Abstract
The current concern about resistance to acaricides and the impact of toxic waste on the environment has led to the search of vegetal alternatives in the control of the brown tick of the dog Rhipicephalus sanguineus. Schinus molle L. (Anacardiaceae) derivatives have been associated with insecticidal, antimicrobial and antiprotozoal activities and essential oil showed to be lethal to R. microplus larvae. This study aimed at evaluating the acaricidal effect of essential oil of S. molle (EOSm) on engorged adult females and larval stages of R. sanguineus. One-hundred engorged females were obtained from the ears, interdigital spaces, neck, groin and base of the tail of two cross-bred dogs. The larvae package test was accomplished with 21-day-old larvae and five concentrations (v/v) of EOSm (0.125, 0.25, 0.50, 1 and 2%) in an anionic detergent, a synthetic acaricide (cypermethrin) and detergent and deionized water as controls. The immersion adult test was carried out with nine concentrations (0.125, 0.25, 0.50, 1, 2, 4, 8, 16, 20%) of the EOSm. At the concentration of 2%, EOSm caused 99.3% of larval mortality. In adults, inhibition of oviposition, egg hatching (EH) and reproductive efficiency (RE) values were dose-dependent from 4 to 20% EOSm; the lowest values of EH (29.62) and RE (22.61) were achieved with 20% EOSm. Strong and negative correlations were found between concentration of EOSm and EH (r = − 0.948) and between concentration of EOSm and RE (r = − 0.985). This study demonstrated for the first time the acaricidal effect of EOSm on larvae and reproductive parameters of engorged adult females of R. sanguineus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhipicephalus sanguineus (s.l.) is the most widespread tick in human dwellings and of great importance as a biological vector of several pathogens that cause severe infections in canines as well in humans (Otranto et al. 2009). The control of the tick is quite difficult due to its ethology: R. sanguineus has an endophilic behavior; a fully engorged adult female may oviposit up to 4000 eggs which are deposited in hidden places and almost inside the ground (Dantas-Torres 2010); due to the cryptic behavior and the small size of the larvae and nymphs, low-level indoor infestations can be difficult to detect, allowing populations to increase rapidly (Eiden et al. 2015).
Industrial, synthetic acaricides have long been used to effectively control the parasitic stages of the tick. However, these molecules have several disadvantages, including development of tick resistance and permanence of residues in the animals and in the environment. Hence, the effect of plant derivatives on several cellular and animal models has been intensively studied in the last years (Bakkali et al. 2008; Castelblanco et al. 2013; Pavela et al. 2016). Compounds from plants are an alternative to industrial, synthetic products; tick resistance usually develops slowly because they are a mixture of several active agents with different mechanisms of action (Borges et al. 2011). Plants accumulate organic substances in significant quantities and concentrations and are a renewable source of these substances; hence, they can be exploited economically and sustainably (Da Silva et al. 2016). The use of plant derivatives in the control of veterinary ectoparasites is an area which holds considerable potential for the future and research into their use is still at an early stage (Ellse and Wall 2014).
Essential oils (EOs) are natural compounds with an important component of volatile molecules with strong odor and generally soluble in organic solvents. EOs are composed primarily of terpenes, terpenoids and other aromatic compounds; two or three major components are at fairly high concentrations (20–70%) compared to others components present in trace amounts. Antimicrobial, insecticide and acaricide effects have been exhaustively demonstrated (Andreotti et al. 2013; Kačániová et al. 2014; Martins et al. 2014; Wanzala et al. 2014).
Schinus molle L. (Anacardiaceae) is known as Peru tree, bolilla, pirú, preconcuahuitl, copalquahuitl, yag lachi ntaka (popoloca), molle, Californian pepper and pink pepper. The molle was the sacred tree of the Incas; they used to plant it on the periphery of their palaces and public buildings (De Mendonça et al. 2012). The tree grows from sea level to 3500 m a.s.l. from Southern Mexico to Northern Chile and Central Argentina in America and in many other tropical and subtropical regions. The red fruits are aromatic and have a peppery, pungent and sharp flavor; fruits contain an essential oil rich in hydrocarbon monoterpenes which contribute to the citrus aroma and sesquiterpenes that provide the pungent woody odor (Bernhard et al. 1983). The “molle” has been used in ethnobotanical medicine to treat bad air and a myriad of disorders, from toothache to labor pain because of its antibacterial, antiviral, antiseptic, topical, antifungal, antioxidant, anti-inflammatory, antitumor and analgesic activities (Tene et al. 2007). The antimicrobial activity of S. molle essential oil (EOSm) has been demonstrated in Gram +, Gram − and in some fungi (Martins et al. 2014), as well as on Trypanosoma cruzi (Molina-Garza et al. 2014). There are reports on the activity of molle as an insecticide on mosquito species such as Aedes aegypti or the housefly Musca domestica (Wimalaratne et al. 1996; Chantraine et al. 1998).
