no product by this PCR, it was considered that DNA extraction was not successful, and the individual tick was discarded from the study.
Serology
Sera samples from dogs, rodents and marsupials were tested by immunofluorescence assay (IFA) against six Rickettsia antigens isolated from Brazil, namely: Rickettsia rickettsii strain Taiaçu, R. parkeri s.s. strain At24, R. amblyommatis strain Ac37, Rickettsia rhipicephali strain HJ5, Rickettsia felis strain Pedreira, and Rickettsia bellii strain Mogi, as previously described (Labruna et al. 2007a). Briefly, sera were diluted in twofold increments with phosphate buffered saline (PBS) from an initial dilution 1:64. Slides were incubated with fluorescein isothiocyanate-labelled rabbit anti-dog IgG (Sigma, St Louis, MO, USA), goat anti-mouse IgG (Sigma) and sheep anti-opossum IgG (CCZ, São Paulo, Brazil) for canine, rodent and marsupial sera, respectively. For each sample, the endpoint IgG titer reacting with each of the six Rickettsia antigens was determined. An endpoint titer at least 4-fold higher for a Rickettsia species than that observed for any other Rickettsia species was considered probably homologous to the first Rickettsia species or to a very closely related species (Labruna et al. 2007a; Szabó et al. 2013). In each slide, a serum previously shown to be non-reactive (negative control) and a known reactive serum (positive control), both from the studies of Szabó et al. (2013) and Krawczak et al. (2016c), were tested at the 1:64 dilution.
Results
Ticks from animals
During the period from June 2013 to January 2014, three field campaigns were carried out, encompassed a study period of 6 months; for this reason, all data were pooled for presentation. From a total of 69 canine examinations, 17% (12/69) revealed tick infestations, which were identified as adults of Amblyomma aureolatum (one male on one dog; 1.5% infestation rate) or A. ovale (17 males and 17 females on 11 dogs; 16%).
A total of 164 small mammals of nine species (two marsupials and seven rodents) were captured, of which 23 (14%) were infested by ticks. Considering the host species that yielded > 1 captured specimen, tick infestations were detected on 100% (7 infested/7 captured) of the marsupial Didelphis aurita (black-eared opossum), 57% (4/7) of the rodent Oxymycterus quaestor = Oxymycterus judex (quaestor hocicudo), 50% (2/4) of the rodent Sooretamys angouya (Paraguayan rice rat), 11% (1/9) of the rodent Oligoryzomys nigripes (pygmy rice rat), 8% (1/13) of the rodent Brucepattersonius iheringi (Ihering’s hocicudo), 5% (6/117) of the rodent Akodon montensis (montane grass mouse), and 0% (0/5) of the rodent Thaptomys nigrita (blackish grass mouse). The single captured specimen of the rodent Euryoryzomys russatus (big-headed rice rat) and the marsupial Cryptonanus guahybae (Guahiba gracile opossum) were both infested by ticks.
Four tick species were found infesting small mammals (Table 1). The most frequent and abundant tick species was A. yucumense, of which larvae and nymphs were collected on the rodent O. quaestor (22 larvae, 11 nymphs) and the marsupial D. aurita (56 larvae, 53 nymphs), and only nymphs were collected on the rodents A. montensis (one nymph), E. russatus (one nymph) and S. angouya (two nymphs). Although most of these records of A. yucumense were previously reported by Krawczak et al. (2015), the following specimens are here reported for the first time: 22 larvae and three nymphs from O. quaestor, 23 larvae from D. aurita, and one nymph from S. angouya; these larvae were identified to species level by generating 16 S rDNA partial sequences, which were 99–100% identical to available sequences of A. yucumense in GenBank (KJ914670, MH282856).
Ixodes loricatus was the second most abundant tick species, with immature stages on two rodent species [A. montensis (three larvae, two nymphs) and O. nigripes (one larva)], and adults (eight males, 17 females) on the marsupial D. aurita. Here we report for the first-time larvae and nymphs of I. loricatus on A. montensis. These four larvae were also identified in the molecular level, generating 16 S rDNA partial sequences that were 99% identical to sequences of I. loricatus from GenBank (AF549840, KX137895). Two other tick species were each found on a single host species: Amblyomma brasiliense (eight nymphs) on D. aurita and A. ovale (one nymph) on S. angouya. Finally, 90 larvae could not be identified to species level and were retained as Amblyomma sp.
