Abstract
Purpose
Rhipicephalus (Boophilus) microplus is one the most significant ectoparasite in cattle farming in tropical and subtropical regions, causing problems to livestock health worldwide. The control of this ectoparasite primarily relies on the use of synthetic acaricides. However, the emergence of acaricide resistance has stimulated the search for new control alternatives, including phytocompounds with acaricidal and insecticidal potential. The aim of this study was to evaluate the acaricidal potential of Lavandula dentata essential oil against the engorged females of R. (B.) microplus.
Methods
Engorged females were obtained from infested bovines in dairy farms in Pernambuco, Brazil. L. dentata essential oil was extracted, and adult immersion test assays were performed using the following oil concentrations: 0.2%, 0.4%, 0.6%, 0.8%, and 1%.
Results
L. dentata essential oil at a concentration of 1% was lethal to all engorged females, and concentrations of 0.6% and 0.8% caused mortality of 98.6% and 99.1%, respectively. These concentrations disrupted the reproductive capacity of engorged females, reducing oviposition by more than 90% and preventing egg hatching by over 87%.
Conclusion
The data revealed that L. dentata essential oil possesses effective pharmacological properties against R. (B.) microplus and could be used for tick control following in vivo evaluation, thus contributing to mitigating the negative impacts of synthetic acaricide use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Using phytochemicals for therapeutic purposes is an ancient practice that originated from the need to cure diseases with the available resources, based on the empirical observation of plants that had beneficial effects in treating various pathologies, both in humans and animals [1, 2]. The use of botanical compounds has gained momentum in various areas of human and veterinary medicine in the modern word. Many plants have shown potential for disease treatment with notable advantages, such as lower toxicity, a favourable cost-to-benefit ratio, absence of dependence and resistance to active principles, and promising acaricidal activity [3].
A common practice among cattle producers is using medicinal plants to prevent and treat diseases that frequently affect herds, particularly parasitic diseases [1]. In this context, infestation by ectoparasites is considered one of the significant challenges in livestock farming, with a particular emphasis on the infestation caused by the Rhipicephalus (Boophilus) microplus tick. This tick is distributed in tropical and subtropical regions worldwide, posing a significant problem for the livestock industry [4]. R. (B.) microplus infestations cause substantial economic losses due to severe blood loss and reduced weight gain in infested cattle, resulting in approximately 3.24 billion dollars in annual losses [5]. The tick’s bite leads to blood depletion, skin irritation, and damage to the hide, which can promote secondary skin infections and the deposition of larvae by myiasis-causing arthropods. Additionally, R. (B.) microplus serves as a vector for various disease-causing pathogens, including the protozoa Babesia bovis and Babesia bigemina and the bacterium Anaplasma marginale. These pathogens are responsible for high morbidity and mortality rates and the costs associated with treating and managing infested animals [5, 6].
The control of R. (B.) microplus involves using synthetic acaricides. However, due to incorrect application, errors in dosage, repetitive bathing of cattle, and the repeated use of the same active ingredient result in increasing resistance of R. (B.) microplus populations to most synthetic acaricides. This phenomenon underscores the need to develop efficient control methods to reduce the presence of these arthropods in cattle herds [7, 8].
Resistance is established through genetic changes that affect the cuticular penetration of the drug, leading to metabolic resistance to the chemical formulation and insensitivity at the target site [9]. Furthermore, the indiscriminate use of these chemicals leads to contamination of animal-derived products and the environment [10]. Therefore, the use of plant-based compounds derived from medicinal plants has proven to be an effective alternative for controlling ectoparasites, given the need for the development of tools that allow the identification and characterization of alternative pharmacological principles that can be used as viable options for controlling this parasite [10].
Essential oils are notable among the herbal products widely used in veterinary medicine. Essential oils are compounds derived from the secondary metabolism of plants and are extensively studied for tick control and prevention [11]. Essential oils from Lavandula species are used in the cosmetic, food, and pharmaceutical industries. Their main components include oxygenated monoterpenes (such as cineol, camphor, fenchone, and linalool) and non-oxygenated monoterpenes (α-pinene, β-pinene, and limonene), as well as oxygenated sesquiterpenes (caryophyllene oxide, limonene oxide) and non-oxygenated sesquiterpenes (trans-caryophyllene, α-copaene, and β-eudesmol). These components contribute to the essential oil’s medicinal properties [12, 13]. Its main therapeutic indications include antihypertensive, antispasmodic, antimicrobial, and antiparasitic properties [13, 14].
Therefore, the present study aimed to evaluate the in vitro acaricidal potential of the essential oil of Lavandula dentata L. against engorged females of R. (B.) microplus.