In order to exploit an alternative mechanism of control of dog ticks using plant derivatives, this work aimed at evaluating the acaricidal effect of the EO obtained from fruits of S. molle L. on the larval stages and engorged adult females of the brown tick of the dog, R. sanguineus.
Materials and methods
Isolation and characterization of the essential oil of Schinus molle (EOSm)
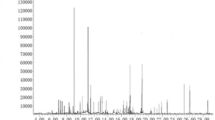
Fruits of S. molle in maturity state were collected in three locations from Catamayo (3°58′S, 79°21′O), Province of Loja; Cuenca (2°54′S, 79°02′O), Province of Azuay and Salcedo (1°02′S, 78°35′O), Province of Tungurahua, Ecuador. The EOSm was isolated from 300 g of mesocarp from fresh fruits by hydrodistillation using a Clevenger-type apparatus for three hours and dried over anhydrous sodium sulfate. EOSm was stored in sealed vials protected from the light at 4 °C until further analysis. Characterization of EOSm was accomplished by (a) quantitative analyses (gas chromatography with a flame ionization detector), (b) qualitative analyses (gas chromatography and mass spectrometry) and (c) determination of physical properties (density and refraction index) as is completely described in Rey-Valeirón et al. (2017).
Obtention of engorged females of Rhipicephalus sanguineus
One-hundred adult engorged females of R. sanguineus were collected manually from the ears, interdigital spaces, neck, groin and base of the tail of two cross-bred, male dogs with no acaricide treatment for at least 45 days. Specimens were kept in plastic flasks with small holes until assays, approximately 1 h after. R. sanguineus females were identified by standard keys and used for the assays.
Larval package test (LPT)
To obtain larvae, eight adult engorged females were stuck to the lid of a glass Petri dish with double-sided sticky tape and maintained in an incubator at 29 ± 1 °C and relative humidity (RH) 80% for 20 days. Eggs were then collected and transferred to a glass tube with a cotton lid to allow air and moisture exchange and incubated to allow hatching in the same conditions as above. Larvae of 21 days old were used for LPT.
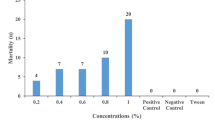
The LPT was carried out as by Stone and Haydock (1962). One-hundred larvae were placed in the center of a sheet of filter paper measuring 6 × 6 cm with pencil drawn grids. Five concentrations of EOSm were used (0.125, 0.25, 0.50, 1 and 2%) diluted in anionic detergent Tween 80 (v/v). Tween 80 diluent alone had been previously evaluated for not causing mortality of larvae. Each sheet with the 100 larvae inside was folded and moistened with 1 mL of each concentration to be tested. The sheet was then placed into an envelope of same filter paper. Additionally, cypermethrin 1:1000 in water was tested as synthetic acaricide. EOSm control group were treated with 1 mL of 2% Tween 80 and cypermethrin control groups with 1 mL of deionized water. Each treatment was repeated five times. To prevent effects due to volatility of some compounds from EOSm, the control groups were kept apart in the same conditions (27 ± 1 °C and RH > 80 ± 10%). After 24 h, the packets were opened and the number of living and dead larvae was counted manually. Only larvae capable of locomotion were considered alive. The percentage of mortality was calculated as Abbott (1925). Mortality in control groups was calculated as number of dead larvae/total number of larvae × 100%.
Adult immersion test (AIT)
AIT was carried out as by Drummond et al. (1973) with minor modifications. Groups of eight engorged adult females were weighed and immersed for 5 min in 10 mL of each EOSm dilution (0.125, 0.25, 0.50, 1, 2, 4, 8, 16, 20% v/v in 2% Tween 80) into a 25 mL beaker which was gently agitated at room temperature. The detergent solution was used as the negative control of EOSm. Cypermethrin at dilution 1:1000 (as recommended by manufacturer) was used as synthetic, commercial acaricide and deionized water as negative control of cypermethrin. The weight of the groups was homogeneous. After the immersion, the females were placed on paper sheets for 15 min to allow the solvents and excess solution to evaporate. Each female was stuck to the lid of glass Petri dishes with double-sided sticky tape. The groups were maintained in a climate-controlled chamber (27 ± 1 °C and RH > 80 ± 10%) and the egg masses were collected until the death of the females, around day fourteen. To obtain larvae, eggs were treated as described above in "Results" section. Larvae and unhatched eggs of each group were counted in a 24-wells culture plates under a stereo microscope to estimate the percentage of hatching.
