Abstract
The developmental stages in the life cycle of Haemaphysalis qinghaiensis were investigated under laboratory conditions. The larval, nymphal and adult ticks were fed on sheep at 25–27 °C, 50 % relative humidity (RH) and exposed to daylight. All free-living stages were maintained in an incubator (28 °C with 90 % RH and a 12-h photoperiod). The whole life cycle of H. qinghaiensis was completed in an average of 176 days (range 118–247 days). The average developmental periods were 34.44 days for egg incubation; 5.83, 4.20 and 33.70 days for larval pre-feeding, feeding and pre-molting; and 3.88, 5.30 and 46.50 days for nymphal pre-feeding, feeding and pre-molting. The average times for pre-feeding, feeding, pre-oviposition and oviposition of female adult ticks were 2.60, 11.40, 8.50, and 19.35 days, respectively. The results confirmed the positive correlation between the weight of the engorged female and the egg mass laid (r = 0.557, P < 0.05). The reproductive efficiency index and reproductive fitness index in females were 5.49 and 4.98, respectively. Engorged nymphs moulting to females (4.53 ± 0.16 mg) were significantly heavier (P < 0.001) than those moulting to males (3.45 ± 0.19 mg). The overall sex ratio of the adult ticks was 1:1.1 (M:F).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Haemaphysalis qinghaiensis, an ixodid tick species, is only recorded in China (Gao et al. 2007a, b, 2008, 2009; Li et al. 2007). It is particularly prevalent in the western plateau, including the provinces of Qinghai, Gansu, Sichuan and Tibet (Li et al. 2007; Teng 1991). This three-host tick infests mainly ruminants (Gao et al. 2008) and its debilitating effects on its hosts mainly attribute to blood loss and transmission of pathogens, such as Theileria uilenbergi, Theileria luwenshuni, Theileria sinensis (Li et al. 2007, 2009; Yin et al. 2002a, b, c) and Babesia sp. BQ1 (Lintan) (Guan et al. 2002, 2010). Both Haemaphysalis qinghaiensis infestation and H. qinghaiensis-transmitted protozoal diseases resulted in a major economic burden for the animal husbandry in Northwestern China (Gao et al. 2008, 2009).
The present work was carried out to describe the life cycle of H. qinghaiensis under laboratory conditions. The results may provide basic background information for further investigations.
Materials and methods
Collection and rearing of ticks
Unfed adult H. qinghaiensis ticks were collected on vegetation in Lintan County (103.35E, 34.7N) of the Gannan Tibetan Autonomous Region during late March when they were actively seeking hosts (Sun et al. 2008). Colonies of the ticks were reared on sheep, and maintained in glass tubes sealed with hydrophilic cotton in an incubator, under controlled conditions (28 °C with 90 % RH and a 12-h photoperiod).
Animals
Six sheep (1-year old Tan mutton breed) were divided into three groups and were infested by larvae, nymph and adult ticks, respectively. They were maintained at 25–27 °C, 50 % RH and exposed to daylight. Muslin cloth bags (25 × 25 cm) were glued to the back of sheep, and ticks were reared in bags. Opening of the bag was bound so that ticks could not come out.
Observation of the biology of larvae and nymph ticks
Two hundred newly hatched larvae and 50 newly emerged nymphs were put onto the back of two sheep daily to determine their pre-feeding periods. Seven days after hatching/moulting, 200 unfed larvae and 50 unfed nymphs were weighed and fed on sheep. Naturally detached engorged ticks were collected, counted, weighed and then placed into separate tubes. These ticks were weighed in pools, in which 10 larvae were a pool and 5 nymphs were a pool with an analytic balance (Denver instrument TB-25, USA). During their non-feeding period, they were maintained in an incubator. The sex was recorded, after engorged nymphs moulting.
Observation of the biology of adult ticks
Freshly emerged adult ticks (25 females and 25 males) were placed on a sheep daily to determine whether they could attach and feed. Freshly males and females were maintained without food for a specified pre-feeding period of 4 days, and then were weighed before put on the back of a sheep for blood-feeding. The engorged females were collected, weighed and put into an incubator. The pre-oviposition and oviposition periods and egg masses laid were observed daily. Daily deposited eggs were collected, counted, and placed into separate glass tubes. In total, 500 eggs were used for observing the incubation period. After hatching, the percentage of hatched larvae was calculated. Finally, the reproductive efficiency index (REI) (amount of eggs/weight of engorged female) (Drummond and Whetstone 1970) and reproductive fitness index (RFI) (amount of eggs incubated to larvae/weight of engorged female) (Chilton 1992) were calculated.
Observation of the longevity of unfed ticks
Freshly emerged larvae (300), nymphs (100) and adults (50) were maintained in an incubator, under controlled conditions (28 °C with 90 % RH and a 12 h-photoperiod), and their longevities were observed.