Materials and Methods
Ethical Aspects
The study was approved by the Ethics Committee on Animal Use (CEUA) of the Federal Rural University of Pernambuco (protocol number 8,717,300,420/ID 192/2020).
Collection and Identification of Botanical Material
The botanical samples (leaves) of L. dentata L. were collected on rural properties in the municipality of Garanhuns, in the southern Agreste region of Pernambuco, from August to October 2022, in the early morning hours. This time of day is when the plants are most saturated with oil [15].
During this period, the Garanhuns region had a climatological classification of a warm, sub-humid, subtropical/tropical high-altitude climate, with a recorded precipitation level of around 11.8 mm, minimum temperatures of 18 °C, maximum temperatures of 26 °C, and relative humidity of 83%. Meteorological data were obtained from of the National Institute of Meteorology (INMET).
A plant sample was deposited in the Dárdano de Andrade Lima herbarium of the Pernambuco Agricultural Research Institute (IPA), where botanical identification was confirmed, and it received the registration number 94,311.
Extraction of the Essential Oil and Chemical Characterization
Leaves were cleaned with distilled water and dried in a circulating air oven at 50 °C for 96 h. The material was ground after drying, and the extraction process was initiated.
The Essential Oil (EO) extraction was performed through hydro distillation using a Clevenger-type graduated apparatus with a 2 L volumetric flask. The extraction involved using a 1:100 ratio of distilled water to crushed botanical material. Distillation, isolated from sunlight, commenced with rapid heating until it reached 100 °C, which remained for two hours.
Upon completion of the distillation period, the essential oil was separated from the hydrolate using a separation funnel[16]. Subsequently, the obtained oil sample was treated with anhydrous sodium sulfate (Na2SO4) to remove excess water and stored in a sealed amber glass container under refrigeration. The extraction yield was calculated by dividing the volume of essential oil obtained by the dry matter mass used in the extraction and multiplying the result by 100.
The chemical composition of the EO was determined using Gas Chromatography-Mass Spectrometry (GC-MS) with a Shimadzu GCMS-QP2010 ULTRA model chromatograph and a mass-selective detector (70 eV electron ionization) at 220 °C. The column used was the NST-01 column with a length of 30 m, an internal diameter of 0.25 mm, and a film thickness of 0.25 μm (NanoSeparation Technology). The initial temperature of the analysis was 50 °C, and the final temperature was 300 °C, with a heating rate of 25 °C/min. A 1.0 µL sample was injected, and the injector and detector were set at 250 °C. Helium was used as the carrier gas at a 1.2 mL/min flow rate. Data were acquired using GCMS Solution software (Shimadzu) (Spectral library: NIST11).
The concentrations of the chemical constituents present in the oil were estimated by comparing mass spectra and retention indices (RI) of the components with those of a standard substance (methyl eugenol) in the GC/MS system library and literature data [17, 18]. For this purpose, a solution was prepared containing 0.1 mg of the standard with 0.2 mg of the essential oil in 1 mL of dichloromethane, and it was analyzed under the previously described conditions. The concentration of each constituent in the injected solution and the standard concentration were multiplied by the compound’s area, and the result was divided by the standard’s area. To obtain the concentration of each compound in the essential oil, taking into account the standard dilution factor (1:10000) and the essential oil: standard ratio (2:1), the concentration of the compound in the injected solution was divided by two and then multiplied by 10,000.
Tick Collection and Adult Immersion Test (AIT)
For the AIT, engorged females of R. (B.) microplus were manually collected from naturally-infested cattle that had not been treated with acaricides in the prior 30 days. All ticks were sampled from animals from rural properties in the Garanhuns microregion, Pernambuco.
After collection, the females were placed in plastic vials, stored in isothermal boxes (12 to 15 °C) and transported to the laboratory. All specimens were cleaned with distilled water, dried with absorbent paper, and individually weighed. The morphological identification was carried out following dichotomous and pictorial keys [19, 20].
The AIT was performed following the methodology of [21]. The engorged females were separated into Petri dishes in groups of ten, with an average weight of 2.0 g per dish. They were then immersed for five minutes in solutions of the EO. Concentrations of 0.2%, 0.4%, 0.6%, 0.8%, and 1% (volume/volume) were prepared using the essential oil diluted in distilled water and Tween 80™ as an emulsifier. Distilled water was used for the negative control, and the positive control consisted of a commercial synthetic pyrethroid based on deltamethrin at the concentration recommended by the manufacturer.