Host-seeking ticks
A total of 319 ticks were collected on vegetation and were identified as A. brasiliense (20 larvae, 61 nymphs, six males, 10 females), Amblyomma incisum (62 nymphs, 15 males, 19 females), A. ovale (three males), A. yucumense (eight nymphs, 13 males, nine females), Haemaphysalis juxtakochi (seven nymphs, two females), and Amblyomma sp. (83 larvae). The 20 A. brasiliense larvae derived from a cluster found on vegetation, and their identification to species relied on a 16 S rRNA gene partial sequence that was generated from this larval pool, which was 100% identical to a corresponding sequence of A. brasiliense from GenBank (FJ424399). Although not foreseen, taxonomic identification of a H. juxtakochi nymph was also confirmed by molecular analysis, generating a 16 S rRNA partial sequence 99% identical to H. juxtakochi from GenBank (AY762323). The above-mentioned host-seeking specimens of A. yucumense were previously reported by Krawczak et al. (2015).
Rickettsial infection in ticks
Attempts to isolate rickettsiae in Vero cell culture were performed individually with the following adult ticks: two A. brasiliense from vegetation, two A. incisum from vegetation, five A. ovale from dogs, two A. yucumense from vegetation, two H. juxtakochi from vegetation and two I. loricatus from marsupial D. aurita. Rickettsiae were successfully established in Vero cell culture only from one I. loricatus tick. By PCR, the infected Vero cells generated a 350 bp fragment (excluding primer sequences) of the rickettsial gltA, which was 100% identical to a corresponding sequence of R. bellii from GenBank (DQ146481). This isolate was further molecularly characterized as R. bellii strain IL-RS1, in another study that performed broader genotypic characterization of R. bellii isolates (Krawczak et al. 2018).
From a total of 266 tick specimens tested by real-time PCR, rickettsial DNA was detected in 22 samples (Table 2), which were further tested by conventional PCR targeting fragments of two rickettsial genes, gltA and ompA. PCR products were DNA sequenced from the 22 ticks, and when submitted to BLAST analyses, the gltA (350 bp) and ompA (588 bp) fragments amplified from A. brasiliense (larvae, nymphs and adults) were 100% identical to available sequences of R. amblyommatis in GenBank (CP015012 and KX434739, respectively). The gltA (350 bp) and ompA (488 bp) fragments amplified from A. yucumense (larvae, nymphs and adults) and H. juxtakochi (nymphs) were 100% identical to available sequences of R. rhipicephali in GenBank (CP013133). The gltA (350 bp) fragment amplified from I. loricatus (adults) and H. juxtakochi nymphs were 100% identical to available sequences of R. bellii in GenBank (CP000849).
Serology
Serum samples were obtained from 198 individuals (36 dogs and 162 small mammals) and tested by IFA against six rickettsial antigens. Overall, the proportions of seroreactive animals to SFG antigens were 24% for R. parkeri, 23% for R. rickettsii, 21% for R. amblyommatis and 17% for R. rhipicephali. Then, only 9% of the sera reacted to R. bellii and none to R. felis antigens (Table 3). Among 36 dogs, only 2 (5.5%) reacted to R. rhipicephali (endpoint titers: 64 and 256) and 1 (2.8%) to R. bellii (endpoint titer: 256). Regarding the small mammals, a total of 116 sera of A. montensis were tested, of which 34 (29%) were reactive to R. parkeri (endpoint titers: 64 to 512) and R. rickettsii (64 to 512), 30 (27%) to R. amblyommatis (64 to 512), 21 (18%) to R. rhipicephali (64 to 512), and 15 (13%) to R. bellii (64 to 1024). Among 12 tested sera of B. iheringi, six (50%) were reactive to R. rickettsii (endpoint titers: 256 to 4096) and R. rhipicephali (128 to 2048), and five (42%) were reactive to R. parkeri (128 to 1024) and R. amblyommatis (128 to 2048). Among seven tested sera of O. quaestor, six (86%) were reactive to R. parkeri (endpoint titers: 64 to 256), R. rickettsii (256 to 512) and R. amblyommatis (128 to 512), five (71%) were reactive to R. rhipicephali (128 to 2048), and one (14%) to R. bellii (128). Only two (29%) out of D. aurita were reactive to R. parkeri (endpoint titers: 128), whereas none of nine O. nigripes, five T. nigrita, four S. angouya, one C. guahybae, or one E. russatus was reactive to any rickettsial antigen (Table 3).