Data analysis
To reveal the relationship among duration of feeding periods, weight of engorged females, length of oviposition and number of eggs laid, several Spearman’s correlations and Student’s t tests were applied. The further statistical analyses were performed using SPSS (version 11.5).
Results
Life cycle of H. qinghaiensis
Under laboratory conditions, the complete life cycle of H. qinghaiensis had a mean duration of 176 days (range 118–247 days), the average developmental periods were 34.44 days for egg incubation, 5.83, 4.20 and 33.70 days for larval pre-feeding, feeding and pre-molting, respectively and 3.88, 5.30 and 46.50 days for the same stages of nymphs, respectively. The average times for pre-feeding, feeding, pre-oviposition and oviposition of female adult ticks were 2.60, 11.40, 8.50, and 19.35 days, respectively. The duration of the stages is shown in Table 1.
Feeding biology and changes of body weight
The average weights of the larvae, nymphs and adults before and after feeding are given in Table 2. In addition, the weights of engorged female and male nymphs ranged from 3.7 to 5.4 mg and from 2.4 to 4.6 mg, respectively. The Student’s t test revealed that the mean weight of engorged nymphs molting to females (4.53 ± 0.16 mg) was significantly heavier (P < 0.001) than that of nymphs molting to males (3.45 ± 0.19 mg). However, their engorged weights overlapped. The relationship between the body weights of H. qinghaiensis nymphs and the resultant sex was recorded. The ratio of males to females was 1:1.1. The Fig. 1 illustrated the distribution emergence rates of males and females from engorged nymphs.
Oviposition
The oviposition data are presented in Table 3. Linear regression analysis revealed a positive correlation between the weight of the engorged females and the egg mass laid (r = 0.557, P < 0.05) (Fig. 2).
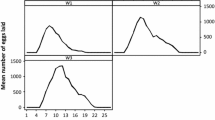
The oviposition pattern of H. qinghaiensis is presented in Fig. 3 (based on 25 females). The number of eggs laid initially was low but increased rapidly to a peak on day 7, and then gradually declined. The percentage of hatched eggs (hatched eggs/total eggs) was on average 90.7 %. The female reproductive efficiency index (REI) (amount of eggs/weight of engorged female) and reproductive fitness index (RFI) (amount of eggs incubated to larvae/weight of engorged female) were calculated to be 5.49 and 4.98 on the basis of the data in Table 3.
Longevity
Under controlled conditions (28 °C with 90 % RH and a 12-h photoperiod), unfed H. qinghaiensis larvae survived for 150–190 days (larvae started to die on the day 150, and they died off on the day 190), nymphs for 170–300 days, and unfed adults for 200–500 days after hatching or molting.
Discussion
Haemaphysalis qinghaiensis, a typical three-host tick, has only been reported from China (Gao et al. 2008). Its natural hosts include sheep, goat, yak, cattle and hare (Lepus oiostolus) (Teng. 1988). It is known that all stages of the tick could develop in sheep, goat, yak and cattle (Li et al. 2007, 2009; Yin et al. 2002a, b, c; Guan et al. 2002, 2010). However, few studies on its life cycle have been published.
The present work revealed that life cycle of H. qinghaiensis needed an average period of 163.39 days (excluding the prefeeding stages of larvae, nymph and adult ticks) under laboratory conditions, which is longer than that of Haemaphysalis doenitzi (100.2 days on average) (Chen et al. 2012a), Hyalomma aegyptium (147 days on average) (Široký et al. 2011), Haemaphysalis punctata (146 days on average) (Yin and Lu 1995), Hyalomma asiaticum kozlovi (144.8 days on average) (Chen et al. 2009) and Rhipicephalus bursa (142.45 days on average) (Yeruham et al. 2000), and shorter than Hyalomma rufipes (173.7 days on average) (Chen et al. 2012b) and Amblyomma ovale (190 days on average) (Martins et al. 2012). This discrepancy in life cycle was vastly observed in different tick species, which might also due to different host species (Labruna et al. 2002; Martins et al. 2012) and temperature (Chilton et al. 2000; Yano et al. 1987), ect.