To establish the acaricidal efficacy of the EOSm or cypermethrin, several biological parameters were calculated using the following formulas (FAO 2004):
-
(a)
Surviving period: number of ticks which remained alive after treatment
-
(b)
Percentage of egg hatching (EH) = (number of larvae)/(total number of unhatched eggs and larvae) × 100
-
(c)
Reproductive index (RI) = egg mass weight/engorged adult female weight before oviposition
-
(d)
Inhibition of oviposition (IOv%) = (RI control group − RI treated group/RI control group) × 100.
-
(e)
Reproductive efficiency (RE) = egg mass weight/engorged adult female weight before oviposition × egg hatching
Statistical analysis
Statistical differences between biological parameters and concentrations were calculated by analysis of variance (α = 0.05). Pearson correlation test was used to calculate the correlations between the EOSm concentration and mortality of larvae or reproductive parameters of adult females. Shared variance (r2) was also estimated to account the effect of EOSm concentrations on reproductive parameters. Calculation of LC90 and LC50 (concentration necessary for 90% and 50% lethality) was carried out. In all the cases, Prism v6.4 for Windows was used (GraphPad Software, USA).
Results
Characterization of the EOSm
Twenty-one components comprising more than 97% of the EOSm were identified. The major components were p-cymene (40.0%) followed by limonene (19.5%), myrcene (7.7%), and camphene (5.6%). The yield (wt/wt) was 3.50 ± 0.30% (Table 1).
LPT
The results of the larval package test are shown in Table 2. Mortality rate was dose-dependent (p < 0.05); the highest value of mortality (> 99%) was achieved with 2% EOSm, but no statistical differences were found between 1 and 2%. LC50 and LC90 were calculated as 0.21 and 0.80%, respectively. Correlation between concentrations and larval mortality was strong and positive (r = 0.849). Cypermethrin in dilution 1:1000 caused 23.1% larval mortality.
Effect of EOSm on biological and reproductive parameters of Rhipicephalus sanguineus
Surviving period
At all the concentrations tested, immersion in EOSm was not lethal to engorged females: the specimens remained alive until the end of oviposition. Identical results were observed in ticks treated with cypermethrin and in control groups immersed in detergent or in deionized water.
Inhibition of oviposition (IOv), egg hatching (EH) and reproductive efficiency (RE)
The highest value of IOv (29.6%) was achieved by 20% EOSm (p < 0.05). Concentrations below 16% resulted in negligible values of IOv (data not shown). A moderate coefficient of correlation (0.681) was found between EOSm concentrations and IOv values and the shared variance was over 46%. IOv in the group treated with cypermethrin was 13.7% (Table 3).
The efficacy of the EOSm was also evaluated on the egg hatchability of R. sanguineus. EH ranged between 53 and 98.3% with concentrations of 16 and 20% EOSm, resulting in the lowest egg hatching values (53 and 59.4%, respectively). A strong negative correlation (r = − 0.948) was found between EOSm concentrations and EH; shared variance was over 89%. In the group treated with cypermethrin, EH was only 73% (Table 3).
RE decrease was dose-dependent starting at a concentration of 2% (Table 3). Lowest RE value (22.6%) was achieved with concentration of 20%. Concentrations below 2% resulted in negligible values of RE (data not shown). A negative and strong correlation (r = − 0.985) was found between EOSm concentrations and RE; shared variance was over 97%. In the group treated with cypermethrin, RE was 61.6% (Table 3).
Discussion
The major components in Ecuadorian EOSm were characterized by monoterpenes p-cymene, limonene, myrcene and camphene, partially consistent with the findings of other studies. Bernhard et al. (1983) reported myrcene, α-phellandrene, δ-cadinene, limonene, α-cadinol and β-phellandrene as major components in the EOSm from California. Zahed et al. (2011) identified limonene and β-phellandrene, α-phellandrene, myrcene and α-pinene in EOSm collected from four locations in Tunisia. Fruit EOSm from Portugal was characterized mainly by β-myrcene, limonene, α-phellandrene and β-phellandrene (Martins et al. 2014). Limonene and myrcene are the common components in all those essential oils. However, Batista et al. (2016) did not detect the presence of myrcene, phellandrene and limonene in samples from Rio de Janeiro, Brazil. Differences may be due to genotype, plantation origin, age, physiology or ecological conditions as seasonality, water availability and soil nutrients (dos Santos et al. 2015, Bhattacharya 2015).