One dog had endpoint titers to R. rhipicephali at least 4-fold higher than those observed for the other five Rickettsia species, indicating a possible homologous reaction to R. rhipicephali or a closely related species. Using the same criterion, one dog and three A. montensis were exposed to R. bellii or a closely related species, two D. aurita were exposed to R. parkeri or a closely related species, and one O. nigrita was exposed to R. rickettsii or a closely related species (Table 3).
Accession numbers
GenBank accession numbers for the DNA partial sequences generated in the present study are KX434748 − KX434754 for the 16 S rRNA gene of A. yucumense, A. brasiliense, H. juxtakochi, I. loricatus; KX434741, KX434739 for the gltA and ompA genes of R. amblyommatis, KX434744, KX434745 for the gltA gene of R. rhipicephali, KX434735, KX434736 for the ompA gene of R. rhipicephali; and KX434740 for the gltA gene of R. bellii. Voucher specimens of the tick species collected in the present study have been deposited in the tick collection ‘Coleção Nacional de Carrapatos Danilo Gonçalves Saraiva’ (CNC) of the University of São Paulo, under accession numbers CNC 3323–3331.
Discussion
Through the investigation of ticks and rickettsial infection/exposure in a natural Reserve of the Atlantic Forest biome and its surroundings in the Rio Grande do Sul, we found a richness of seven tick species harboring three Rickettsia species, and serological evidence of rickettsial exposure in domestic dogs and small mammals.
The two tick species found on dogs in the present study, A. aureolatum and A. ovale, have been reported in another area of the Atlantic Forest biome in southern Brazil (Barbieri et al. 2014). In both studies, there was a predominance of A. ovale, which is implicated as the most important vector of R. parkeri strain Atlantic rainforest in southern Brazil (Barbieri et al. 2014; Krawczak et al. 2016b; Voizzoni et al. 2016). In endemic areas for tick-borne spotted fever of southern Brazil, 20–60% of the dogs were reported to be seroreactive to SFG rickettsiae, with highest endpoint titers to R. parkeri (Barbieri et al. 2014; Krawczak et al. 2016b). Herein, only three (8%) out of 36 rural dogs, with free access to forest, were reactive to rickettsiae; however, none of them was reactive to R. parkeri or R. rickettsii, the agents that cause tick-borne SFG rickettsiosis in Brazil (Parola et al. 2013). Moreover, we found no SFG agent infecting A. ovale ticks in the present study. These results indicate that the surroundings of the PET are not endemic for R. parkeri rickettsiosis, in contrast to the transition area of Atlantic Forest and Pampa biomes of Rio Grande do Sul, where human cases of rickettsiosis have been reported and 15% of the A. ovale ticks from dogs were infected by R. parkeri strain Atlantic rainforest (Krawczak et al. 2016b). In other R. parkeri rickettsiosis-endemic areas of Brazil, 8–15% infection rates by R. parkeri strain Atlantic rainforest have been reported in A. ovale ticks (Szabó et al. 2013; Barbieri et al. 2014).
Immature stages of A. yucumense were found on four rodent species (A. montensis, E. russatus, O. quaestor, S. angouya) and one marsupial (D. aurita). Moreover, > 70% of the larvae and nymphs were collected from D. aurita, suggesting a more important role as host for immature stages of A. yucumense. Contrastingly, hosts for adults of A. yucumense have never been reported; however, their host-seeking behavior inside the forest is compatible with ticks of tapirs (Krawczak et al. 2015). Regarding I. loricatus, our records of immature stages parasitizing two rodent species (A. montensis, O. nigripes) and the adult stage on D. aurita agrees with the typical host pattern reported for I. loricatus in South America, i.e., immature stages on Cricetidae rodents, and adult ticks on marsupials, chiefly Didelphis spp. (Nava et al. 2017).