Additionally, a control was performed using Tween 80™ at the same concentration used to emulsify the EO at the studied concentrations. All concentrations, including control samples, were prepared in duplicate. After immersion, the females were removed, placed in sterile Petri dishes, and kept under controlled temperature and environmental conditions (25 ± 1 °C, 80 ± 5% RH) for egg-laying over 18 days.
During the incubation period, daily checks were conducted to assess female mortality. At the end of the egg-laying period, the eggs were weighed and placed in adapted plastic syringes for hatching and assessing the hatchability rate. The evaluation of larval hatching was done by visually estimating the number of hatched larvae.
Data Analysis
Descriptive statistics were employed to obtain absolute and relative frequencies. Acaricidal Efficiency (AE) was calculated based on the weight of engorged females, percentages of egg weight, and egg hatching. The botanical products were considered efficient if AE ≥ 95% [10,11,12,13,14,15,16,17,18,19,20,21].
Pearson’s correlation (r) was used to calculate the correlation between the oil concentrations and tick reproductive parameters. Shared correlation (r²) was calculated to assess the effect of lavender oil concentrations on tick reproductive parameters. The mean concentration for inhibition of the oviposition (IC50) parameter was determined. The significance level was set at 5%, and all statistical analyses were conducted using the BioEstat and Prism 9.0 software for Windows (GraphPad Software, USA).
Results
During each extraction process, 1 mL of L. dentata L. essential oil was obtained with a yield of 1%, and three extraction processes were conducted.
In the chromatographic analyses, 18 compounds were identified, with concentrations ranging from major constituents to trace concentrations. Among these compounds, three were found in significant concentrations, and these peaks were identified as Eucalyptol (1,8-cineole), Camphor, and Fenchone (Table 1).
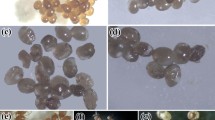
The results obtained in the acaricidal tests demonstrated that the L. dentata L. essential oil (EO) has lethal effects on engorged females of R. (B.) microplus (Fig. 1). Acaricidal efficacy was achieved at 1%, followed by concentrations of 0.8% and 0.6% (Table 2). Furthermore, the lavender EO also showed significant reductions in oviposition rates of the engorged females (OvR). A positive correlation was observed between the concentrations of lavender oil and OvR (r = 0.9482; r² = 90%; p = 0.01).
There was a negative correlation between oil concentrations and larval eclosion (r = -0.9530; r² = 91%; p = 0.01). Regarding reproductive efficiency (ER), a decline was observed from the 1.00% concentration, with values ranging from 0 to 5.25%. Thus, a negative correlation was detected between concentrations and ER (r = -0.8272; r² = 68%; p = 0.08) (Table 2).
The positive control group showed no efficacy against R. (B.) microplus, indicating the development of parasitic resistance to this acaricide class, according to [10,11,12,13,14,15,16,17,18,19,20,21]. The negative control exhibited the expected behaviour, causing no alterations in the engorged females, with all laying eggs and a 100% larval eclosion rate. It is worth noting that the Tween 80 control did not induce changes in the engorged females of R. (B.) microplus. Therefore, it can be observed that this product did not interfere with the experiment, as it had no impact on engorged females’ mortality, oviposition, and larval eclosion (Table 2).
The IC50 was obtained at 0.21% of L. dentata L. essential oil.
Discussion
This study assessed the in vitro efficacy of L. dentata L. essential oil (EO) on engorged females of R. (B.) microplus, and the results demonstrated a significant potential of the EO for bovine tick control. The 1% concentration achieved a 100% efficacy rate, being lethal for all the engorged females in the first eight days of the experiment. It is worth noting that the 1% EO solution had a fluid presentation that did not compromise the breathing and locomotionof the ticks. However, the actions of the bioactive components led to the mortality of all females exposed to this concentration. Probably, camphor, a significant constituent of L. dentata L. EO, acted by blocking the olfactory receptors located on the spiracular plates of the ticks, hindering the passage of oxygen and leading to the ectoparasite’s death due to suffocation [22]. Concentrations of 4% and 8% of Lavandula angustifolia EO on engorged females of Rhipicephalus (Boophilus) annulatus caused mortality within the first 24 h of exposure, corroborating the findings of the present study [23].
Additionally, 0.6% and 0.8% of L. dentata L. EO concentrations showed acaricidal efficacy (Table 2). They interfered with the reproductive indices of the engorged females, causing inhibition of oviposition with a reduction of over 90% in the egg mass compared to the negative control. The EO provided an 88% reduction in egg hatching, likely due to the presence of 1,8-cineol. It is known that this compound affects the reproductive efficiency of engorged females, causing morphological changes in the ovaries and oocyte tissue [13].