The results presented in this paper proved the efficacy of the phytotherapy upon R. sanguineus in vitro. At a concentration of 2%, EOSm was lethal to R. sanguineus larvae. The results were not surprising because the EOSm obtained from fruits showed 99.9% of mortality of R. (B.) microplus larvae at concentration of 2.5% (Rey-Valeirón et al. 2017). A fraction of the EOSm obtained from the whole plant demonstrated acaricidal effect in vitro on R. (B.) microplus (Cidade-Torres et al. 2012). A recently report showed insecticidal effect of EOSm from leaves and fruits on adult stages of Ctenocephalides felis felis in vitro (Batista et al. 2016).
Although comparisons are difficult due the units used to record the concentrations and it is extremely difficult to compare the efficacy of oils tested in one study with that of oils tested in another (Ellse and Wall 2014), several reports focused on acaricidal effect of EOs on ungorged larvae. Ribeiro et al. (2008) observed that the oil of bitter cinnamon, Drimys brasiliensis Miers (Winteraceae) at 6.25 µL/ml was lethal, killing 100% of the larvae of R. sanguineus. Lippia sidoides (Verbenaceae) EO caused 96% of mortality at 14.10 mg/mL (Gomes et al. 2014). However, a concentration of 20% of Tagetes minuta (Asteraceae) EO was required to cause 100% of mortality in larvae (da Silva et al. 2016). The hydrophobic nature of the oils may exert mechanical effects on the parasite—particularly larvae—by disrupting the cuticular waxes and blocking the spiracles, which leads to death by water stress or suffocation (Ellse and Wall 2014).
This is the first report about the effect of EOSm on adult stages of R. sanguineus. Although the susceptibility of R. sanguineus engorged females was lower than that of the larvae and EOSm was not lethal, the oil had a remarkable effect over biological parameters as shown by decreases in oviposition, EH and RE if compared with untreated group. In this work, the best results were obtained with 20% EOSm: 29.6% of IOv, 59.4% of EH and 22.6% of RE. The EO of Tagetes patula caused no deaths in engorged adult females of R. sanguineus subjected to treatment but reduced the oviposition (Politi et al. 2012). The effect on oil over tick reproductive system was showed by Sampieri et al. (2012). They reported ultrastructural changes in the somatic and germ cells of ovaries, in females engorged on rabbits with castor oil diets. Vendramini et al. (2012) demonstrated that andiroba (Carapa guianensis) seed oil was able to cause severe changes in the oocytes and the reproductive system of R. sanguineus.
The results were obtained from female ticks manually collected before they had completely finished blood feeding; nonetheless, the control group was able to lay viable eggs with a hatching rate of 95%. Compared with the untreated group, the EH value of 59.43% with EOSm concentration of 20% was certainly due to the essential oil.
The low impact of cypermethrin on larval mortality and the regular efficacy on reproductive parameters of R. sanguineus obtained in the present study requires further revision in upcoming research, because cypermethrin is one of the most used in tick control on dogs from Venezuela and Ecuador (data unpublished). Rodriguez-Vivas et al. (2017) reported high levels of cypermethrin resistance in R. sanguineus populations in dogs in Yucatán, Mexico.
The results obtained in this work with the EOSm suggest that effects on mortality and reproductive parameters observed in the assays are related to the presence of hydrogenated monoterpenoids (MH), since major components of EOSm were MH (limonene, p-cymene, α-phellandrene, myrcene, and camphene). Monoterpenoids were the first inhibitors from plants which were considered to have anticholinesterasic properties; advantageously, these compounds penetrate quickly the membranes due to volatile and lipophilic characteristics. Limonene has also been reported as broad spectrum arthropod and insect repellent due to a potent acetylcholinesterase (AChE) inhibitory activity whereas other compounds as geraniol or R-carvone have strong insecticidal activity but are weak inhibitors of AChE (López and Pascual-Villalobos 2010). As the AChE from insects differs from the mammalian for a single residue, known as the insect-specific cysteine residue, AChE can be an insect-selective target (Pang et al. 2012) for one or several major components of EOs. Prado-Rebolledo et al. (2017) reported up to 80.9% of mortality in larvae treated with d-limonene. p-Cymene, the major constituent of EOSm, was found to be toxic against two stored pests (Sitophilus granarius and Tribolium confusum) (Kordali et al. 2008).