Our findings of nymphs of A. brasiliense on D. aurita is supported by a recent study in the Argentinean Atlantic Forest, where D. aurita was reported as occasional hosts for immature stages of A. brasiliense (Lamattina et al. 2018). While a broad range of mammal hosts has been reported for A. brasiliense (Guglielmone et al. 2021), all records of have been from areas inhabited by peccaries (D. tajacu and/or Tayassu pecari), suggesting that these mammals are primary hosts for this tick species (Szabó et al. 2009). The present record of a nymph of A. ovale on the rodent S. angouya also agrees with current literature, which includes a vast list of Cricetidae species as hosts for immature stages of A. ovale, whose adults are primarily associated with Carnivora (Martins et al. 2016; Guglielmone et al. 2021).
Among the five tick species collected from vegetation, A. brasiliense, A. ovale, A. yucumense, A. incisum, and H. juxtakochi, only the latter two were not collected from small mammals. This result is expected for A. incisum, as one study in another Atlantic Forest Reserve showed that larvae, nymphs and adults of this tick species quest for hosts on the vegetation at heights usually above 30 cm, suggesting that they are primarily associated to large mammals, i.e., tapirs and peccaries (Szabó et al. 2009). In fact, A. incisum has never been reported on small rodents (Guglielmone et al. 2021). Although H. juxtakochi is primarily associated to deer (Mazama spp.), there have been a few records of larvae and nymphs on small mammals (Guglielmone et al. 2021). Therefore, the absence of H. juxtakochi on small mammals of the present study could be a result of low tick density, as this tick represented < 3% of the collected host-seeking ticks.
Regarding the Rickettsia species detected in ticks of the present study, the presence of R. bellii in I. loricatus is corroborated by several studies in southeastern and southern Brazil (Horta et al. 2007; Szabó et al. 2013; Krawczak et al. 2016b), suggesting a widespread tick-rickettsia association among I. loricatus populations. Similarly, our findings of R. rhipicephali in H. juxtakochi is corroborated by previous studies in southeastern, midwestern and northern Brazil (Labruna et al. 2007b; Soares et al. 2015; Acosta et al. 2016). On the other hand, we report two tick-rickettsia association for the first time, R. rhipicephali in A. yucumense, and R. amblyommatis in A. brasiliense. Indeed, the latter adds A. brasiliense to the broad list of Amblyomma species that have been found infected by R. amblyommatis in Central and South America (Parola et al. 2013; Soares et al. 2015; Binetruy et al. 2020; Bermúdez et al. 2021).
Serological analyses revealed that a few dogs, opossums (D. aurita) and some individuals of three rodent species (A. montensis, B. iheringi, O. quaestor) were reactive to rickettsial antigens, indicating previous exposure to Rickettsia spp. Since there are serological cross-reactions between different Rickettsia species notably within the SFG (Parola et al. 2013), most of the seroreactive animals reacted to two or more Rickettsia antigens, although this result could also be strengthened by the exposure to multiple species of Rickettsia. The fact that two D. aurita reacted solely to R. parkeri antigens, and a single O. quaestor reacting to R. rickettsii with endpoint titers ≥ 4-fold higher than those observed for the other five Rickettsia species do not necessarily indicate exposure to these specific rickettsial pathogens, as it could be exposure to closely related agents, pathogenic or not, yet to be investigated in the study area. On the other hand, the ≥ 4-fold higher antibody titers to R. rhipicephali in a dog or to R. bellii in three A. montensis and one dog suggest previous exposure to these specific agents because they were shown to be present in the ticks of the study area.
None of the tested dogs or small mammals were reactive to R. felis, an agent primarily associated with fleas of the genus Ctenocephalides infesting dogs in all regions of Brazil (Horta et al. 2014). It is noteworthy that some of the sampled dogs of the present study were infested by fleas (Ctenocephalides sp.), which were collected from two dogs and shown in our laboratory to harbor ‘Candidatus Rickettsia asemboensis’ (GenBank acc. nr. KX533943) (data not shown). Indeed, ‘Ca. R. asemboensis’ is an agent very closely related to R. felis (Jiang et al. 2013); therefore, if this agent had infected the sampled dogs, we would have detected some serological reactiveness to R. felis.