L. dentata L. EO can delay and interfere with various stages of the tick’s developmental cycle. Lavandula angustifolia EO also caused egg-laying failures in R. (B.) annulatus and reduced their weight in a concentration-dependent manner [23]. These characteristics are attributed to the major constituents found in this plant’s cellular matrix, which contribute to its acaricidal action. Studies in different regions have found compounds like camphor and fenchone with similar efficacy to the present analysis [12, 24]. Research on monoterpene 1,8-cineole confirms its antimicrobial, antifungal, analgesic, antispasmodic, and anti-inflammatory action [13]. Previous studies using Lippia gracilis EO with 3.9% 1,8-cineole were found to be lethal to 90% of R. (B.) microplus engorged females in vitro [25]. This compound’s mode of action involves inhibiting interleukin’s activity, capable of activating arachidonic acid metabolism [25]. Fenchone acts on the receptors of the enzyme acetylcholinesterase, inhibiting its synthesis [26].
The employment of plant-based alternative to control ticks might be stimulated because the acaricide resistance has raised everywhere. It is known that resistance to different classes of synthetic acaricides occurs all around the world [10,11,12,13,14,15,16,17,18,19,20,21].
Plant-derived compounds are desirable as alternatives to conventional acaricides for tick management. However, regulatory approval challenges by government organizations can delay the commercialization of various combinations of interest [27]. The results of the present study demonstrate that L. dentata L. EO, contains active principles potentially effective for R. (B.) microplus control. Further research is necessary to assess the product’s effectiveness in field-infested animals.
References
Rezende HA, Cocco MIM (2002) A utilização da fitoterapia no cotidiano de uma população rural. Rev Esc Enferm USP 36:282–288. https://doi.org/10.1590/S0080-62342002000300011
Bakkali F, Averbeck D, Idaomar M (2008) Biological effects of essential oils: a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Bressanin GGN, Melo ALT, Perinotto WMS (2020) Óleos Essenciais com Atividade Acaricida para o Controle De Rhipicephalus (Boophilus) microplus no Brasil. Ens Cien 24:480–488. https://doi.org/10.17921/1415-6938.2020v24n5-esp.p480-488
Mendes MC, Duarte FC, Martins JR, Klafke GM, Fiorini LC, Barros ATM (2013) Characterization of the pyrethroid resistance profile of Rhipicephalus (Boophilus) microplus populations from the States of Rio Grande do sul and Mato Grosso do sul, Brazil. Rev Bras Parasitol Vet 22:379–384. https://doi.org/10.1590/51984-29612013000300010
Grisi L, Leite RC, Martins JRS, Barros ATM, Andreotti R, Cançado PHD, Léon AAP, Pereira JB, Villela HS (2014) Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet 23:150–156. https://doi.org/10.1590/51984-29612014042
Raynal JT, Silva AAB, Sousa TJ, Bahiense TC, Meyer R, Portela RW (2013) Acaricides efficiency on Rhipicephalus (Boophilus) microplus from Bahia state North-Central region. Rev Bras Parasitol Vet 22:71–77. https://doi.org/10.1590/S1984-29612013005000006
Garcia MV, Rodrigues VS, Koller WW, Andreotti K (2019) Biologia E importância do carrapato Rhipicephalus (Boophilus) microplus. In: Andreotti R, Garcia MV (eds) Koller WW carrapatos na cadeia produtiva de bovinos. Embrapa, Brasília, Distrito Federal, pp 17–27
Klafke GM, Miller RJ, Tidwell JP, Thomas DB, Sanchez D, Arroyo TPF, Pérez de Léon AA (2019) High-resolution melt (HRM) analysis for detection of SNPs associated with pyrethroid resistance in the southern cattle fever tick, Rhipicephalus (Boophilus) microplus. Int J Parasitol Drugs Drug Resist 9:100–111. Acari: Ixodidaehttps://doi.org/10.1016/j.ijpddr.2019.03.001
Guerrero FD, Lovis L, Martins JR (2012) Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Rev Bras Parasitol Vet 21:1–6. https://doi.org/10.1590/s1984-29612012000100002
Food and Agriculture Organization– FAO (2004) Guidelines: Resistance Management and Integrated Parasite Control in Ruminants. Disponível em: http://ftp.fao.org/docrep/fao/010/ag014e 2004 Accessed 11 may 2023