In arthropods, the group of biogenic amine messengers consists of dopamine, tyramine, octopamine, serotonin and histamine (Blenau et al. 2012); the tyraminergic/octopaminergic pathway is the main regulatory system for oviposition control in insects, arachnids, crustaceans and mollusks (Li et al. 2015). The identification of the orthologous octβ 2R octopamine receptor in R. microplus as well as the inhibition of oviposition by adrenergic ligands strongly suggest a role in ticks (Cossió-Bayúgar et al. 2015). Importantly, EOs can be considered as agonists of all types of octopamine and tyramine receptors (Jankowska et al. 2018). Hence, the study of the effects of EOSm on insect-specific octopamin receptor should be considered in the quest of candidates for the control of R. sanguineus. However, there are many potential targets in the tick nervous system that propose EO components as candidates for acaricides.
In addition to the toxicity of a plant against arthropods, the product should not be toxic to mammals. Plant derivatives are not always harmless; in fact, it has been shown that some compounds have good antioxidants or antimutagenic activities at low concentrations but at higher concentrations induce cellular DNA damage or can have deleterious effects on the behavior and health of the host (Bakkali et al. 2008; George et al. 2008); some essential oils or some of their constituents may be considered as secondary carcinogens after metabolic activation (Guba 2001). Most studies on acaricides report the bioactivity of EOs and their compounds in vitro, but there is a minority of reports about the use in vivo of natural acaricides (Manzoor et al. 2013). In the case of S. molle, Bras et al. (2011) studied the acute dermal exposure to ethanolic and hexanic extracts in rats. A slight and reversible skin irritation was observed, which indicated that the topical use of these extracts was safe, either as a therapeutic agent or as an insect repellent in that animal model.
This study presents an evaluation of the acaricidal effect of EOSm in vitro and the results showed an important effect on larvae and female reproductive parameters of R. sanguineus. However, before their use on dogs, several issues concerning acaricide potential of EOSm have to be evaluated. A toxicology profiling including excipient development, research into residual activities and the effects on the behavior of dogs are required. Graham et al. (2005) showed that dog’s behavior changed significantly with the length of exposure to odours of lavender (Lavandula angustifolia), chamomile (Anthennis nobilis), rosemary (Cymbopogon citrates) and peppermint (Mentha piperata) essential oils.
EOs have a limited half-life because their volatile components with repellent effect tend to being short-lived in their effectiveness (Nerio et al. 2010). However, 2.5% turmeric essential oil sprayed on dogs demonstrated repellency comparable to 20% DEET and was more effective than 5% PMD (para-Menthane-3,8-diol, a monotorpene product of Corymbia citriodora ssp. citriodora) (Goode et al. 2018). The use of EOs is less difficult to manage in pet habitat than in livestock, because pets are usually kept singly and owners are often willing to reapply products (Ellse and Walls 2014).
A drawback in the development of EOs as acaricides is the high cost of extraction and low yields. Nonethless, it is possible to develop synthetic analogs of the major components with acaricide or repellent effects. TT-4302, a plant-based repellent with 5% geraniol, showed 100% of efficacy against R. sanguineus in vitro (Bissinger et al. 2014).
Finally, the results obtained in the present study contribute to a further research in acaricidal/repellent effects of synthetic components based on EOSm, which would be better controlled in terms of preparation, reproducibility and a higher economical viability.