The present study reveals a richness of ticks and associated-rickettsiae in the largest Atlantic Forest Reserve of the state of Rio Grande do Sul, which is characterized by a rich fauna of wild mammals (tapirs, peccaries, jaguar), typical of more preserved areas of this biome. Noteworthy, none of the detected Rickettsia species have been associated to human or animal diseases. This result contrasts to other areas of this biome in Brazil, which are endemic for tick-borne spotted fever caused by R. rickettsii (Ogrzewalska et al. 2012; Luz et al. 2019) or R. parkeri (Barbieri et al. 2014; Krawczak et al. 2016b). These spotted fever-endemic areas have in common a notable anthropization process, reflected by more fragmented areas of the Atlantic Forest, devoid of a rich fauna of wild large mammals. Similarly, to the present study, studies in the largest Atlantic Forest Reserve of the state of São Paulo (southeastern Brazil) also revealed a richness of ticks and associated-rickettsiae, albeit not R. rickettsii or R. parkeri (Labruna et al. 2007b; Pacheco et al. 2008, 2011; Szabó et al. 2009). Future studies are warranted to investigate key elements that could trigger the emergence of tick-borne spotted fever in areas of the Atlantic Forest biome.
Data Availability
The data presented in this study are available within the article.
References
Acosta ICL, Martins TF, Marcili A et al (2016). Ticks (Acari: Ixodidae, Argasidae) from humans, domestic and wild animals in the state of Espírito Santo, Brazil, with notes on rickettsial infection. Vet Parasitol Reg Stud Reports. 3–4:66–69. https://doi.org/10.1016/j.vprsr.2016.08.001
Arya R, Antonisamy B, Kumar S (2012) Sample size estimation in prevalence studies. Indian J Pediatr 79:1482–1488. https://doi.org/10.1007/s12098-012-0763-3
Barbieri ARM, Filho JM, Nieri-Bastos FA et al (2014) Epidemiology of Rickettsia sp. strain Atlantic rainforest in a spotted fever-endemic area of southern Brazil Ticks Tick Borne Dis. 5:848–853. https://doi.org/10.1016/j.ttbdis.2014.07.010
Barros-Battesti, Darci Moraes, Arzua MBGH (2006) Comentários e chaves para as espécies do gênero Amblyomma, In: Barros-Battesti DM, Arzua M, Bechara GH. (Eds.) Carrapatos de importância médico-veterinária da Região Neotropical: um guia ilustrado para identificação de espécies. Vox/ICTTD-3/Butantan, São Paulo. 223p. https://repositorio.butantan.gov.br/handle/butantan/3153 (accessed 18 July 2022)
Barros e Silva PM, Pereira S, Fonseca L, et al (2014) Febre maculosa: uma análise epidemiológica dos registros do sistema de vigilância do Brasil. Scientia Plena 10:1–9. https://www.scientiaplena.org.br/sp/article/view/1758
Bermúdez SC, Zaldivar Y, Domínguez LA et al (2021) Rickettsia amblyommatis isolated from Amblyomma mixtum (Acari: Ixodida) from two sites in Panama. Ticks Tick Borne Dis 12:101597. https://doi.org/10.1016/j.ttbdis.2020.101597
Binetruy F, Buysse M, Barosi R, Duron O (2020) Novel Rickettsia genotypes in ticks in French Guiana, South America. Sci Rep. 10(1):2537. https://doi.org/10.1038/s41598-020-59488-0
Bonvicino CR, Oliveira JA, D’Andrea PS (2008) Guia dos roedores do Brasil, com chaves para gêneros baseadas em caracteres externos. Organização Pan-Americana da Saúde. 120p. http://www.fiocruz.br/ioc/media/livro%20roedores.pdf (accessed 18 July 2022)
Brazil (2022) Ministério da Saúde. Casos confirmados de febre maculosa. Brasil, Grandes Regiões e Unidades Federadas. 2007 a 2021*. Sistema de Informação de Agravos de Notificação. https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/f/febre-maculosa-1/arquivos/casos_conf_anexo1.pdf (accessed 02 July 2022)