It is known there is a significant relationship between the weight of the engorged nymph and the resulting sex (Chilton et al. 2000), which is often used as an index to predict tick sex. For example, in Ixodes scapularis, fully engorged nymphs molting into females weighed between 3.8 and 6.4 mg, while those nymphs molting into males weighed between 2.0 and 3.2 mg, with the difference in nymphal weight being statistically significant (Hu and Rowley 2000). However, despite this discrepancy in the nymphal weight of some other tick species, such as Dermacentor silvarum, there was no relationship between engorged nymphal body weight and the resulting sex of the adult (58.4–70.2 mg for males and 57.1–73.3 mg for females) (Liu et al. 2005). In the current work, engorged H. qinghaiensis nymphs molting into females were significantly heavier (4.53 ± 0.16 mg) than those molting into males (3.45 ± 0.19 mg). However, the weight range of engorged female nymphs (3.7–5.4 mg) showed some overlaps with that of the engorged male nymphs (2.4–4.6 mg). Similar results are also observed in Hyalomma asiaticum kozlovi (Chen et al. 2009), Dermacentor variabilis (Hu and Rowley 2000) and Hyalomma rufipes (Chen et al. 2012b). In the current study, the adult sex ratio observed was nearly equal, and the ratio of male to female was 1:1.1. Similar results were found for Haemaphysalis doenitzi (Chen et al. 2012a) and H. aegyptium (Široký et al. 2011). Such a trait could be caused by mating habits with long-lasting host attachment by males (Široký et al. 2011; Siroky et al. 2006).
In comparison with other tick species, H. qinghaiensis (193.72 mg of engorged female) is a medium-size tick. Before and after feeding, the average weights of H. qinghaiensis adult females increased by approximately 99.34-fold. Other ticks, such as H. doenitzi, the increase was 174.42-fold (Chen et al. 2012a), in H. longicornis 102.6-fold (Zheng et al. 2011), in H. rufipes 51.36-fold (Chen et al. 2012b) and in D. silvarum 76.1-fold (Liu et al. 2005). Linear regression analysis revealed a positive correlation between the weights of engorged H. qinghaiensis females and the number of eggs laid (r = 0.557, P < 0.05). The mean number of eggs laid was approximately 1,063 in H. qinghaiensis, compared with 2,852 in H. doenitzi (Chen et al. 2012a) and 3,335 in H. punctata (Yin and Lu 1995). Therefore, the mean number of eggs laid of H. qinghaiensis is lower than those of other Haemaphysalis species.
The oviposition pattern of Haemaphysalis qinghaiensis was similar to other species of Haemaphysalis (Chen et al. 2012b; Zheng et al. 2011), laid most of the eggs in the first week. Oviposition peaked at day 5–7 and then declined rapidly throughout the remaining oviposition period. A similar curve has been record for other tick species (Chen et al. 2012b; Rechav and Knight 1983).
References
Chen Z, Yu Z, Yang X, Zheng H, Liu J (2009) The life cycle of Hyalomma asiaticum kozlovi Olenev, 1931 (Acari: Ixodidae) under laboratory conditions. Vet Parasitol 160:134–137
Chen X, Yu Z, Guo L, Li L, Meng H, Wang D, Liu R, Liu J (2012a) Life cycle of Haemaphysalis doenitzi (Acari: Ixodidae) under laboratory conditions and its phylogeny based on mitochondrial 16S rDNA. Exp Appl Acarol 56:143–150
Chen Z, Li Y, Liu Z, Yang J, Yin H (2012b) The life cycle of Hyalomma rufipes (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 56:85–92
Chilton NB (1992) An index to assess the reproductive fitness of female ticks. Int J Parasitol 22:109–111
Chilton NB, Andrews RH, Bull CM (2000) Influence of temperature and relative humidity on the moulting success of Amblyomma limbatum and Aponomma hydrosauri (Acari: Ixodidae) larvae and nymphs. Int J Parasitol 30:973–979
Drummond RO, Whetstone TM (1970) Oviposition of the Gulf Coast tick. J Econ Entomol 63:1547–1551
Gao J, Luo J, Fan R, Guan G, Ren Q, Ma M, Sugimoto C, Bai Q, Yin H (2007a) Molecular characterization of a myosin alkali light chain-like protein, a “concealed” antigen from the hard tick Haemaphysalis qinghaiensis. Vet Parasitol 147:140–149
Gao J, Luo J, Li Y, Fan R, Zhao H, Guan G, Liu J, Wiske B, Sugimoto C, Yin H (2007b) Cloning and characterization of a ribosomal protein L23a from Haemaphysalis qinghaiensis eggs by immuno screening of a cDNA expression library. Exp Appl Acarol 41:289–303
Gao J, Luo J, Fan R, Fingerle V, Guan G, Liu Z, Li Y, Zhao H, Ma M, Liu J (2008) Cloning and characterization of a cDNA clone encoding calreticulin from Haemaphysalis qinghaiensis (Acari: Ixodidae). Parasitol Res 102:737–746