References
Abbott W (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. https://doi.org/10.1093/jee/18.2.265a
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured, Illinois, USA
Andreotti R, Garcia MV, Cunha RC, Barros J (2013) Protective action of Tagetes minuta (Asteraceae) essential oil in the control of Rhipicephalus microplus (Canestrini, 1887) (Acari: Ixodidae) in a cattle pen trial. Vet Parasitol 197:341–345. https://doi.org/10.1016/j.vetpar.2013.04.045
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils: a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Batista LCDSO, Cid Y, De Almeida A, Prudêncio E, Riger C, De Souza M, Coumendouros K, Chaves D (2016) In vitro efficacy of essential oils and extracts of Schinus molle L. against Ctenocephalides felis felis. Parasitology 143:627–638. https://doi.org/10.1017/S0031182016000081
Bernhard RA, Shibamoto T, Yamaguchi K, White E (1983) The volatile constituents of Schinus molle L. J Agric Food Chem 31:463–466. https://doi.org/10.1021/jf00116a075
Bhattacharya S (2015) Cultivation of essential oils. In: Preedy V (ed) Essential oils in food preservation, flavor and safety. Elsevier, Amsterdam, pp 19–29. https://doi.org/10.1016/b978-0-12-416641-7.00003-1
Bissinger BW, Schmidt JP, Owens JJ, Mitchell SM, Kennedy MK (2014) Activity of the plant-based repellent, TT-4302 against the ticks Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis and Rhipicephalus sanguineus (Acari: Ixodidae). Exp Appl Acarol 62:105–113. https://doi.org/10.1007/s10493-013-9719-1
Blenau W, Rademacher E, Baumann A (2012) Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 43:334–347. https://doi.org/10.1007/s13592-011-0108-7
Borges LMF, de Sousa LAD, Barbosa C (2011) Perspectives for the use of plant extracts to control the cattle tick Rhipicephalus (Boophilus) microplus. Rev Bras Parasitol Vet 20:89–96. https://doi.org/10.1590/S1984-29612011000200001
Bras C, Gumilar F, Gandini N, Minetti A, Ferrero A (2011) Evaluation of the acute dermal exposure of the ethanolic and hexanic extracts from leaves of Schinus molle var areira L. in rats. J Ethnopharmacol 137:1450–1456. https://doi.org/10.1016/j.jep.2011.08.036
Castelblanco L, Sanabria O, Cruz A, Rodríguez C (2013) Preliminary report of the ixodicidal effect of some plant extracts on ticks Boophilus microplus. Rev Cub Plantas Med 18:118–130
Chantraine J, Laurent D, Ballivian C, Saavedra G, Ibañez R, Vilaseca LA (1998) Insecticidal activity of essential oils on Aedes aegypti larvae. Phytother Rev 12:350–354. https://doi.org/10.1002/(SICI)1099-1573(199808)
Cidade-Torres F, Machado Lucas A, Sardá Ribeiro VL, Martins JR, von Poser G, Guala MS, Elder HV, Cassel E (2012) Influence of essential oil fractionation by vacuum distillation on acaricidal activity against the cattle tick. Braz Arch Biol Technol 55:613–621
Cossió-Bayúgar R, Miranda-Miranda E, Fernández-Rubalcaba M, Narvaéz Padilla V, Reynaud E (2015) Adrenergic ligands that block oviposition in the cattle tick Rhipicephalus microplus affect ovary contraction. Sci Rep. https://doi.org/10.1038/srep15109
Da Silva EMG, Rodrigues VS, Jorge JO, Osava CF, Szabó MPJ, Garcia MV, Andreotti R (2016) Efficacy of Tagetes minuta (Asteraceae) essential oil against Rhipicephalus sanguineus (Acari: Ixodidae) on infested dogs and in vitro. Exp Appl Acarol 70:483–489. https://doi.org/10.1007/s10493-016-0092-8
Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3:26. https://doi.org/10.1186/1756-3305-3-26
De Mendonça P, Rodilla JM, Díez D, Elder H, Guala MS, Silva LA, Baltazar E (2012) Synergistic Antibacterial activity of the essential oil of Aguaribay (Schinus molle L.). Molecules 17:12023–12036. https://doi.org/10.3390/molecules171012023
dos Santos A, Alves MS, da Silva LCP, Patrocínio DS, Sanches MN, Chaves DSA, Souza MAA (2015) Volatiles composition and extraction kinetics from Schinus erebinthifolius and Schinus molle leaves and fruit. Rev Bras Farmacogn 25:356–362. https://doi.org/10.1016/j.bjp.2015.07.003
Drummond R, Ernst S, Trevino J, Gladney W, Graham O (1973) Boophilus annulatus and Boophilus microplus: laboratory test of insecticides. J Econ Entomol 66:130–133. https://doi.org/10.1093/jee/66.1.130
Eiden A, Kaufman P, Oi F, Allan S, Miller R (2015) Detection of permethrin resistance and fipronil tolerance in Rhipicephalus sanguineus (Acari: Ixodidae) in the United States. J Med Entomol 52:429–436. https://doi.org/10.1093/jme/tjv005