Faccini-Martínez ÁA, Muñoz-Leal S, Krawczak FS, et al (2020) Epidemiological aspects of Rickettsia parkeri in the Atlantic Forest biome of Espírito Santo state, Brazil. Ticks Tick Borne Dis 11:101319. https://doi.org/10.1016/j.ttbdis.2019.101319
Faccini-Martínez ÁA, Oliveira SV, Junior CC, Labruna MB (2018) Rickettsia parkeri spotted fever in Brazil: Epidemiological surveillance, diagnosis and treatment. J. Health Biol Sci 6(3): 299–312. https://doi.org/10.12662/2317-3076jhbs.v6i3.1940
Guedes E, Leite RC, Prata MCA, et al (2005) Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem Inst Oswaldo Cruz 100:841–845. https://doi.org/10.1590/S0074-02762005000800004
Guglielmone A, Nava S, Robbins R (2021) Neotropical hard ticks (Acari: Ixodida: Ixodidae); a critical analysis of their taxonomy, distribution, and host relationships. Springer International Publishing: Berlin/Heidelberg, Germany, 486 pp
Horta MC, Labruna MB, Pinter A et al (2007) Rickettsia infection in five areas of the state of São Paulo, Brazil. Mem Inst Oswaldo Cruz 102:793–801. https://doi.org/10.1590/S0074-02762007000700003
Horta MC, Ogrzewalska M, Azevedo MC et al (2014) Rickettsia felis in Ctenocephalides felis felis from five geographic regions of Brazil. Am J Trop Med Hyg. 91(1):96–100. https://doi.org/10.4269/ajtmh.13-0699
Jiang J, Maina AN, Knobel DL et al (2013) Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya (2013) Vector Borne Zoonotic Dis. 13(8):550–558. https://doi.org/10.1089/vbz.2012.1123
Kasper CB, Mazim FB, Soares JBG et al (2007). Composição e abundância relativa dos mamíferos de médio e grande porte no Parque Estadual do Turvo, Rio Grande do Sul, Brasil. Revista Brasileira de Zoologia 24(4):1087–1100. https://doi.org/10.1590/S0101-81752007000400028
Krawczak FS, Nieri-Bastos FA, Nunes FP, et al (2014) Rickettsial infection in Amblyomma cajennense ticks and capybaras (Hydrochoerus hydrochaeris) in a Brazilian spotted fever-endemic area. Parasites and Vectors 7:1–7. https://doi.org/10.1186/1756-3305-7-7
Krawczak FS, Martins TF, Oliveira CS, et al (2015) Amblyomma yucumense n. sp. (Acari: Ixodidae), a parasite of wild mammals in Southern Brazil. J Med Entomol 52:28–37. https://doi.org/10.1093/jme/tju007
Krawczak FS, Agostinho WC, Polo G, et al (2016a) Comparative evaluation of Amblyomma ovale ticks infected and noninfected by Rickettsia sp. strain Atlantic rainforest, the agent of an emerging rickettsiosis in Brazil. Ticks Tick Borne Dis 7:502–507. https://doi.org/10.1016/j.ttbdis.2016.02.007
Krawczak FS, Binder LC, Oliveira CS, et al (2016b) Ecology of a tick-borne spotted fever in southern Brazil. Exp Appl Acarol 70:219–229. https://doi.org/10.1007/s10493-016-0070-1
Krawczak FS, Muñoz-Leal S, Guztzazky AC, et al (2016c) Case report: Rickettsia sp. strain Atlantic rainforest infection in a patient from a spotted fever-endemic area in southern Brazil. Am J Trop Med Hyg 95:551–553. https://doi.org/10.4269/ajtmh.16-0192
Krawczak FS, Labruna MB, Hecht JA et al (2018). Genotypic characterization of Rickettsia bellii reveals distinct lineages in the United States and South America. BioMed Research International. https://doi.org/10.1155/2018/8505483
Labruna MB (2009) Ecology of rickettsia in South America. Ann. N. Y. Acad. Sci. 1166:156–166. https://doi.org/10.1111/j.1749-6632.2009.04516.x
Labruna MB, Whitworth T, Horta MC, et al (2004) Rickettsia Species Infecting Amblyomma cooperi Ticks from an Area in the State of São Paulo, Brazil, Where Brazilian Spotted Fever Is Endemic. J Clin Microbiol 42:90–98. https://doi.org/10.1128/JCM.42.1.90-98.2004