Rey–Valeirón C, Pérez K,·Guzmán, Lopez–Vargas L, Valarezo J E (2018) Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae). Exp Appl Acarol 76:399–411. https://doi.org/10.1007/s10493-018-0303-6
Masetto MAM, Deschamps C, Mórgor AF, Bizzo HR (2011) Teor E composição do óleo essencial de inflorescências e folhas de Lavandula Dentata L. em diferentes estádios de desenvolvimento floral e épocas de colheita. Rev Bras Plantas Med 13:413–421. https://doi.org/10.1590/s1516-05722011000400007
Batiha GES, Teibo JO, Wasef L, Shaheen HM, Akomolafe AP, Teibo TKA, Al-Kuraishy HM, Al-Garbeeb AL, Alexiou A, Papadakis M (2023) A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn Schmiedebergs Arch Pharmacol 396:877–900. https://doi.org/10.1007/s00210-023-02392-x
Baracuhy JGV, Furtado DA, Francisco PRM, Lima FJLS, Pereira JPG (2016) Plantas medicinais de uso comum no Nordeste do Brasil. EDUFCG, Campina Grande, PB, p 205
Ribeiro PGF, Diniz RC (2008) Plantas aromáticas e medicinais: cultivo e utilização. IAPAR, Londrina, PR, p 218
Hocayen PAS, Pimenta DS (2013) Extrato De Plantas Medicinais como carrapaticida de Rhipicephalus (Boophilus) microplus. Rev Bras Plantas Med 15:627–631. https://doi.org/10.1590/s1516-05722013000500001
Adams RP (2007) Identification of essential oils components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Illinois, p 804
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr 11:463–471. https://doi.org/10.1016/s0021-9673(01)80947-x
Aragão H, Fonseca F (1961) Notas De Ixodologia. VIII. Lista E Chave Para Os Representantes Da Fauna Ixodológica Brasileira. Mem Inst Oswaldo Cruz 59:115–130. https://doi.org/10.1590/s0074-02761961000200001
Vieira AML, Souza CC, Labruna MB, Mayo RC, Souza SSL (2004) Manual de Vigilância Acarológica. Superintendência de controle de Endemias, SUCEN, São Paulo, p 66
Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus Annulatus and Boophilus microplus: laboratory tests of insecticides. J Econ Entomol 66:130–133. https://doi.org/10.1093/jee/66.1.130
Chagas ACS, Prates HT, Leite RC, Furlong J (2002) Ação larvicida de derivados arilsulfonílicos da (+)– cânfora e da (+)– isopinocanfona sobre o carrapato Boophilus microplus. Arq Bras Med Vet Zootec 54:462–477. https://doi.org/10.1590/s0102-09352002000500002
Pirali-Kheirabadi K, Silva JAT (2010) Lavandula angustifolia essential oil as a novel and promising natural candidate for tick (Rhipicephalus (Boophilus) annulatus) control. Exp Parasitol 126:184–186. https://doi.org/10.1016/j.exppara.2010.04.012
Wells R, Truong F, Adal AM, Sarker LS, Mahmoud SS (2018) Lavandula essential oils: a current review of applications in medicinal, food, and cosmetic industries of lavender. Nat Prod Commun 13:1403–1417. https://doi.org/10.1177/1934578X1801301038
Cruz EMO, Costa-Junior LM, Oliveira-Pinto JA, Santos DA, Alves S, Arrigoni-Blank MF, Bacci L, Barreto-Alves P, Cavalcanti SCH, Fitzgerald-Blank A (2013) Acaricidal activity of Lipia Gracilis essential oil and its major constituents on the tick Rhipicephalus (Boophilus) microplus. Vet Parasitol 195:198–202. https://doi.org/10.1016/j.vetpar.2012.12.046
Miyazawa M, Yamafuji C (2005) Inhibition of acetylcholinesterasic activity by biciclic monoterpenoids. J Agric Food Chem 53:1765–1768. https://doi.org/10.1021/jf040019b
Quadros DG, Johnson TL, Whitney TR, Oliver JD, Oliva Chávez AS (2020) Plant-Derived Natural compounds for Tick Pest Control in Livestock and Wildlife: pragmatism or Utopia? Insects 11(490). https://doi.org/10.3390/insects11080490
Acknowledgements
This article is based on the Masters of Sciences dissertation (Postgraduate Program in Health and Reproduction of Production Animals) of the first author, which was developed at the Federal Rural University of Pernambuco, with support from a fellowship from the Brazilian funding agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Xavier, C.M., Silva, E.H.A., de Siqueira, I.V.M. et al. Chemical Composition and Acaricidal Activity of Essential Oil of Lavandula dentata L. on Engorged Females of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Acta Parasit. 69, 1141–1147 (2024). https://doi.org/10.1007/s11686-024-00835-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-024-00835-w