Ellse L, Wall R (2014) The use of essential oils in veterinary ectoparasite control: a review. Med Vet Entomol 28:233–243. https://doi.org/10.1111/mve.12033
Food and Agriculture Organization (FAO) (2004) Resistance management and integrated parasites control in ruminants. Guidelines, module 1: ticks acaricide resistance, diagnosis, management and prevention. Food and Agriculture Organization (FAO), Rome, pp 25–77
George DR, Callaghan K, Guy JH, Sparagano OAE (2008) Lack of prolonged activity of lavender essential oils as acaricides against the poultry red mite (Dermanyssus gallinae) under laboratory conditions. Res Vet Sci 85:540–542. https://doi.org/10.1016/j.rvsc.2008.02.001
Gomes GA, Monteiro CMO, Julião LS, Maturano R, Senra TOS, Zeringóta V, de Carvalho MG (2014) Acaricidal activity of essential oil from Lippia sidoides on unengorged larvae and nymphs of Rhipicephalus sanguineus (Acari: Ixodidae) and Amblyomma cajennense (Acari: Ixodidae). Exp Parasitol 137:41–45. https://doi.org/10.1016/j.exppara.2013.12.003
Goode P, Ellse L, Wall R (2018) Preventing tick attachment to dogs using essential oils. Ticks Tick Borne Dis 9:921–926. https://doi.org/10.1016/j.ttbdis.2018.03.029
Graham L, Wells DL, Hepper PG (2005) The influence of olfactory stimulation on the behaviour of dogs housed in a rescue shelter. Appl Anim Behav Sci 91:143–153. https://doi.org/10.1016/j.applanim.2004.08.024
Guba R (2001) Toxicity myths—essential oils and their carcinogenic potential. Int J Aromather 11:76–83. https://doi.org/10.1016/s0962-4562(01)80021-7
Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M (2018) Molecular targets for components of essential oils in the insect nervous system—a review. Molecules 23:34. https://doi.org/10.3390/molecules23010034
Kačániová M, Vukovič N, Horská E, Salamon I, Bobková A, Hleba L, Fiskelová M, Vatľák A, Petrová J, Bobko M (2014) Antibacterial activity against Clostridium genus and antiradical activity of the essential oils from different origin. J Environ Sci Health B 49:505–512. https://doi.org/10.1080/03601234.2014.896673
Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E (2008) Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour Technol 99:8788–8795. https://doi.org/10.1016/j.biortech.2008.04.048
Li Y, Fink C, El-Kholy S, Roeder T (2015) The octopamine receptor octß2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch Insect Biochem Physiol 88:168–178. https://doi.org/10.1002/arch.21211
López MD, Pascual-Villalobos MJ (2010) Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crops Prod 31:284–288. https://doi.org/10.1016/j.indcrop.2009.11.005
Manzoor F, Fazal S, Munir N, Naz S, Khalid A (2013) Acaricidal activity of essential oils from tulsi (Ocimum basilicum), bach (Acorus calamus), and mint (Mentha arvensis) against Rhipicephalus sanguineus (Latreille). Asian J Chem 25:6787–6790. https://doi.org/10.14233/ajchem.2013.14680
Martins M, Arantes S, Candeias F, Tinoco M, Cruz J (2014) Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol 151:485–492. https://doi.org/10.1016/j.jep.2013.10.063
Molina-Garza Z, Bazaldúa A, Quintanilla R, Galaviz L (2014) Anti-Trypanosoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Tropic 136:14–18. https://doi.org/10.1016/j.actatropica.2014.04.006
Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: a review. Bioresour Technol 101:372–378. https://doi.org/10.1016/j.biortech.2009.07.048
NIST 05 Mass Spectral Library (NIST/EPA/NIH) (2005) National Institute of Standards and Technology, Gaithersburg, Maryland, USA
Otranto D, Dantas-Torres F, Breitschwerdt E (2009) Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol 25:57–163. https://doi.org/10.1016/j.pt.2009.01.003
Pang YP, Brimijoin S, Ragsdale DW, Zhu KY, Suranyi R (2012) Novel and viable acetylcholinesterase target site for developing effective and environmentally safe insecticides. Curr Drug Targets 13:471–482. https://doi.org/10.2174/138945012799499703
Pavela R, Canale A, Mehlhorn H, Benelli G (2016) Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: a review. Res Vet Sci 109:1–9. https://doi.org/10.1016/j.rvsc.2016.09.001