Labruna MB, Horta MC, Aguiar DM, et al (2007a) Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro Municipality, Western Amazon, Brazil. Vector-Borne Zoonotic Dis 7:249–255. https://doi.org/10.1089/vbz.2006.0621
Labruna MB, Pacheco RC, Richtzenhain, LJ et al (2007b) Isolation of Rickettsia rhipicephali and Rickettsia bellii from Haemaphysalis juxtakochi ticks in the state of São Paulo, Brazil. Appl. Environ. Microbiol. 73: 869–873. https://doi.org/10.1128/AEM.02249-06
Lamattina, D., Venzal, J.M., Costa, S.A., Arrabal, J.P., Flores, S., Berrozpe, P.E., González-Acuña, D., Guglielmone, A.A. & Nava, S. (2018) Ecological characterization of a tick community across a landscape gradient exhibiting differential anthropogenic disturbance in the Atlantic Forest ecoregion in Argentina. Medical and Veterinary Entomology 32(3): 271–281. https://doi.org/10.1111/mve.12295
Luz HR, Costa FB, Benatti HR, Ramos VN, de A. Serpa MC, et al. (2019) Epidemiology of capybara-associated Brazilian spotted fever. PLOS Neglected Tropical Diseases 13(9): e0007734. https://doi.org/10.1371/journal.pntd.0007734
Mangold AJ, Bargues MD M-CS (1998) Mitochondrial 16S rDNAsequences and phylogenetic relationships of species of Rhipicephalus and othertick genera among Metastriata (Acari: Ixodidae). Parasitol Res 84:478–484. https://doi.org/10.1007/s004360050433
Marques S, Barros-Battesti DM, Onofrio VC et al (2004). Redescription of larva, nymph and adults of Ixodes (I.) loricatus Neumann, 1899 (Acari: Ixodidae) based on light and scanning electron microscopy. Syst Parasitol. 59(2):135 − 46. https://doi.org/10.1023/B:SYPA.0000044430.05551.44
Martins TF, Onofrio VC, Barros-Battesti DM et al (2010) Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 1:75–99. https://doi.org/10.1016/j.ttbdis.2010.03.002
Martins, TF et al. (2016) Carrapatos infestando pequenos roedores silvestres em três municípios do estado de São Paulo, Brasil. Ciência Rural 46(5):871–875. https://doi.org/10.1590/0103-8478cr20150671
Melo GL, Sponchiado J, Machado AF et al (2011) Small-mammal community structure in a South American deciduous Atlantic Forest. Community Ecol 12:58–66. https://doi.org/10.1556/ComEc.12.2011.1.8
Nava S, Venzal JM, Acuña DG, Martins TF, Guglielmone AA (2017) Ticks of the Southern Cone of America: diagnosis, distribution, and hosts with taxonomy, ecology and sanitary importance. Academic Press: London, 348 pp.
Ogrzewalska M, Uezu A, Jenkins CN et al (2011) Effect of forest fragmentation on tick infestations of birds and tick infection rates by Rickettsia in the Atlantic Forest of Brazil. Ecohealth 8:320–331. https://doi.org/10.1007/s10393-011-0726-6
Ogrzewalska M, Saraiva DG, Moraes-Filho J et al. (2012). Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of São Paulo, Brazil. Parasitology 139(10):1283 − 300. https://doi.org/10.1017/S0031182012000546
Oliveira PR, Borges LMF, Lopes CML et al (2000) Population dynamics of the free-living stages of Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) on pastures of Pedro Leopoldo, Minas Gerais State, Brazil. Vet Parasitol 92:295–301. https://doi.org/10.1016/S0304-4017(00)00322-8
Oliveira SV, Guimarães JN, Reckziegel GC, et al (2016) An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 22:1–8. https://doi.org/10.1186/s40409-016-0077-4
Pacheco R, Rosa S, Richtzenhain L, MPJ et al. (2008) Isolation of Rickettsia bellii from Amblyomma ovale and Amblyomma incisum ticks from southern Brazil. Rev. MVZ Córdoba 13:1273–1279.