Gao J, Luo J, Fan R, Schulte-Spechtel UC, Fingerle V, Guan G, Zhao H, Li Y, Ren Q, Ma M (2009) Characterization of a concealed antigen Hq05 from the hard tick Haemaphysalis qinghaiensis and its effect as a vaccine against tick infestation in sheep. Vaccine 27:483–490
Guan G, Yin H, Luo J, Lu W, Zhang Q, Gao Y, Lu B (2002) Transmission of Babesia sp. to sheep with field-collected Haemaphysalis qinghaiensis. Parasitol Res 88:22–24
Guan G, Moreau E, Liu J, Hao X, Ma M, Luo J, Chauvin A, Yin H (2010) Babesia sp. BQ1 (Lintan): Molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol Int 59:265–267
Hu R, Rowley WA (2000) Relationship between weights of the engorged nymphal stage and resultant sexes in Ixodes scapularis and Dermacentor variabilis (Acari: Ixodidae) ticks. J Med Entomol 37:198–200
Labruna MB, Kasai N, Ferreira F, Faccini JLH, Gennari SM (2002) Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet Parasitol 105:65–77
Li Y, Luo J, Liu Z, Guan G, Gao J, Ma M, Dang Z, Liu A, Ren Q, Lu B (2007) Experimental transmission of Theileria sp. (China 1) infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol Res 101:533–538
Li Y, Luo J, Guan G, Ma M, Liu A, Liu J, Ren Q, Niu Q, Lu B, Gao J (2009) Experimental transmission of Theileria uilenbergi infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol Res 104:1227–1231
Liu J, Liu Z, Zhang Y, Yang X, Gao Z (2005) Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 36:131–138
Martins TF, Moura MM, Labruna MB (2012) Life-cycle and host preference of Amblyomma ovale (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 56:151–158
Rechav Y, Knight M (1983) Life cycle of Rhipicephalus oculatus (Acari: Ixodidae) in the laboratory. Ann Entomol Soc Am 76:470–472
Siroky P, Petrzelkova K, Kamler M, Mihalca A, Modry D (2006) Hyalomma aegyptium as dominant tick in tortoises of the genus Testudo in Balkan countries, with notes on its host preferences. Exp Appl Acarol 40:279–290
Široký P, Erhart J, Petrželková KJ, Kamler M (2011) Life cycle of tortoise tick Hyalomma aegyptium under laboratory conditions. Exp Appl Acarol 54:1–8
Sun C, Liu Z, Gao J, Guan G, Ma M, Luo J, Yin H (2008) Investigations into the natural infection rate of Haemaphysalis qinghaiensis with Piroplasma using a nested PCR. Exp Appl Acarol 44:107–114
Teng K (1991) Economic insect fauna of China. Fasc 39, Acari: Ixodidae Science Press, Beijing (in Chinese)
Yano Y, Shiraishi S, Uchida T (1987) Effects of temperature on development and growth in the tick, Haemaphysalis longicornis. Exp Appl Acarol 3:73–78
Yeruham I, Hadani A, Galker F (2000) The life cycle of Rhipicephalus bursa Canestrini and Fanzago, 1877 (Acarina: Ixodidae) under laboratory conditions. Vet Parasitol 89:109–116
Yin H, Lu W (1995) The life cycle of Haemaphysalis punctata. Chin J Vet Sci Tech 25:8–10 (in Chinese)
Yin H, Guan G, Ma M, Luo J, Lu B, Yuan G, Bai Q, Lu C, Yuan Z, Preston P (2002a) Haemaphysalis qinghaiensis ticks transmit at least two different Theileria species: one is infective to yaks, one is infective to sheep. Vet Parasitol 107:29–35
Yin H, Luo J, Guan G, Gao Y, Lu B, Zhang Q, Ma M, Lu W, Lu C, Yuan Z (2002b) Transmission of an unidentified Theileria species to small ruminants by Haemaphysalis qinghaiensis ticks collected in the field. Parasitol Res 88:25–27
Yin H, Luo J, Guan G, Lu B, Ma M, Zhang Q, Lu W, Lu C, Ahmed J (2002c) Experiments on transmission of an unidentified Theileria sp. to small ruminants with Haemaphysalis qinghaiensis and Hyalomma anatolicum anatolicum. Vet Parasitol 108:21–30
Zheng H, Yu Z, Chen Z, Zhou L, Zheng B, Ma H, Liu J (2011) Development and biological characteristics of Haemaphysalis longicornis (Acari: Ixodidae) under field conditions. Exp Appl Acarol 53:377–388
Acknowledgments
This study was financially supported by the NSFC (30972182, 30800820, 31072130, 31101621 and 31001061), 948 (2010-S04), Beef and Yak Production System Programme, Specific Fund for Sino-Europe Cooperation, MOST. The research was also facilitated by EPIZONE (FOOD-CT-2006-016236), ASFRISK (211691), ARBOZOONET (211757), and PIROVAC (KBBE-3-245145) of the European Commission, Brussels, Belgium.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, M., Guan, G., Chen, Z. et al. The life cycle of Haemaphysalis qinghaiensis (Acari: Ixodidae) ticks under laboratory conditions. Exp Appl Acarol 59, 493–500 (2013). https://doi.org/10.1007/s10493-012-9617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9617-y