Politi FAS, Figueira GM, Araújo AM, Sampieri BR, Mathias MIC, Szabó MPJ, Bechara GH, dos Santos LC, Vilegas W, Pietro RCLR (2012) Acaricidal activity of ethanolic extract from aerial parts of Tagetes patula L. (Asteraceae) against larvae and engorged adult females of Rhipicephalus sanguineus (Latreille, 1806). Parasit Vectors 5:295–306. https://doi.org/10.1186/1756-3305-5-295
Prado-Rebolledo O, Molina-Ochoa J, Lezama-Gutiérrez R, García-Márquez L, Minchaca-Llerenas Y, Morales-Barrera E et al (2017) Effect of Metarhizium anisopliae (Ascomycete), cypermethrin, and d-limonene, alone and combined, on larval mortality of Rhipicephalus sanguineus (Acari: Ixodidae). J Med Entomol 54:1323–1327. https://doi.org/10.1093/jme/tjx092
Rey-Valeirón C, Guzmán L, Saa LR, López-Vargas J, Valarezo E (2017) Acaricidal activity of essential oils of Bursera graveolens (Kunth) Triana & Planch and Schinus molle L. on unengorged larvae of cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). J Essent Oil Res 29:344–350. https://doi.org/10.1080/10412905.2016.1278405
Ribeiro VLS, Rolim V, Bordignon S, Henriques AT, Dorneles GG, Limberger RP, Von Poser G (2008) Chemical composition and larvicidal properties of the essential oils from Drimys brasiliensis Miers (Winteraceae) on the cattle tick Rhipicephalus (Boophilus) microplus and the brown dog tick Rhipicephalus sanguineus. Parasitol Res 102:531–535. https://doi.org/10.1007/s00436-007-0799-x
Rodriguez-Vivas RI, Ojeda-Chi MM, Trinidad-Martinez I, Bolio-González ME (2017) First report of amitraz and cypermethrin resistance in Rhipicephalus sanguineus sensu lato infesting dogs in Mexico. Med Vet Entomol 31:72–77. https://doi.org/10.1111/mve.12207
Sampieri B, Arnosti A, Nunes P, Furquim K, Chierice G, Mathias M (2012) Ultrastructural changes in the ovary cells of engorged Rhipicephalus sanguineus female ticks treated with esters of ricinoleic acid from castor oil (Ricinus communis). Microsc Res Tech 75:683–690. https://doi.org/10.1002/jemt.21112
Stone B, Haydock K (1962) A method for measuring the acaricide susceptibility of the cattle tick Boophilus microplus (Can.). Bull Entomol Res 53:563–578. https://doi.org/10.1017/S000748530004832X
Tene V, Malagón O, Finzi P, Vidari G, Armijos Ch, Zaragoza T (2007) An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J Ethnopharmacol 11:63–81. https://doi.org/10.1016/j.jep.2006.10.032
Vendramini MCR, Mathias MIC, De Faria AU, Furquim KCS, De Souza LP, Bechara GH, Roma GC (2012) Action of andiroba oil (Carapa guianensis) on Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females: morphophysiological evaluation of reproductive system. Microsc Res Tech 75:1745–1754. https://doi.org/10.1002/jemt.22126
Wanzala W, Hassanali A, Mukabana W, Takken W (2014) Repellent activities of essential oils of some plants used traditionally to control the brown ear tick, Rhipicephalus appendiculatus. J Parasitol Res. https://doi.org/10.1155/2014/434506
Wimalaratne P, Slessor K, Borden J, Chong L, Abate T (1996) Isolation and identification of house fly, Musca domestica L., repellents from pepper tree, Schinus molle L. Chem Ecol 22:49–59. https://doi.org/10.1007/BF02040199
Zahed N, Hosni K, Brahim NB, Sebei H (2011) Essential oil composition of Schinus molle L. Fruits: an ornamental species used as condiment. J Food Biochem 35:400–408. https://doi.org/10.1111/j.1745-4514.2010.00391.x
Acknowledgements
To Lic. Víctor García and DVM Yoselín Dietes, Universidad Nacional Experimental Francisco de Miranda, Venezuela, for their invaluable technical assistance. To Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT), República del Ecuador, for making possible the scientific cooperation between Catalina Rey-Valeirón, Lucía Guzmán y Eduardo Valarezo through Proyecto Prometeo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The ticks were collected from dogs under qualified veterinary supervision without causing pain or distress, as the Code of Bioethics and Biosecurity by Fondo Nacional de Ciencia, Tecnología e Innovación, Venezuela. Acquiescence of owner for tick sampling was previously asked. Participation was voluntary.
Rights and permissions
About this article
Cite this article
Rey-Valeirón, C., Pérez, K., Guzmán, L. et al. Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae). Exp Appl Acarol 76, 399–411 (2018). https://doi.org/10.1007/s10493-018-0303-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0303-6