Pacheco RC, Moraes-Filho J, Marcili A et al (2011). Rickettsia monteiroi sp. nov., infecting the tick Amblyomma incisum in Brazil. Appl Environ Microbiol 77(15):5207-11. https://doi.org/10.1128/AEM.05166-11
Parola P, Paddock CD, Socolovschi C, et al (2013) Update on tick-borne rickettsioses around the world: A geographic approach. Clin Microbiol Rev 26:657–702. https://doi.org/10.1128/CMR.00032-13
Roux V, Fournier PE, Raoult D (1996) Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 34:2058–2065. https://doi.org/10.1128/jcm.34.9.2058-2065.1996
Sangioni LA, Horta MC, Vianna MCB, et al (2005) Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis 11:265–270. https://doi.org/10.3201/eid1102.040656
Silva N, Eremeeva ME, Rozental T, et al (2011) Eschar-associated spotted fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 17:275–278. https://doi.org/10.3201/eid1702.100859
Soares HS, Barbieri AR, Martins TF, Minervino AH, de Lima JT, Marcili A, Gennari SM, Labruna MB (2015) Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Exp Appl Acarol 65:125–140. https://doi.org/10.1007/s10493-014-9851-6
Soto FRM, Ferreira F, Pinheiro SR, et al (2006) Dinâmica populacional canina no Município de Ibiúna-SP estudo retrospectivo. Brazilian J Vet Res Anim Sci 43:178. https://doi.org/10.11606/issn.1678-4456.bjvras.2006.26497
Souza U, Dall’Agnol B, Michel T, et al (2018) Molecular survey of Rickettsia spp. in the Neotropical deer tick Haemaphysalis juxtakochi from Brazilian Pampa. Parasitol Res 117: 3293–3298. https://doi.org/10.1007/s00436-018-5996-2
Spolidorio MG, Labruna MB, Mantovani E et al (2010) Novel spotted fever group rickettsiosis, Brazil. Emerg Infect Dis 16:521–523. https://doi.org/10.3201/eid1603.091338
Szabó MP, Labruna MB, Garcia MV et al. (2009). Ecological aspects of the free-living ticks (Acari: Ixodidae) on animal trails within Atlantic rainforest in south-eastern Brazil. Ann Trop Med Parasitol. 103(1):57–72. https://doi.org/10.1179/136485909X384956
Szabó MP, Nieri-Bastos FA, Spolidorio MG et al (2013) In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology 140(6):719 − 28. https://doi.org/10.1017/S0031182012002065
Terassini FA, Barbieri FS, Albuquerque S, et al (2010) Comparison of two methods for collecting free-living ticks in the Amazonian Forest. Ticks Tick Borne Dis 1:194–196. https://doi.org/10.1016/j.ttbdis.2010.08.002
Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the neotropics. J Mammal 87:757–765. https://doi.org/10.1644/05-MAMM-A-285R2.1
Voizzoni VF, Silva AB, Cardoso KM et al (2016). Genetic identification of Rickettsia sp. strain Atlantic rainforest in an endemic area of a mild spotted fever in Rio Grande do Sul state, Southern Brazil. Acta Trop.162:142–145. https://doi.org/10.1016/j.actatropica.2016.06.018
Weck B, Krawczak FS, Costa FB et al (2020) Rickettsia parkeri in the Pampa biome of southern Brazil: Isolation, molecular characterization, and serological evidence of canine infection. Vet Parasitol Reg Stud Reports 22:100448. https://doi.org/10.1016/j.vprsr.2020.100448
Funding
This work was financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP process 2012/21915-4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq process 474307/2013-1), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES Finance Code 001).
Author information
Authors and Affiliations
Contributions
FSK and MBL conceived and designed the study, and critically revised the manuscript. FSK, CS, FBC, FG, GLM, GP, GTP, JS and LCB performed the experiment, analyzed the data, and drafted the manuscript. FSK, CS, FBC, LCB, JS, MBL and TFM helped in the implementation and execution of the study. FSK, LCB, MBL, FG and TFM performed and interpreted the laboratory analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was previously approved by the Chico Mendes Institute for biodiversity (ICMBio Permit No. 38502-1) and the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Medicine of the University of São Paulo (protocol 2908/2013) and State Secretary for the Environment of the Rio Grande do Sul (protocol 05/2013 registration number 428).
Consent for publication
All authors consent to publication of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krawczak, F.d.S., Binder, L.C., Sobotyk, C. et al. Rickettsial infection in ticks from a natural area of Atlantic Forest biome in southern Brazil. Exp Appl Acarol 88, 371–386 (2022). https://doi.org/10.1007/s10493-022-00754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00754-3